Back to Journals » International Medical Case Reports Journal » Volume 15
Beneficial Effect of Minocycline as Additional Treatment to Prednisone for Pustular Erythema Nodosum Leprosum
Authors Gunawan H , Faldian Y , Hindritiani R
Received 27 March 2022
Accepted for publication 11 May 2022
Published 31 May 2022 Volume 2022:15 Pages 263—268
DOI https://doi.org/10.2147/IMCRJ.S368213
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ronald Prineas
Hendra Gunawan, Yogi Faldian, Reti Hindritiani
Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin Hospital, Bandung, West Java, Indonesia
Correspondence: Hendra Gunawan, Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin Hospital, Jl. Pasteur 38, Bandung, West Java, Indonesia, 40161, Tel/Fax +62 222032426 ext 3449, Email [email protected]
Introduction: Pustular erythema nodosum leprosum (ENL) is an atypical manifestation associated with chronic ENL. The use of corticosteroid alone might not be sufficient for this condition, and addition of another anti-inflammatory drug is often necessary. Minocycline is a tetracycline antibiotic with anti-neutrophilic properties, which may accelerate the treatment of pustular ENL. This case report aimed to elaborate on the beneficial effect of minocycline for pustular ENL.
Case: We report a case of pustular ENL in a 23-year-old male who had been released from treatment (RFT) of lepromatous leprosy (LL). The patient had been on prednisone for six months as treatment for ENL. The condition recurred when prednisone was tapered to 10 mg daily. Eventually, pustules developed on the erythematous nodules, and the lesions did not improve despite seven weeks of treatment with 40– 60 mg prednisone. Later, 100 mg minocycline once daily was given in addition to 60 mg prednisone once daily and improvement was rapidly observed on the ninth day after minocycline administration. This condition was sustained for four weeks with prednisone tapering, and no side effects were reported during the treatment.
Discussion: Minocycline is an antibiotic with anti-inflammatory properties. Only a few studies have been conducted regarding the use of minocycline in chronic ENL, but there was no reported case of minocycline use for pustular ENL in RFT patient. The addition of minocycline to prednisone may accelerate the improvement of pustular ENL. We observed an improvement after the ninth day of minocycline administration compared to seven weeks of prednisone monotherapy. No new ENL lesions occurred during four weeks of minocycline administration therapy.
Conclusion: Pustular ENL is an atypical manifestation of chronic ENL, and the addition of minocycline to prednisone may accelerate its therapeutic effect on the patient.
Keywords: erythema nodosum leprosum, minocycline, pustular ENL
Introduction
Leprosy reactions are episodes of acute or subacute inflammation, which interrupt the relatively uneventful typical chronic course of leprosy,1,2 and may occur before, during, or after leprosy treatment course (released from treatment or RFT).1 Leprosy reaction is classified into two type, which are type 1 (reversal reaction) and type 2 reaction (erythema nodosum leprosum or ENL).1,3,4 ENL often occurs in lepromatous leprosy (LL) or borderline lepromatous (BL) patients.1 The clinical manifestation of ENL commonly presents as a crop of painful erythematous nodules.5 Less commonly, it may present as vesicle, bullae, ulcerations, erythema multiforme-like lesions, and pustules.1,5–7 ENL with pustular lesions is a sign of severe ENL,8 and is associated with chronic ENL.9 This condition may require a combination of corticosteroids with either thalidomide or clofazimine.8 However, these drugs often cannot be administered because, apart from their side effects, they are also not available in some countries. In Indonesia, thalidomide is not authorized and clofazimine is only available as multidrug treatments (MDT) pack. Therefore, alternative anti-inflammatory drugs are needed to be combined with corticosteroids for the treatment of pustular ENL.
Minocycline is a second-generation, semi-synthetic tetracycline antibiotic,10 and because of its antimicrobial activity, it has been used as an alternative drug for leprosy.11,12 This drug has been reported to exert various biological actions in addition to its antimicrobial activity, including analgesic and anti-inflammatory properties.10 Due to its anti-inflammatory property, minocycline can be combined with corticosteroids for the treatment of ENL, yet there are only a limited number of studies regarding the use of corticosteroid and minocycline for chronic ENL.13,14 A recent study has shown the effectiveness of minocycline for chronic ENL,13 but no case of minocycline use in an RFT patient with pustular ENL has been reported. This case report aimed to show the beneficial effect of additional minocycline to prednisone for pustular ENL treatment.
