Back to Journals » Patient Preference and Adherence » Volume 17
Attitudes Toward Providing Open Access for Use of Biospecimens and Health Records: A Cross-Sectional Study from Jordan
Authors Al-Shami KM, Ahmed WS , Alzoubi KH
Received 12 January 2023
Accepted for publication 18 March 2023
Published 28 March 2023 Volume 2023:17 Pages 895—903
DOI https://doi.org/10.2147/PPA.S402769
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Kamal M Al-Shami,1,2,* Wesam S Ahmed,3,* Karem H Alzoubi1
1Department of Clinical Pharmacy, Faculty of Pharmacy, Jordan University of Science and Technology, Irbid, 22110, Jordan; 2Faculty of Biosciences, University of Heidelberg, Heidelberg, 69120, Germany; 3College of Health and Life Sciences, Hamad Bin Khalifa University, Qatar Foundation, Doha, Qatar
*These authors contributed equally to this work
Correspondence: Wesam S Ahmed, Email [email protected]; [email protected]
Purpose: Biospecimen repositories and big data generated from clinical research are critically important in advancing patient-centered healthcare. However, ethical considerations arising from reusing clinical samples and health records for subsequent research pose a hurdle for big-data health research. This study aims to assess the public’s opinions in Jordan toward providing blanket consent for using biospecimens and health records in research.
Participants and Methods: A cross-sectional study utilizing a self-reported questionnaire was carried out in different cities in Jordan, targeting adult participants. Outcome variables included awareness of clinical research, participation in clinical research, and opinions toward providing open access to clinical samples and records for research purposes. Descriptive analysis was utilized for reporting the outcome as frequency (percentages) out of the total responses. Univariate and multivariate logistic regression were used to investigate the association between independent variables and the outcome of interest.
Results: A total of 1033 eligible participants completed the questionnaire. Although the majority (90%) were aware of clinical research, only 24% have ever participated in this type of research. About half (51%) agreed on providing blanket consent for the use of clinical samples, while a lower percentage (43%) agreed on providing open access to their health records. Privacy concerns and lack of trust in the researcher were cited as major barriers to providing blanket consent. Participation in clinical research and having health insurance were predictors for providing open access to clinical samples and records.
Conclusion: The lack of public trust in Jordan toward data privacy is evident from this study. Therefore, a governance framework is needed to raise and maintain the public’s trust in big-data research that warrants the future reuse of clinical samples and records. As such, the current study provides valuable insights that will inform the design of effective consent protocols required in data-intensive health research.
Keywords: biospecimens, health records, Jordan, open access, blanket consent, clinical research
Introduction
Clinical research is a fundamental tool for understanding diseases and designing preventive and therapeutic strategies.1–3 Jordan is an Arab country in the Middle East and North Africa (MENA) region with progressive research agenda.1,4,5 The county is ranked among the top countries in the Arab region based on clinical studies per capita (Table 1). Additionally, it was the first Arab country to enact clinical research regulations.6 The country is known for its flourished pharmaceutical industry, which exports its products to more than 60 countries globally, making it the second-largest exporting industry in the country.7–9 Moreover, Jordan hosts several clinical study centers, some of which conduct clinical trials on behalf of international pharmaceutical companies.10 The country also has a well-established cancer biobank, the King Hussein Cancer Center Biobank, which is the first ISO-accredited cancer biobank from a diverse ethnic MENA population.11
 |
Table 1 Comparison Between the Number of Clinical Studies in the Arab MENA Region |
Establishing clinical sample repositories and the digitalization of medical health records, accompanied by the ease of communication and the over-sees research collaboration, has opened the door for big-data clinical research that, if utilized effectively, can provide the premise for precision medicine and patient-centered healthcare. However, the need to obtain repeated patient consent for subsequent clinical research creates a hurdle for researchers and delays the commencement of research projects. Therefore, consenting models, such as “blanket consent” and “broad consent”, have been adopted by several biobank projects to balance the interest of the donors and researchers.12,13 Blanket consent implies providing open access to a broad range of future studies without restriction.14,15 This is slightly different from broad consent, which means consenting to a framework of future research subjected to limited predefined restrictions.16,17 Hence, given the active clinical research agenda in country and the importance of having a robust consenting model for big-data clinical research, the current study explores Jordan’s public views toward providing blanket consent for use of biospecimens and health records, aiming to overcome barriers and enhance positive attitudes toward providing the same.
