Back to Journals » Clinical Ophthalmology » Volume 15
Baseline Subfoveal Choroidal Thickness as a Predictor for Response to Short-Term Intravitreal Bevacizumab Injections in Diabetic Macular Edema
Authors Dweikat A , Jarrar A, Akkawi M, Shehadeh M, Aghbar A, Qaddumi J, Akkawi M
Received 27 June 2021
Accepted for publication 6 September 2021
Published 18 October 2021 Volume 2021:15 Pages 4175—4180
DOI https://doi.org/10.2147/OPTH.S325951
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Alaa Dweikat,1 Arkan Jarrar,1 Mohammad Akkawi,2,3 Mohammad Shehadeh,2,3 Ammar Aghbar,3 Jamal Qaddumi,1 Maha Akkawi1
1Department of Medicine, Faculty of Medicine and Health Sciences, An-Najah National University, Nablus, Palestine; 2Department of Special Surgeries, Faculty of Medicine and Health Sciences, An-Najah National University Hospital, An-Najah National University, Nablus, Palestine; 3Ophthalmology Department, An-Najah National University Hospital, Nablus, Palestine
Correspondence: Mohammad Akkawi; Alaa Dweikat Email [email protected]; [email protected]
Purpose: This article aims to evaluate how the subfoveal choroidal thickness (SFCT) and best-corrected visual acuity (BCVA) respond to the intravitreal injection of bevacizumab and to assess the correlation between these changes. It will also assess the use of the baseline SFCT as a predictor for BCVA changes in eyes of treatment-naive, diabetic macular edema (DME) patients.
Methods: This retrospective, consecutive case series comprised 59 eyes of 39 treatment-naive DME patients. Complete slit-lamp assessment, swept-source optical coherence tomography (SS-OCT) scans to measure SFCT and BCVA values were performed at two stages: baseline and one month after the third monthly injection of intravitreal bevacizumab.
Results: Patients’ ages ranged from 46.3 to 76.4 years (mean: 62.6 ± 2.3). The mean SFCT was 318 ± 82 μm at baseline, which decreased after 3 months to 300 ± 66 μm (P-value = 0.021). There was an improvement in the mean of the logMAR best-corrected visual acuity (BCVA) from 0.7 (decimal equivalent: 0.2) to 0.5 (decimal equivalent: 0.3) (P-value = 0.019). There was no association between SFCT changes and BCVA changes (P-value = 0.180). Wilcoxon signed-rank test disclosed that a better BCVA improvement was related to a greater subfoveal choroidal thickness at baseline P-value < 0.00.
Conclusion: Eyes with a higher baseline subfoveal choroidal thickness (SFCT) attained greater BCVA improvement than eyes with a lower baseline SFCT. In addition to this, changes to SFCT do not appear to correlate with BCVA changes. These findings do not support using OCT SFCT changes as a prognostic factor for changes to BCVA after intravitreal bevacizumab treatment in evaluating treatment-naive DME eyes.
Keywords: diabetic macular edema, subfoveal choroidal thickness, best-corrected visual acuity, bevacizumab, anti-vascular endothelial growth factor
Introduction
Diabetic Macular Edema (DME) is a serious microvascular complication of diabetes mellitus. It is a common sight-threatening retinopathy and a significant cause of irreversible vision loss among adults with diabetic retinopathy worldwide,1 with a global prevalence of 6.8%.2
In DME, oxidative stress and inflammation due to hyperglycemia lead to abnormalities in retinal vasculature. This plays a major role in the pathophysiology of the disease, causing breakdown and malfunction of the blood-ocular barrier, which leads to increased retinal vascular permeability, causing plasma, protein, and lipid leakage within the macula and choroid.3 The fluid subsequently accumulates within the retina and choroid,4 causing impairment to the choriocapillaris (the major blood supply for the outer retina), leading to retinal ischemia, and can critically diminish visual acuity.
Reviewing choroidal changes on a macrovascular level in patients with diabetic macular edema (DME), several studies have shown increased retinal vascular permeability that led to choroidal thickening.4 However, other studies of choroidal thickness changes in DME patients produced diverging results; some reporting thinning,5,6 while others found no changes at all. Despite this range of findings in the choroidal vasculature in DME, it all leads to the same conclusion; abnormal choriocapillaris, ie, impaired retinal vasculature, leads to impaired vision.