Case Report
A 23-year-old male, with a history of LL who had been RFT, came to the emergency department with a chief complaint of painful red bumps accompanied by purulent blisters on both arms, hands, legs, and feet. He also complained of fever, joint, and body ache, rendering him to not be able to move properly. The patient had been diagnosed with leprosy in the past 17 months before admission and had finished the multibacillary leprosy treatment course with MDT multibacillary (MDT-MB). He had a history of ENL without pustules for seven months before admission and was treated with prednisone. A 40 mg prednisone was initially given, and the dose was tapered according to the World Health Organization (WHO) guideline. However, the lesions consistently recurred when prednisone was tapered at 10 mg daily, and did not experience complete remission for six months. The first pustules were discovered a month before admission. Skin biopsy was conducted for both histopathology examination and drug resistance test. Histopathology examination from pustular lesion revealed thinning of the epidermis with a massive number of foam cells deposition in the dermis. There was no RpoB and FolP gene mutation, indicating no rifampicin and dapsone resistance. He was diagnosed with pustular ENL and received 40 mg a day prednisone, but there was no improvement despite five weeks of treatment. Instead, the ENL worsened as pustules kept appearing, accompanied by systemic symptoms such as fever, severe arthralgia, and myalgia. Because of the severity of the symptoms, he came to the emergency department afterward and was hospitalized.
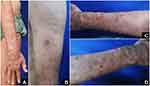
The patient admitted that he was in psychological distress due to his condition. Physical examination showed pendulous earlobes, moon face, and gynecomastia. There were pustules on the center of erythematous nodules on the extremities (Figure 1A–C), erythematous nodules, and shallow ulcers (Figure 1D). Neurological examination revealed that both ulnar nerves were enlarged and rubbery, without tenderness. There was hypesthesia of the skin lesions on both arms, hands, legs, and feet, without gloves and stockings anesthesia. On slit-skin smear, mean bacterial index (BI) and morphological index (MI) were 6+ and 0, respectively. Direct microscopic examination of the pus with Ziehl-Neelsen (ZN) staining revealed acid-fast bacilli (AFB) (Figure 2A), and Gram staining revealed no bacteria (Figure 2B). Upon hospitalization, the patient was initially treated with 60 mg prednisone once daily. After three days of prednisone administration, no clinical improvement was observed. Therefore, we substitute the prednisone with intravenous dexamethasone 10 mg once daily. Despite the administration of intravenous dexamethasone, new pustules still appeared. Therefore, oral minocycline 100 mg once daily was administered in addition to the intravenous dexamethasone on the seventh day of hospitalization. After a day of minocycline administration, no new pustule was observed. The patient was discharged on the ninth day of hospitalization, and treatment was continued with prednisone 60 mg once daily and minocycline 100 mg once daily.
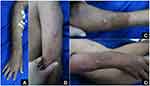 |
Figure 1 Pustules on upper extremities (A–C). Pustules, erythematous nodules and shallow ulcers on right lower arm (D). |
At a 7-day follow-up after hospitalization in the outpatient clinic, the patient showed a significant improvement as the arthralgia and myalgia almost entirely subsided, and no new skin lesion was identified. The minocycline dose was sustained for four weeks, while the prednisone was able to be tapered without any recurring lesions (Figure 3A–D). There were no adverse effects such as gastrointestinal disorders and skin discoloration during minocycline and prednisone treatment. As the pustules no longer recurred, the minocycline was discontinued while the prednisone was continued at 30 mg a day and tapered according to WHO guideline.
Discussion
During the chronic course of leprosy, there is a period of acute inflammation called leprosy reaction.1 ENL is a type 3 hypersensitivity reaction as described in Coombs and Gell’s classification.1 The risk factors for ENL are LL,2 receiving the anti-leprosy drug, BI of more than 4+, age younger than 40 years, recurrent infection, as well as physical and psychological stress.1,15 In this case report, the patient was receiving MDT-MB, with a BI of 6+, aged younger than 40 years, and was under psychological stress. Those might be the risk factors for ENL in this patient.