To achieve this, a self-administered questionnaire was distributed to eligible participants. Results showed a lack of social license to reuse medical samples and records in clinical research. Participants were more stringent on providing access to health records than to clinical samples. To encourage social license, there is a need to identify conditions that lead to public distrust, and to establish a governance framework that considers patient and public views, needs, values, and interests.
Methods
Study Design and Participants
A questionnaire survey was carefully constructed after reviewing similar work from the region.18–22 The survey was distributed by experienced interviewers to eligible participants in public places in different cities in Jordan, such as Amman, Zarqa, Karak, Irbid, and Mafraq, from Feb 2019 to March 2019 using a convenience sampling approach. Eligibility criteria included autonomy, competency, an age of more than 18, and the ability to read and understand the Arabic language. The questionnaire was distributed in Arabic since it is the native language of the country. Informed oral consent was obtained from all participants after providing them with a detailed description of the study as well as contact information should they decide to withdraw or have any concerns regarding the survey. The participants were then provided with a brief description of clinical research, its scope, the consent process, and its different forms, including the definition of “open access”. Response formats utilized in the three-sectioned questionnaire included multiple-choice, “Yes” or “No”, multiple check boxes, and Likert scale items. The first section solicited sociodemographic information from respondents such as age, gender, marital status, nationality, education level, health insurance status, and whether they have any chronic medical conditions. The second section assessed participants’ awareness of “clinical research”, whether they have previously participated in clinical research, and their willingness to participate in clinical research in the future. The last section solicited participants’ views toward providing open access to their biospecimens and health records for research purposes. The study was verified by the Institutional Review Board (IRB) committee of Jordan University of Science and Technology (Ref# 38/117/2018) with no consent form requirement.
Data Analysis
Data were analyzed using statistical package for social science (SPSS) version 22 (SPSS Inc., Chicago, IL, USA). Power analysis was performed, ensuring power is more than 80%. Descriptive analysis was performed to summarize the data by reporting the frequency (percentages) out of the total responses approximated to two decimals in the tables and zero decimals in the text. Answers to the follow up, open ended questions were mapped into general categories and reported as frequency (percentages) out of the total answers. Univariate and multivariate logistic regression was performed to screen for variables associated with hesitancy toward providing open access to clinical samples and medical records. Following the univariate logistic regression analysis, any variable that was significant on the single predictor level (P-value <0.25) was entered into the multivariate logistic regression analysis to explore predictors significantly and independently associated with hesitancy toward providing blanket consent. Odds ratio were calculated to estimate the effect of each predictor on the outcome. A P value of ≤ 0.05 was considered statistically significant. Figures were prepared using Microsoft Excel 13.
Results
Demographics
The sociodemographic characteristics are summarized in Table 2. A total of 1033 participants completed the survey. The response rate was 85%. Approximately half of the participants (48%) were less than 24 years old. There was an almost equal distribution of male to female participants (50%, n=512, 50%, n=521). Most participants were Jordanians (84%, n=865), single (75%, n=777), and have received a diploma or a higher education degree (84%, n=869). Most were medically insured (67%, n=696) and had no chronic clinical conditions (90%, n=934).
 |
Table 2 Sociodemographic Characteristics of the Participants (n=1033) |
Awareness and Participation in Clinical Research
Most participants (74%, n=768) reported understanding the “clinical research” terminology. A minority reported (21%, n=212) prior participation in clinical research. However, the majority (63%, n=650) were willing to participate if they were invited in the future (Figure 1).