With the advent of Swept-Source Optical Coherence Tomography (SS-OCT), an imaging technique that displays high-resolution cross-sectional images (introduced to clinical practice in 2012), a detailed visualisation of the retinal structures and choroid are now possible. This is due to its deeper penetration and longer wavelength compared to spectral-domain OCT (SD-OCT).7,8
Even with the growing evidence in elucidating choroidal circulation changes in DME,4 the correlation between the subfoveal choroidal thickness at baseline and vision response to anti-vascular endothelial growth factor (anti-VEGF) therapy has not been verified. Therefore, we studied the use of the baseline subfoveal choroidal thickness (SFCT) as a predictor for best-corrected visual acuity changes in response to bevacizumab injections, as well as the correlation between changes of best-corrected visual acuity (BCVA) and OCT findings of changes in SFCT one month after the third monthly intravitreal bevacizumab injection for treatment-naive patients with DME.
Methods
This retrospective, consecutive case series study was conducted at the Ophthalmology Department at An-Najah National University Hospital (NNUH) in Nablus-Palestine, after obtaining the ethical approval from An-Najah National University Institutional Review Board (IRB) and following the guidelines of the Declaration of Helsinki. Written and verbal informed consent was waived due to the retrospective nature of the study, the data was anonymized and maintained with confidentiality.
The population included 59 eyes of 39 patients, who had been diagnosed with DME, according to the following inclusion criteria: treatment-naive patients before their first anti-VEGF injection, given only bevacizumab as a monthly anti-VEGF injection for 3 months with a standard dose of 1.25 mg (including patients with systemic conditions such as hypertension (10)), and patients determined to have clinically significant macular edema based on the Criteria of the Early Treatment of Diabetic Retinopathy Study (ETDRS) guidelines.9
We excluded patients who had received any type of medication related to diabetic retinopathy before or during the 3-month course of bevacizumab; such as steroid injection or a different anti-VEGF therapy, any patients with previous laser therapy or history of intraocular surgery, patients with presence of high refractive errors (>+5 and <-5; as choroidal thickness may change with the high refractive state), and any patients diagnosed with concomitant ocular disease that might affect their vision (significant cataract/glaucoma/age-related macular degeneration, uveitis, etc.). We also excluded patients with non-proliferative diabetic retinopathy.
Patients’ relevant sociodemographic and clinical data were recorded, including age, sex, systemic disease, diabetes mellitus duration, baseline glycosylated hemoglobin (HbA1C), complete slit-lamp biomicroscopic examination baseline and follow-up findings, BCVA at baseline and one month after the third injection, injection dates, and length of follow-up. BCVA values were recorded in a decimal unit and converted to LogMar. According to ETDRS and FrACT study, CF (counting fingers) at 30 cm can be replaced by 0.014 in decimal and visual acuity in the HM-range (hand motion), replaced by VA of 0.005 in decimal.10
Intervention
Until recently laser therapy was the main treatment of DME, reducing the risk of blindness and increasing the opportunity for vision improvement compared with conservative management. However, these valuable effects are also associated with considerable side effects due to the destructive nature of the laser photocoagulation on the retina. Recently, clinical trials of regular intravitreal injections of anti-VEGF have shown higher efficacy regarding vision preservation and decreased vision loss compared to laser photocoagulation.11–13
In this study, all participants were treatment-naive before their first anti-VEGF injection, and given bevacizumab only, as a monthly anti-VEGF injection for 3 months with a standard dose of 1.25 mg.
Imaging
Swept-source optical coherence tomography (SS-OCT) [Topcon DRI OCT Triton software 2015, version 10.15.003.01] findings: SFCT was recorded at the first visit as a baseline and one month after the third bevacizumab injection. The SFCT was measured manually as a vertical line from the outer surface of the retinal pigment epithelium to the lower border of the choroid (choroid-sclera interface), by three different researchers separately to ensure the reliability and reproducibility of measurements. Each value was an average of 12 scans, with each scan centered at the fovea.
Statistical Analysis
All analyses were carried out by the Statistical Package for Social Sciences (SPSS) version 21. The data were described as mean ± standard deviation, median interquartile range], or frequency (percentage). The Kolmogorov–Smirnov test was conducted to assess the normality of data distribution, and the data was recognised as non-normally distributed data. Univariate analysis was accomplished to assess the influence of demographic and clinical characteristics (age, gender, systemic diseases, diabetes mellitus duration, and glycosylated hemoglobin (HbA1C)) on the continuous dependent variables (BCVA and SFCT). Additionally, the Wilcoxon signed-rank test was used to make a comparison between the findings at baseline and one month after the third injection. It was also used to assess whether baseline SFCT can predict BCVA response to bevacizumab therapy one month after the third injection. Then, by using the continuous measurements (BCVA and SFCT changes), a bivariate analysis (Spearman’s rank correlation coefficients) was conducted to investigate the correlation between these changes. A p-value less than 0.05 is statistically significant.