ENL is often described as a neutrophilic immune-complex mediated condition. The clinical manifestation may be in the form of corps of evanescent papule and nodules, which are often accompanied by constitutional symptoms.1 The atypical form of ENL are vesicles, bullae, ulcerations, erythema multiforme-like lesions, and pustules.1,5–7 Pustular ENL is a sign of severe ENL,8 which often occur in chronic ENL.9 Chronic ENL is an episode of continuous ENL lasting for more than six months, while acute ENL is one episode of ENL lasting for less than six months, characterized by a steady decrease on steroid tapering without recurrence. Patients with the chronic course are 3.2 times more likely to develop severe ENL than acute ENL.9 In this case report, the patient had a history of ENL without remission episodes for more than six months, which is in accordance with chronic ENL. This condition was worsened by development of pustular lesions along with fever, body aches, and arthritis. Thus, the diagnosis of pustular ENL was established.
Pustular ENL might respond well to the combination of prednisone and thalidomide as reported by Sirka et al.16 However, thalidomide is unavailable in Indonesia. Another alternative is clofazimine, which is commonly used for chronic recurrent ENL, but the use of clofazimine for pustular ENL have not yet been reported. Moreover, clofazimine is mostly unavailable apart from MDT blister packs while it should be administered in high doses for management of ENL, making this drug virtually inaccessible. Other agents such as pentoxifylline, azathioprine, and methotrexate had also been used for chronic ENL with various results,8 but there is no case report or study regarding their use for pustular ENL. In this case report, we use a combination of minocycline and corticosteroids as treatment of pustular ENL.
Minocycline, a second-generation tetracycline analogue, has been known to suppress neutrophilic chemotaxis in vitro that has been associated to its anti-inflammatory properties.17,18 It also exhibit various anti-inflammatory mechanisms by inhibiting inflammatory enzymes such as inducible nitric oxide synthase (iNOS), matrix metalloproteinase (MMP), cyclooxygenase-2 (COX-2), and prostaglandin A2, (PLA2). It inhibits the activity the inflammatory cells such as neutrophil, monocytes, T-cells, as well as preventing reactive oxygen species formation. These mechanisms contribute to the prevention of tissue injury induced by inflammation.10 Different studies have also evaluated its effectiveness in cutaneous inflammation,19 such as bullous and neutrophilic dermatoses.20 It has also been reported to show neuroprotective properties and alleviate neuropathic pain.10,21 Minocycline was first studied in a clinical trial for leprosy by Gelber et al12 in 1992 and later by Fajardo et al11 in 1995 because of the rising concern of rifampicin resistance in leprosy patients.11,12 Recently, the use of minocycline in leprosy reactions such as ENL has been studied, but the number remains limited.13,14 Anti-inflammatory and antibacterial properties in minocycline are thought to be the primary mechanism of minocycline in treating ENL. It is also hypothesized that, in addition to possess a strong bactericidal effect against Mycobacterium leprae (M. leprae), minocycline can also prevent ENL by treating remote concomitant bacterial infections.14 A randomized controlled trial by Hanumanthu et al13 demonstrated that minocycline exhibited significant and faster control of ENL compared to clofazimine. In terms of side effects, minocycline has fewer adverse events than clofazimine,13 is generally well-tolerated by patients,22 and safer for extended period of use due to its minimum side effects.14 The most common side effects of minocycline are mild gastrointestinal disorders and blue-grey skin pigmentation.10,14,22 A study described that, on an extended time, minocycline might induce skin pigmentation in 14.8% of patients.22 Meanwhile, another study also reported that clofazimine, which was also used for treating ENL, induced skin pigmentation in 94% of patients.23 Despite being temporary in nature, those skin pigmentation may affect the patient’s compliance in prolonged use.
We observed ineffective prednisone monotherapy in our patient. He had received prednisone for more than a month, but the pustules still recurred. Moreover, the symptoms had worsened to the point that the patient had to be hospitalized. After the addition of minocycline to the corticosteroid treatment, a rapid improvement was observed. In this case, the rationale for minocycline use is based on pustular lesions of ENL and minocycline’s suppression effect on neutrophilic chemotaxis. During the four weeks of minocycline administration, we did not observe any new ENL lesions, and the prednisone was able to be tapered. There were also no adverse events reported during the treatment.
