Back to Journals » International Medical Case Reports Journal » Volume 13
Bickerstaff’s Brainstem Encephalitis Suspected as Functional Neurologic Disorders
Authors Okayasu H , Yasui-Furukori N , Kadowaki T, Funakoshi K, Hirata K, Shimoda K
Received 14 February 2020
Accepted for publication 27 April 2020
Published 18 May 2020 Volume 2020:13 Pages 177—181
DOI https://doi.org/10.2147/IMCRJ.S249818
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Hiroaki Okayasu,1 Norio Yasui-Furukori,1 Taro Kadowaki,2 Kei Funakoshi,2 Koichi Hirata,2 Kazutaka Shimoda1
1Department of Psychiatry, Dokkyo Medical University, Tochigi, Japan; 2Department of Neurology, Dokkyo Medical University, Tochigi, Japan
Correspondence: Hiroaki Okayasu Tel +81-282-86-1111
Email [email protected]
Abstract: Functional neurologic disorders feature nervous system symptoms that cannot be explained by a neurological disease or other medical condition. The patient described here was a 21-year-old Japanese woman who was initially diagnosed with a functional neurologic disorder based on numbness and weakness of the limbs with no abnormalities in routine examinations. Further detailed examinations revealed monocytes in cerebrospinal fluid (CSF), and electroencephalography revealed widespread, low-voltage, slow waves with concentrated spindle waves. Thus, encephalitis was suspected, and steroid pulse therapy was initiated. Her symptoms subsequently improved. Afterward, CSF analysis was positive for serum anti-GQ1b IgG antibodies. We made a final diagnosis of Bickerstaff’s brainstem encephalitis (BBE). Our report describes the difficult differentiation of functional neurologic disorders from BBE. Physicians and psychiatrists should be aware of BBE.
Keywords: Bickerstaff’s brainstem encephalitis, functional neurologic disorder, Guillain-Barré syndrome, Miller Fisher syndrome, consciousness disturbance
Introduction
Functional neurologic disorders feature nervous system symptoms that cannot be explained by a neurological disease or other medical conditions.1 However, the symptoms are real and cause significant distress or problems in functioning.1 The cause of functional neurologic disorders is unknown.2 These conditions may be triggered by a neurological disorder or as a reaction to stress or psychological or physical trauma, but that is not always the case.1,2 Signs and symptoms include weakness or paralysis, abnormal movement, loss of balance, dysphagia, seizures or episodes of shaking and apparent loss of consciousness, episodes of unresponsiveness, numbness or loss of touch sensation, speech problems, vision problems, and hearing problems or deafness.1
We report our experience with a patient who had been suspected as a functional neurologic disorder and was diagnosed with Bickerstaff’s brainstem encephalitis (BBE), a very rare disease of the central nervous system, after admission to our hospital. This case report was approved by the ethics committees of Dokkyo Medical University. The patient gave written consent for the publication of this case report.
Case Presentation
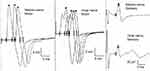
The patient was a 21-year-old woman who suffered from numbness and weakness of the limbs. She had no abnormalities on blood tests or physical examination and was accordingly suspected of having a psychological disorder. On the same day, she was examined at a psychiatric clinic. She was diagnosed with a functional neurologic disorder triggered by psychological stress, such as conflicts with her supervisor at her workplace and her parents. She was treated with the anxiolytic alprazolam (1.2 mg). She then developed symptoms such as numbness of the tongue, headache, and difficulty opening her eyes. She also complained that her vision was unfocused and that she had noticed circular eye motions (nystagmus) in the mirror. The next day, she suddenly felt exhausted and was unable to walk unaided, whereupon she presented at the Department of Psychiatry. On examination, she sat slumped backward in a wheelchair and responded to questions with a slight nod of the head. When asked if there were any people she found disagreeable at her workplace, she suddenly raised her voice. She was thus provisionally diagnosed as being catatonic due to a functional neurologic disorder triggered by psychological stressors from her medical and occupational history. We admitted her to our hospital on the same day for a detailed evaluation. Immediately after admission, she responded only by nodding when spoken to, until her elder sister arrived to visit, whereupon she suddenly became excited and started crying and talking loudly. She later became unresponsive and started groaning as if she was in pain, crying out in a loud voice, and making backward jerking motions with her body. She had a temperature of 37.2°C, a pulse of 72 beats/min, and blood pressure of 106/67 mmHg. Her pupillary diameter was 5 mm, and the light reflex was normal bilaterally. The deep tendon reflex was normal, and the Babinski sign was negative. Blood tests revealed no abnormalities in liver or kidney function or electrolytes. Thyroid function indices (free thyroxine, free triiodothyronine, thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibody (TRAb)) were within the normal range; however, serum anti-thyroglobulin antibody (TgAb) and anti-thyroid peroxidase antibody (TPOAb) were positive (630.0 IU/mL and 15.4 IU/mL). Magnetic resonance imaging (MRI) of the head region revealed no obvious abnormalities in the brain parenchyma, cerebral blood vessels, or intracranial structures. Electroencephalography (EEG) revealed widespread, low-voltage, slow waves between 5 and 7 Hz, with 4- and 