Back to Journals » International Medical Case Reports Journal » Volume 10
Bilateral herpes zoster in a patient with end-stage kidney disease
Authors Akimoto T , Muto S, Nagata D
Received 30 March 2017
Accepted for publication 27 May 2017
Published 19 June 2017 Volume 2017:10 Pages 209—212
DOI https://doi.org/10.2147/IMCRJ.S138398
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Tetsu Akimoto, Shigeaki Muto, Daisuke Nagata
Division of Nephrology, Department of Internal Medicine, Jichi Medical University, Shimotsuke-Shi, Tochigi, Japan
Abstract: Herpes zoster (HZ) is caused by the reactivation of a latent varicella-zoster virus (VZV) infection within the cranial or dorsal root ganglia. The cutaneous lesions of HZ are typically limited to a single dermatome, while non-contiguous HZ involving two or more dermatomes is a very rare clinical entity. In this report, we describe a case of HZ involving the left and right side of the abdomen corresponding to the T11 dermatome in a 63-year-old man on chronic peritoneal dialysis. The characteristic cutaneous manifestation encouraged us to ascribe the disease to HZ duplex bilateralis, and the patient was given a single dose of oral valacyclovir and achieved a favorable outcome. The therapeutic concerns regarding the reactivation of VZV in patients with end-stage kidney disease are also discussed.
Keywords: herpes zoster duplex bilateralis, end-stage kidney disease, peritoneal dialysis, valacyclovir, dermatome
Introduction
Herpes zoster (HZ), which is caused by the reactivation of a latent varicella-zoster virus (VZV) infection within the cranial or dorsal root ganglia, typically manifests as a characteristic vesicular rash with a unilateral distribution limited to a single dermatome.1 Bilateral dermatomal involvement is a rare phenotype of this disease and has been referred to as HZ duplex or multiplex bilateralis.2,3 In this report, we describe our unique experience with one such case involving a man with end-stage kidney disease.
Case report
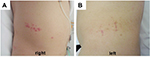
A 63-year-old male with end-stage kidney disease due to diabetic nephropathy had been undergoing continuous ambulatory peritoneal dialysis for 3 years when he sought medical advice regarding the appearance of grouped red crusts on the left and right sides of his abdomen. The problem initially started as the development of painful erythematous vesicles on the left flank corresponding to the T11 dermatome approximately 15 days prior to presentation and on the right side of his abdomen 5 days previously. The patient reported a history of prodromal pain on the left side of the abdominal region approximately 3 weeks prior to the onset of this disease. A few days later, grouped vesicular eruptions developed, lasting approximately 1 week. Thereafter, the lesions became encrusted; some of them healed with residual scarring and pigmentation, but new painful vesicular lesions appeared on the right side of his abdomen at almost the same level as that observed on the left side (T11 dermatome) (Figure 1). A physical examination revealed the patient to be afebrile, alert, and oriented, and no abnormalities other than the cutaneous manifestation were found. Laboratory evaluations revealed hemoglobin 11.7 g/dL, hematocrit 35.4%, white blood cell count 10,800/μL (neutrophils 71.1%, lymphocytes 14.1%, monocytes 6.3%, eosinophils 6.5%, basophils 2.0%), platelet counts 33,0000/μL, blood urea nitrogen 40 mg/dL, serum creatinine 17.68 mg/dL, fasting blood sugar 151 mg/dL, and hemoglobin A1c 8.4%, while the chest X-ray findings and liver function were normal. The characteristic cutaneous manifestation encouraged us to ascribe the disease to HZ duplex bilateralis, and he was given a single dose of 250 mg oral valaciclovir. Overall, the patient’s clinical course was favorable, and the pain subsided without any complications. Finally, elevated serum titers of both anti-VZV immunoglobulin (Ig)M (IgM index 2.9, cut-off index 0.8) and IgG (3,400 IU/mL) were confirmed.
Discussion
HZ is a not uncommon neurocutaneous disease, and a reduction in the cell-mediated immunity, either as a natural consequence of aging or as a result of immunosuppression, has received focus as the main pathogenic basis for herpes reactivation.1,4,5 Diabetes, genetic susceptibility, mechanical trauma, psychological stress, malignancies, and renal failure have all been included in the list of risk factors for HZ as well.1,5–7 Furthermore, even the mode of renal replacement therapy may predispose subjects with end-stage chronic kidney disease to be at an increased risk for HZ. Indeed, it has been demonstrated that peritoneal dialysis patients are at a higher risk of developing HZ as well as experiencing complications of zoster than hemodialysis patients.8 In this regard, the clinical picture of the current patient may not be very surprising. However, the clinical impact of the present report should be evaluated carefully given the few studies describing cases of bilateral HZ in patients on chronic peritoneal dialysis.