There was only one case report by Dave et al15 describing minocycline use for pustular ENL.15 They reported a drug-resistant leprosy patient initially treated with ofloxacin as a substitute for rifampicin. After ofloxacin administration, the patient developed pustules on the face, trunk, and limbs. As ofloxacin is thought to trigger the patient’s reaction, they switched ofloxacin with minocycline as a leprosy drug and the patient showed improvement afterward. In our case report, the patient was RFT, and there was no indication of drug resistance as there was no RpoB and FolP mutation. This implies that minocycline is still beneficial in pustular ENL without active leprosy infection. However, minocycline might also cause ENL, as described by Travassos et al24 in 2012, which ENL was developed after the patient took minocycline for six weeks. The patient had a history of leprosy with a non-compliance treatment and had not yet finished her leprosy treatment.24 The exact mechanism of this occurrence was not explained. Nonetheless, unlike in our case, minocycline was combined with prednisone for pustular ENL in an RFT patient and gave an excellent result.
The limitation of this case report might be the shorter duration of minocycline administration. For minocycline to give a sustained remission on chronic ENL, we should have continued the drug for at least three months, as described by Narang et al14 and Hanumanthu et al13 in their studies. The high bacillary load in this patient should also be taken into account for further prevention of recurrence as fragmentation of M. leprae can induce ENL. Several studies administering immunotherapy with Mycobacterium indicus pranii (MIP) or Bacillus Calmette–Guérin (BCG) vaccines demonstrated acceleration of bacterial clearance, along with decrease in B cells recruitment,25 and eventually decreases in frequency and severity of ENL.25–28 Nevertheless, in a limited-resources setting without the availability of all anti-reaction drugs for pustular ENL, minocycline has been proved to be beneficial and can be used as an alternative for an additional drug to prednisone. This case report demonstrated that the therapeutic effect might be achieved immediately after adding minocycline to corticosteroid. Although further study with a larger number of subjects is necessary, minocycline is a promising option the treatment of pustular ENL.
Conclusion
Pustular lesion in ENL is an uncommon form of ENL and is criteria for severe ENL. Minocycline is known to have anti-inflammatory, neuroprotective, and analgesic properties that may be beneficial for pustular ENL treatment.
Ethical Statement
The Publication of images were included in the patient’s consent for publication of the case. Institutional approval to publish the case details has been obtained.
Consent Statement
The authors certify that they have obtained all appropriate patient consent forms. The patient signed a consent form for the publication of the case details and images.
Acknowledgments
The authors would like to thank Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin Hospital Bandung for providing meeting, editorial, and general administrative support.
Funding
There is no funding to report.
Disclosure
The authors have no conflicts of interest in relation to this work to declare.
References
1. Kar HK, Chauhan A. Leprosy reaction: pathogenesis and clinical features. In: Kumar B, Kar HK, editors. IAL Textbook of Leprosy. New Delhi: Jaypee Brothers Medical; 2016:416–437.
2. Ramesh V, Pahwa M. Some unusual type 2 reactions in leprosy. Int J Dermatol. 2010;49(2):172–175.
3. Kementrian Kesehatan Republik Indonesia. Pedoman nasional program pengendalian penyakit kusta. In: Direktorat pengendalian penyakit dan penyehatan lingkungan. Jakarta: Kementrian Kesehatan Republik Indonesia; 2012.
4. Salgado CG, de Brito AC, Spencer JS, et al. Leprosy. In: Kang S, Amagai M, Bruckner A, editors. Fitzpatrick’s Dermatology,
5. Jacobson RR, Trautman JR. The diagnosis and treatment of leprosy. South Med J. 1976;69(8):979–985.
6. Agarwal US, Mehta S, Kumar R, Besarwal RK, Agarwal P. Bullous lesions in leprosy: a rare phenomenon. Indian J Dermatol Venereol and Leprol. 2013;79(1):107.
7. Gunawan H, Yogya Y, Hafinah R, Marsella R, Ermawaty D, Suwarsa O. Reactive perforating leprosy, erythema multiforme-like reactions, sweet’s syndrome-like reactions as atypical clinical manifestations of Type 2 leprosy reaction. Int J Mycobacteriology. 2018;7(1):97–100.