Results
The demographic and clinical characteristics of patients with DME are shown in Table 1. The study examined 59 eyes of 39 participants, 18 females [46%] and 21 males [54%]. The mean age was 62.6 ± 2.3 years (ranging between 46.3 and 76.4 years). Diabetes mellitus duration was 19 ± 9 years, glycosylated hemoglobin (HbA1C) mean was 8.7 (± 2) with a range from 6 to 14. There were 6 patients (15%) with systemic disease (eg, hypertension) and 33 patients (85%) free of systemic disease (other than diabetes mellitus).
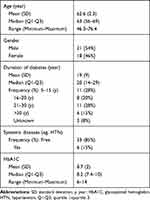 |
Table 1 Socio-Demographic and Clinical Characteristics of Diabetic Macular Edema Eyes Treated with Bevacizumab Therapy (39 Patients, 59 Eyes) |
In univariate analysis, there was no statistically significant effect of independent variables (age, gender, systemic diseases, diabetes mellitus duration, or glycosylated hemoglobin (HbA1C)) on the dependent variables including BCVA (P-value=0.062) and SFCT (P-value=0.400).
Ocular characteristics data are shown in Table 2. After intravitreal bevacizumab injections, the mean LogMar BCVA was significantly improved from 0.7± 1 (Decimal equivalent: 0.2 ± 0.1) at baseline to 0.5± 0.7 (Decimal equivalent: 0.3 ± 0.2) at one month after the third injection (P=0.019). In regard to the SFCT characteristics shown in Figure 1; after three months of treatment, the subfoveal choroidal thickness mean was significantly reduced to 300 ± 66 μm when compared to the baseline mean value of 318 ± 82 μm (P=0.021). Bivariate analysis results have shown no significant correlation between BCVA changes and SFCT changes with a p-value of 0.18. In non-parametric univariate analysis, Wilcoxon signed-rank test results have demonstrated that a greater BCVA improvement was significantly related to a higher baseline subfoveal choroidal thickness at one month after the third injection (P-value <0.00).
 |
Table 2 Comparison of Outcomes for Patients with Diabetic Macular Edema Eyes (39 Patients, 59 Eyes) at Baseline and After 3 Months of Bevacizumab Therapy |
 |
Figure 1 SFCT characteristics at baseline, 3 months and changes. |
Discussion
This retrospective case series study aims to ascertain whether the subfoveal choroidal thickness at baseline is an indicator for treatment outcome, and to assess the correlation between the changes in BCVA and SFCT after intravitreal bevacizumab therapy in treatment naive DME patients.
The choroidal vasculature delivers nutrients to and removes metabolic wastes from the outer retinal layers, and therefore plays a significant role in the preservation of healthy visual function.14 Diabetic retinopathy has a clinical characteristic of ischemic retinopathy, causing localised hypoxia, triggering VEGF and other angiogenic factor overproduction. This in turn triggers degeneration and edematous leakage of the choriocapillaris, leading to impaired choroidal circulation, potentially progressing to retinal dysfunction and visual impairment. The results from this study indicate that the baseline subfoveal choroidal thickness (SFCT) may prognosticate the short-term bevacizumab response. Patients with a thicker baseline subfoveal choroidal thickness were more likely to experience an improvement in visual function at the 3-month follow-up. The potential mechanism behind this finding, pre-existing thicker choroid, may be associated with more intact choriocapillaris, less outer retinal layer ischemia, and more functional photoreceptors compared with patients with a thinner choroidal thickness. The findings of this study are consistent with the findings of Rayess et al, which concluded that baseline subfoveal choroidal thickness may prognosticate which patients with DME will experience BCVA improvement.15
Conversely, this study showed a non-significant correlation between BCVA and SFCT changes, contradicting the Nourinia et al study,16 which suggested SFCT changes were significantly associated with vision improvement. In our study, a small percentage of our patients showed the same results, but it did not reach statistical significance. We attribute this difference to the small sample size in the previous study (only 20 eyes of 20 patients); therefore, these results clearly warrant further study.