A characteristic zoster rash may occur in any dermatome, with thoracic, cranial and cervical distributions being the most common.5,9,10 While the segmental nature of the disease may depend on the VZV genome load distribution in different dorsal root ganglia, with the highest viral genome load leading to clinical HZ,2,11,12 subsequent reactivations should presumably be prevented by concurrent VZV-specific immuno-boosting associated with clinical zoster eruptions.2 Non-contiguous HZ involving two or more dermatomes is a very rare phenotype, with an incidence ranging from 0.1% to 0.5%.2,11 It is believed that the occurrence of such a pathology mirrors random, independent, and simultaneous VZV reactivation in two or more separate locations, thus lending support to the concept that VZV remains latent in the majority of the sensory dorsal root ganglia.2,13 Indeed, the deoxyribonucleic acid of VZV has been detected in the trigeminal, cervical, thoracic, lumbar, and sacral dorsal root ganglia in autopsy studies.13 Considering the anatomical proximity of the left and right side of zoster rash, viral diffusion from one ganglion to another or spread across the spinal cord may be one of presumable mechanisms for the development of HZ duplex bilateralis in the current patient although this concept is quite speculative.14,15 Finally, it is difficult to determine the precise pathogenic basis for the disease in the present patient, although the administration of peritoneal dialysis as well as the presence of concurrent diabetes might be at least partially responsible for predisposing our patient to the milieu facilitating the reactivation of VZV in two separate ganglia independently and concurrently.5,6,8
Other atypical cutaneous manifestations of HZ include nodular, keratotic papular, ulcerative, ecthymatous, and gangrenous lesions, which can be seen especially in patients with immunosuppressed conditions.16,17 Most cases with HZ duplex bilateralis have also occurred in immunocompromised patients, while bilateral HZ occurring in immunocompetent subjects has been reported and described anecdotally2,3,11 and does not seem to represent a risk factor for a poor prognosis, since most subjects with this disease present without visceral dissemination or lethal complications.2 As such, conventional managements, including the administration of an antiviral agent, pain relief, and treating the skin lesions—the same treatments applied for the common form of HZ—have been practiced empirically for this condition, despite its atypical rash distribution.3,11,18 One may argue that the timing of our patient’s presentation might have been too late for the initiation of any beneficial antiviral treatment, since various clinical trials have shown the clinical benefit of starting antiviral treatments within the first 72 h after the onset of cutaneous HZ.19 Nonetheless, we believe that it is still prudent to treat subjects who present after this therapeutic window if new vesicular lesions are clearly continuing to appear, as was seen in the current patient.9 After oral administration, valacyclovir undergoes first-pass intestinal and/or hepatic metabolism to produce active moiety acyclovir, which is mainly eliminated through the renal route.20 It is increasingly recognized that subjects with chronic renal insufficiency are susceptible to valacyclovir neurotoxicity, so dosing adjustments proportionate to renal impairment should be carried out.21–23 Although the dosage of valacylovir used in the current case was obviously below that recommended for subjects with severely reduced renal clearance,20 the therapeutic response seemed to be favorable, and the clinical course of the present patient was consistently uneventful. While the validity of our therapeutic policy remains to be confirmed, the fact that the valacyclovir-mediated neuropsychotic events, the diverse manifestation of which may include unconsciousness, confusion, hallucination, dysarthria, and ataxia, can occasionally develop in patients with end-stage kidney disease, even at an adjusted dosage in accordance with the recommendation,21–23 obliged us to proactively take such a careful approach. We believe the accumulation of more experience with additional cases similar to ours is a matter requiring continuous attention and will aid in the establishment of an optimal management protocol for end-stage kidney disease patients with VZV reactivation as well as help elucidate the pathogenic basis for HZ duplex bilateralis.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Research on Advanced Chronic Kidney Disease, Practical Research Project for Renal Diseases from the Japan Agency for Medical Research and Development (AMED).
Written informed consent was obtained from the patient to publish this case report and any accompanying images.
Disclosure
The authors report no conflicts of interest in this study.