8. Kar HK, Gupta R. Management of leprosy reaction. In: Kumar B, Kar HK, editors. IAL Textbook of Leprosy. New Delhi: Jaypee Brothers Medical; 2016:465–477.
9. Pocaterra L, Jain S, Reddy R, et al. Clinical course of erythema nodosum leprosum: an 11-year cohort study in Hyderabad, India. Am J Trop Med Hyg. 2006;74(5):868–879.
10. Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169(2):337–352.
11. Fajardo T, Villahermosa LG, Dela Cruz EC, Abalos RM, Franzblau SG, Walsh GP. Minocycline in lepromatous leprosy. Int J Lepr Other Mycobact Dis. 1995;63:8.
12. Gelber RH, Fukuda K, Byrd S, et al. A clinical trial of minocycline in lepromatous leprosy. Br Med J. 1992;304(6819):91.
13. Hanumanthu V, Thakur V, Narang T, Dogra S. Comparison of the efficacy and safety of minocycline and clofazimine in chronic and recurrent erythema nodosum leprosum—A randomized clinical trial. Dermatol Ther. 2021;34(6):e15125.
14. Narang T, Sawatkar GU, Kumaran MS, Dogra S. Minocycline for Recurrent and/or Chronic Erythema Nodosum Leprosum. JAMA Dermatol. 2015;151(9):1026–1028.
15. Dave S, Thappa DM, Nori AV, Jayanthi S. A rare variant of erythema nodosum leprosum: a case report. Dermatol Online J. 2003;9(5):11.
16. Sirka CS, Rout AN, Purkait S. Recurrent Pustular Erythema Nodosum Leprosum: a Rare Case Report. Indian Dermatol Online J. 2021;12(3):439–440.
17. Esterly NB, Koransky JS, Furey NL, Trevisan M. Neutrophil Chemotaxis in Patients With Acne Receiving Oral Tetracycline Therapy. Arch Dermatol. 1984;120(10):1308–1313.
18. Leite LM, Carvalho AGG, Tavares Ferreira PL, et al. Anti-inflammatory properties of doxycycline and minocycline in experimental models: an in vivo and in vitro comparative study. Inflammopharmacology. 2011;19(2):99–110.
19. Ishikawa C, Tsuda T, Konishi H, Nakagawa N, Yamanishi K. Tetracyclines Modulate Protease-Activated Receptor 2-Mediated Proinflammatory Reactions in Epidermal Keratinocytes. Antimicrob Agents Chemother. 2009;53(5):1760–1765.
20. Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54(2):258–265.
21. Condon SC, Isada CM, Tomecki KJ, et al. Systemic and topical antibiotics. In: Kang S, Amagai M, Bruckner A, editors. Fitzpatrick’s Dermatology,
22. Dwyer C, Cuddihy A, Kerr R, Chapman R, Allam B. Skin pigmentation due to minocycline treatment of facial dermatoses. Br J Dermatol. 1993;129(2):158–162.
23. Murashov MD, LaLone V, Rzeczycki PM, et al. The Physicochemical Basis of Clofazimine-Induced Skin Pigmentation. J Invest Dermatol. 2018;138(3):697–703.
24. Travassos AR, Antunes J, Pacheco D, Almeida LS, Filipe P, Marques MS. Erythema nodosum leprosum associated with minocycline. Acta Dermatovenerol APA. 2012;21:39–41.
25. Sharma A, Saqib M, Sheikh JA, et al. Mycobacterium indicus pranii protein MIP_05962 induces Th1 cell mediated immune response in mice. Int J Med Microbiol. 2018;308(8):1000–1008.
26. Gupta SK, Kumari S. Chronic recalcitrant erythema nodosum leprosum: therapeutic dilemma and role of mycobacterium indicus pranii vaccine. An Bras Dermatol. 2022;97:49–53.
27. Narang T, Kaur I, Kumar B, Radotra BD, Dogra S. Comparative evaluation of immunotherapeutic efficacy of BCG and mw vaccines in patients of borderline lepromatous and lepromatous leprosy. Int J Lepr Other Mycobact Dis. 2005;73(2):105–114.
28. Zaheer SA, Misra RS, Sharma AK, et al. Immunotherapy with Mycobacterium w vaccine decreases the incidence and severity of type 2 (ENL) reactions. Lepr Rev. 1993;64(1):7–14.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.