15-Hz spindle waves concentrated in the frontal, parietal, and occipital regions. The frequencies of these waves remained the same after photic stimulation. Five days after admission, she showed no response to stimulation. We concluded that her altered consciousness was more likely to be due to an organic disease than catatonia; thus, she was referred to a neurologist. The patient also had pyrexia and nuchal rigidity after admission. Therefore, we performed cerebrospinal fluid (CSF) analysis, which showed a slight increase in monocytes (7 cells/µL) and normal glucose (0 mg/dl) and protein (41 mg/dl) levels. Herpes simplex virus antibody and herpes simplex virus DNA testing were not performed because we thought that the possibility was low due to the cell count, glucose and protein levels in CSF. TPOAb and TgAb in CSF were negative. CSF did not show albuminocytologic dissociation, with 7 cells/µL and protein at 41 mg/dl. Oligoclonal bands were not evaluated. Compound motor action potentials of the median and ulnar nerve were in the normal range, and sensory conditions of the median and ulnar nerve were in the normal range in a nerve conduction velocity examination (Figure 1). Ultrasonography revealed distinct thyroid enlargement. Consequently, Hashimoto’s encephalopathy was initially suspected as more likely than infectious brain inflammation, such as herpetic encephalitis, and the patient was started on steroid pulse therapy (methylprednisolone at 1 g for 3 days). Her consciousness level improved soon after completing steroid pulse therapy, and by day 4 posttreatment (day 10 after admission), she was able to converse and eat. She underwent further neurological examinations because of the improved state of consciousness and complained of double vision and numbness of the left arm from the shoulder to the fingertips. She also had abductive movement disorder of the right eye, reduced grip strength (9.6/8.2 kg), and cerebellar ataxia (clumsiness while performing rapid finger-nose, heel-knee, and hand pronation/supination movements). At that point (11 days after admission), we started another round of steroid pulse therapy (methylprednisolone at 1 g for 3 days), which resulted in almost total amelioration of the abductive dyskinesia of the right eye (with just a small degree of double vision in the right field). Subsequently, she underwent rehabilitation, regained the ability to walk and was discharged from the hospital 35 days after admission. At discharge, she had improved and had no ataxia and only residual numbness in the left index finger. Her grip strength had also improved to 21.6/21.4 kg. Subsequently, CSF was negative for anti-N-methyl-D-aspartate receptor (NMDAR) antibody. However, serum anti-GQ1b and anti-GT1a antibodies were positive; therefore, the patient’s final diagnosis was BBE. She has not undergone chronic immunosuppressive treatment; however, she has not had a recurrence during the follow-up period (approximately 1 year).
Discussion and Conclusion
Our report describes the difficult differentiation of functional neurologic disorders from BBE for the first time in the literature worldwide. A study of 100 consecutive patients who were newly admitted to a neurology ward found that 14% had no objective evidence of neurologic disease and might be diagnosed with functional neurologic disorders.3 We made a definitive diagnosis of BBE based on positivity for anti-GQ1b and anti-GT1a IgG antibodies and typical symptoms, such as limb weakness and numbness, ophthalmoplegia and ataxia, and disturbance of consciousness.4 We ruled out the possibility of bacterial or viral encephalitis based on low cell counts, normal glucose levels and protein levels. Therefore, we performed various autoantibody tests (TPOAb, TgAb, TRAb, anti-NMDAR antibody, anti-GQ1b and anti-GT1a IgG antibodies), although we could not fully exclude the possibility of concomitant Hashimoto’s encephalopathy due to the lack of anti-NAE antibody testing. Serum IgG antibodies against various gangliosides (GM1, GM1b, GD1a, GalNAc-GD1a, GD1b, GT1a and GQ1b; 5 pmol/well) were measured by ELISA, as previously described.5 In this case, serum was considered positive when the optical density was 0.5 or more at a dilution of 1:500, which is a higher threshold than we use in most studies. The present definition is based on our previous report.6
BBE develops from a prodrome that includes headache and pyrexia, followed by progressive alteration of consciousness, and then symptoms such as ataxia, external ophthalmoplegia, facial paralysis, dysphagia, and peripheral muscle weakness develop.4 Symptoms become fixed at 1 to 8 weeks after onset, and patients mostly recover by 3 to 18 months later. Yuki et al and Chiba et al have shown that BBE, Miller Fisher syndrome (MFS), and Guillain-Barré syndrome (GBS) are immunological disorders with common features. Their reports describe the detection of anti-GQ1b IgG antibodies in BBE patients; these are the same autoantibodies that are detected in MFS and GBS (which also feature elevated anti-GQ1b IgG antibody levels), and GBS is accompanied by ocular motility disorders.7–10 Ogawara et al investigated electrophysiological conditions in anti-GQ1b IgG antibody-positive patients with somnolence and unilateral sensory disorders in addition to the three classic signs of MFS (external ophthalmoplegia, ataxia, and loss of deep tendon reflexes).11 Their findings included central and peripheral nervous system abnormalities, and they classified BBE as a subtype of MFS with accompanying central nervous system disorders. Thus, BBE has become recognized as a distinct disease entity that overlaps with MFS and GBS.