References
Weinberg JM. Herpes zoster: epidemiology, natural history, and common complications. J Am Acad Dermatol. 2007;57(6 Suppl):S130–S135. | ||
Castronovo C, Nikkels AF. Chronic herpes zoster duplex bilateralis. Acta Derm Venereol. 2012;92(2):148–151. | ||
Agrawal S, Aara N, Bumb R. Herpes zoster duplex bilateralis symmetricus in an immunocompetent subject. Int J Dermatol. 2014;53(4): e281–e282. | ||
Akimoto T, Yamazaki T, Saito O, Muto S, Kusano E, Nagata D. A Supraglottic Pseudotumor in an Immunocompromised Patient with Nephrotic Syndrome, Herpes Zoster, and a Cytomegalovirus Infection. Clin Med Insights Case Rep. 2016;9:61–65. | ||
Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol. 2010;48 Suppl 1:S2–S7. | ||
Hata A, Kuniyoshi M, Ohkusa Y. Risk of Herpes zoster in patients with underlying diseases: a retrospective hospital-based cohort study. Infection. 2011;39(6):537–544. | ||
Wu MY, Hsu YH, Su CL, Lin YF, Lin HW. Risk of herpes zoster in CKD: a matched-cohort study based on administrative data. Am J Kidney Dis. 2012;60(4):548–552. | ||
Lin SY, Liu JH, Lin CL, et al. A comparison of herpes zoster incidence across the spectrum of chronic kidney disease, dialysis and transplantation. Am J Nephrol. 2012;36(1):27–33. | ||
Cohen JI, Brunell PA, Straus SE, Krause PR. Recent advances in varicella-zoster virus infection. Ann Intern Med. 1999;130(11):922–932. | ||
Gross G, Schöfer H, Wassilew S, et al. Herpes zoster guideline of the German Dermatology Society (DDG). J Clin Virol. 2003;26(3):277–289. | ||
Takaoka Y, Miyachi Y, Yoshikawa Y, Tanioka M, Fujisawa A, Endo Y. Bilateral disseminated herpes zoster in an immunocompetent host. Dermatol Online J. 2013;19(2):13. | ||
Pevenstein SR, Williams RK, McChesney D, Mont EK, Smialek JE, Straus SE. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J Virol. 1999;73(12):10514–10518. | ||
Kennedy PG, Cohrs RJ. Varicella-zoster virus human ganglionic latency: a current summary. J Neurovirol. 2010;16(6):411–418. | ||
Peretz A, Nowatzky J, Steiner I. Neurological picture. Herpes zoster duplex bilateralis. J Neurol Neurosurg Psychiatry. 2007;78(8):818. | ||
Yi A, Chernev I. The pathogenesis of herpes zoster duplex bilateralis symmetricus. Int J Dermatol. 2015;54(3):e89–90. | ||
Gnann JW Jr. Varicella-zoster virus: atypical presentations and unusual complications. J Infect Dis. 2002;186 Suppl 1:S91–S98. | ||
Tanaka A, Hayaishi N, Kondo Y, Kurachi K, Tanemura A, Katayama I. Severe gangrene accompanied by varicella zoster virus-related vasculitis mimicking rheumatoid vasculitis. Case Rep Dermatol. 2014;6(1):103–107. | ||
Shin JW, Kim DH, Whang KU, et al. A case of zoster duplex bilateralis. Ann Dermatol. 2009;21(4):423–425. | ||
Wood MJ, Shukla S, Fiddian AP, Crooks RJ. Treatment of acute herpes zoster: effect of early (< 48 h) versus late (48-72 h) therapy with acyclovir and valaciclovir on prolonged pain. J Infect Dis. 1998;178 Suppl 1:S81–S84. | ||
Ormrod D, Goa K. Valaciclovir: a review of its use in the management of herpes zoster. Drugs. 2000;59(6):1317–1340. | ||
Izzedine H, Mercadal L, Aymard G, et al. Neurotoxicity of valacyclovir in peritoneal dialysis: a pharmacokinetic study. Am J Nephrol. 2001;21(2):162–164. | ||
Okada T, Nakao T, Matsumoto H, et al. Valacyclovir neurotoxicity in a patient with end-stage renal disease treated with continuous ambulatory peritoneal dialysis. Clin Nephrol. 2002;58(2):168–170. | ||
Asahi T, Tsutsui M, Wakasugi M, et al. Valacyclovir neurotoxicity: clinical experience and review of the literature. Eur J Neurol. 2009;16(4):457–460. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.