There were some limitations of this study including the retrospective nature, the manual calculation of choroidal thickness using OCT scans, and owing to the short follow-up time being unable to deduce whether these outcomes would remain significant with continuing longer-term therapy. In addition to this, all of our patients received bevacizumab treatment due to its cost-effectiveness,17 without taking the baseline visual acuity into consideration, disregarding the DRCR.net protocol T. However, there were also several strengths of this study including a relatively good sample size (59 eyes) in comparison to other studies,6,16 and the use of SS-OCT, rather than SD-OCT; which gives more accurate measurements.7 Also, stipulating treatment-naive patients only mitigated the influence of any previous intravitreal injections on subfoveal choroidal thickness.
Conclusion
DME is a common cause of visual impairment in diabetic patients. The results of this study suggest that subfoveal choroidal thickness at baseline could be used as a predictive factor for visual outcomes in treatment-naive patients with DME after a short course of bevacizumab. Consequently, patients who had a thicker subfoveal choroidal thickness at baseline were more likely to experience a notable improvement in vision with bevacizumab therapy.
Acknowledgments
All study authors would like to thank the optometrists in the ophthalmology department of An-Najah National University Hospital for their assistance in the current work, the clinical research center team at An-Najah National University Hospital for their support of this research study, and Samantha Kearly from Southmead Hospital, Bristol, UK for her assistance in language editing.
Disclosure
The authors declare that we have no conflict of interest in this study.
References
1. Romero-Aroca P. Managing diabetic macular edema: the leading cause of diabetes blindness. World J Diabetes. 2011;2(6):98–104. doi:10.4239/wjd.v2.i6.98
2. Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi:10.2337/dc11-1909
3. Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology. 2015;122(7):1375–1394. doi:10.1016/j.ophtha.2015.03.024
4. Kim JT, Lee DH, Joe SG, Kim JG, Yoon YH. Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2013;54(5):3378–3384. doi:10.1167/iovs.12-11503
5. Adhi M, Brewer E, Waheed NK, Duker JS. Analysis of morphological features and vascular layers of choroid in diabetic retinopathy using spectral-domain optical coherence tomography. JAMA Ophthalmol. 2013;131(10):1267–1274. doi:10.1001/jamaophthalmol.2013.4321
6. Esmaeelpour M, Povazay B, Hermann B, et al. Mapping choroidal and retinal thickness variation in type 2 diabetes using three-dimensional 1060-nm optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(8):5311–5316. doi:10.1167/iovs.10-6875
7. Miller AR, Roisman L, Zhang Q, et al. Comparison between spectral-domain and swept-source optical coherence tomography angiographic imaging of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2017;58(3):1499–1505. doi:10.1167/iovs.16-20969
8. Kishi S. Impact of swept source optical coherence tomography on ophthalmology. Taiwan J Ophthalmol. 2016;6(2):58–68. doi:10.1016/j.tjo.2015.09.002
9. Musat O, Cernat C, Labib M, et al. Diabetic macular edema. Rom J Ophthalmol. 2015;59(3):133–136.
10. Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the Freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47(3):1236–1240. doi:10.1167/iovs.05-0981
11. Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report number 1. Arch Ophthalmol. 1985;103(12):1796–1806. doi:10.1001/archopht.1985.01050120030015
12. Do DV, Nguyen QD, Boyer D, et al. One-year outcomes of the da Vinci Study of VEGF trap-eye in eyes with diabetic macular edema. Ophthalmology. 2012;119(8):1658–1665. doi:10.1016/j.ophtha.2012.02.010
13. Solaiman KA, Diab MM, Dabour SA. Repeated intravitreal bevacizumab injection with and without macular grid photocoagulation for treatment of diffuse diabetic macular edema. Retina. 2013;33(8):1623–1629. doi:10.1097/IAE.0b013e318285c99d
14. Linsenmeier RA, Padnick-Silver L. Metabolic dependence of photoreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci. 2000;41(10):3117–3123.
15. Rayess N, Rahimy E, Ying GS, et al. Baseline choroidal thickness as a predictor for response to anti-vascular endothelial growth factor therapy in diabetic macular edema. Am J Ophthalmol. 2015;159(1):
16. Nourinia R, Ahmadieh H, Nekoei E, Malekifar P, Tofighi Z. Changes in central choroidal thickness after treatment of diabetic macular edema with intravitreal bevacizumab correlation with central macular thickness and best-corrected visual acuity. Retina. 2018;38(5):970–975. doi:10.1097/IAE.0000000000001645
17. Ross EL, Hutton DW, Stein JD, et al. Cost-effectiveness of Aflibercept, Bevacizumab, and Ranibizumab for diabetic macular edema treatment: analysis from the diabetic retinopathy clinical research network comparative effectiveness trial. JAMA Ophthalmol. 2016;134(8):888–896. doi:10.1001/jamaophthalmol.2016.1669
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