Although this finding was limited to pediatric patients, Michev et al reported that 64% of patients diagnosed with BBE presented alterations in a nerve conduction study.12 However, in this patient, the results of nerve conduction velocity examinations were within the normal range.
As in this case, routine physical examinations and blood tests may not include abnormal findings, despite the presence of various neurological and psychological symptoms. We should not exclude the possibility of associations between symptoms and a physical condition when examining such patients.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Consent for Publication
Written informed consent was obtained from the patient for the publication of this case report. This case report was approved by the ethics committees of Dokkyo Medical University.
Acknowledgments
The authors are grateful to Takashi Kanbayashi, M.D., Ph.D. of the International Institute for Integrative Sleep Medicine, University of Tsukuba for performing anti-NMDA receptor antibody analysis for this case.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version for publication, and agreed to be accountable for all aspects of the work.
Funding
There was no funding for this case report.
Disclosure
Kazutaka Shimoda has received research support from Novartis Pharma K.K., Dainippon Sumitomo Pharma Co., Astellas Pharma Inc., Meiji Seika Pharma Co., Ltd., Eisai Co., Ltd., Pfizer Inc., Otsuka Pharmaceutical Co., Ltd., Daiichi Sankyo Co., and Takeda Pharmaceutical Co., Ltd. and has received honoraria from Eisai Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd., Janssen Pharmaceutical K.K., Shionogi & Co., Ltd., Dainippon Sumitomo Pharma Co., Daiichi Sankyo Co., and Pfizer Inc. Koichi Hirata has received research support from Otsuka Pharmaceutical Co., Takeda Pharmaceutical Co., Novartis Pharma, AbbVie Pharmaceutical Co., MSD Pharmaceutical Co., Esai Pharmaceutical Co., Pfizer Pharmaceutical Co., Kyowa Hakko-Kirin Pharmaceutical Co. and Asteras Pharmaceutical Co. Norio Yasui-Furukori has received research support from Otsuka Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Dainippon Sumitomo Pharmaceutical Co., and MSD Co. The funders did not have any role in data collection or in the study design, analysis, decision to publish, or preparation of the manuscript. The remaining authors declare that they have no competing interests to report.
References
1. Carson A, Hallett M, Stone J. Assessment of patients with functional neurologic disorders. Handb Clin Neurol. 2016;139:169–188.
2. Edwards MJ. Neurobiologic theories of functional neurologic disorders. Handb Clin Neurol. 2017;139:131–137.
3. Ewald H, Rogne T, Ewald K, et al. Somatization in patients newly admitted to a neurology department. Acta Psychiatr Scand. 1994;89:174–179. doi:10.1111/j.1600-0447.1994.tb08088.x
4. Sharma V, Chan YC, Ong THL, Wilder-Smith EP. Bickerstaff’s brainstem encephalitis: can it recur? J Clin Neurosci. 2006;13:277–279. doi:10.1016/j.jocn.2005.01.011
5. Yuki N, Tagawa Y, Irie F, et al. Close association of Guillain–Barré syndrome with antibodies to minor monosialogangliosides GM1b and GM1a. J Neuroimmunol. 1997;74:30–34. doi:10.1016/S0165-5728(96)00201-9
6. Tagawa Y, Yuki N, Hirata K. High anti-GM1 and anti-GD1a IgG antibody titers are detected in Guillain–Barré syndrome but not in chronic inflammatory demyelinating polyneuropathy. Eur Neurol. 2002;48:118–119. doi:10.1159/000062988
7. Yuki N, Sato S, Tsuji S, et al. An immunologic abnormality common to Bickerstaff’s brain stem encephalitis and Fisher’s syndrome. J Neurol Sci. 1993;118:83–87. doi:10.1016/0022-510X(93)90250-3
8. Chiba A, Takashima M, Hamaguchi M, et al. Phosphate metabolism during muscular contraction in starved frogs (Rana catesbeiana). Comp Biochem Physiol Comp Physiol. 1993;106:725–729. doi:10.1016/0300-9629(93)90388-K
9. Yuki N, Sato S, Tsuji S, et al. Frequent presence of anti-GQ1b antibody in Fisher’s syndrome. Neurology. 1993;43:414–417. doi:10.1212/WNL.43.2.414
10. Yuki N. Acute paresis of extraocular muscles associated with IgG anti-GQ1b antibody. Ann Neurol. 1996;3:668–672. doi:10.1002/ana.410390517
11. Ogawara K, Kuwabara S, Yuki N. Fisher syndrome or Bickerstaff brainstem encephalitis? Anti-GQ1b IgG antibody syndrome involving both the peripheral and central nervous systems. Muscle Nerve. 2002;26:845–849. doi:10.1002/mus.10246
12. Michev A, Musso P, Foiadelli T, et al. Bickerstaff Brainstem Encephalitis and overlapping Guillain-Barré syndrome in children: report of two cases and review of the literature. Eur J Paediatr Neurol. 2019;23(1):43–52. doi:10.1016/j.ejpn.2018.11.008
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.