Attitudes Toward Providing Open Access to Clinical Samples and Health Records
Around 51% (n=528) and 44% (n=454) of the participants agreed on providing blanket consent for the use of clinical samples (blood) and health records, respectively. Participants’ reasons for refusing to provide blanket consent are summarized in Table 3 and Table 4. Results from univariate and multivariate logistic regression revealed predictors associated with participants’ hesitancy toward giving open access to blood samples and health records (Table 5 and Table 6).
 |
Table 3 Participants Reported Barriers to Providing Blanket Consent for the Use of Blood Samples (n=505, 48.89%) |
 |
Table 4 Participants Reported Barriers for Providing Blanket Consent for the Use of Health Records (n=579, 56.05%) |
 |
Table 5 Variables Associated with Hesitancy Toward Providing Open Access to Blood Samples |
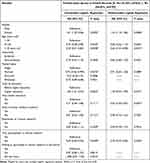 |
Table 6 Variables Associated with Hesitancy Toward Providing Open Access to Health Records |
Discussion
In this study, we investigated the public attitudes in Jordan toward providing open access to their clinical samples and health records for research purposes. Results indicated a public’s unease toward providing free access to medical samples and records, with more stringent attitudes toward providing indefinite access to health records than medical samples.
People older than 35 were more reluctant to provide blanket consent for the use of health records. This may be related to the social stigma that may arise if sensitive information is revealed about older participants, especially since older age is a risk factor for several diseases.23,24 Respecting one’s privacy was the most cited reason in our study for rejecting to provide open access to health records. For similar reasons, the female gender was associated with more reluctance to provide blanket consent for the use of health records, as women privacy is critically valued in the region.25–27 Moreover, health insurance was positively associated with the rejection of providing open access to blood samples and health records. Those who are medically insured may fear losing their insurance if some of their sensitive information were released.28,29
Most participants (90%) reported being aware of clinical research. This could be attributed to the high education level of the participants. After all, the country reportedly has the highest literacy rate in the Arab world.30,31 The country is also well known in the region for its pharmaceutical industry and has several clinical research centers scattered across its cities.7–9 As such, the familiarity with the “clinical research” in the country may be higher than in other countries in the region, for instance, in Lebanon, where only 45% reported recognizing the same terminology.21
Participation in research is an important aspect that may influence participants’ decision to provide blanket consent.32–34 Assessing participation in clinical research revealed that even though a minority (21%) have participated in clinical research, the majority (63%) were willing to participate in the future. This may suggest that most recruitments in the country occur in clinical settings and, therefore, the need for outreach strategies to recruit more of the general public, which is expected to reflect more accurately the research findings.35,36 Studies from other countries in the region, such as Qatar and Saudi Arabia, also showed that most participants (63% and 74%, respectively) had positive attitudes toward participation.18,19 More importantly, multivariate analysis revealed that willingness to participate in research was positively associated with attitudes toward providing blanket consent for the use of blood samples. A similar association was reported in previous studies.34,37–39 On the other hand, there was a positive association between prior participation in clinical research and the reluctance to provide open access for the use of health records. As such, it would seem that there is a need to modify the current communication strategies between researchers and participants and make the recruitment process as seemingly as possible to apparently enhance participants’ experience and elevate the public trust in researchers.40–44 After all, lack of trust in researchers was cited as the second most common barrier to providing open access to blood samples and health records in our study.
The consent process is a fundamental step in clinical research that involves human subjects.45–47 Almost half of the participants were willing to provide open access to their blood samples, while less than half agreed to provide open access to their health records. Fear of privacy being negatively affected was the most reported reason for rejecting to provide blanket consent, followed by a lack of trust in researchers. Indeed, other studies also cited privacy concerns as barriers.39,48 On the other hand, trust is a fundamental aspect of research and has been cited as a positive predictor for providing blanket consent.34,37,38 Therefore, strengthening the measures taken to preserve data confidentiality and informing the potential participants about these measures that protect their privacy could be effective strategies to enhance the public trust in researchers.
Limitations
This study has some limitations that can be addressed and mitigated through future research. For instance, although the current study investigated the public attitude toward providing indefinite access to clinical samples and health records, it did not investigate the reasons that may contribute to the social acceptance of a blanket consent protocol. In addition, despite that, the recruitment process taking place in different places in other cities in Jordan, the sampling technique that was followed in this study is still a convenience sampling approach by definition, and the ability to infer generalizability from a convenience sample is still limited compared to some other sampling techniques such as random sampling.
Conclusion
The study revealed a lack of social licensing for the indefinite use of biospecimens and health records in clinical research. Results from the current study shall inform the decision makers about the current public attitude toward integrating a blanket consent protocol and call for further research to explore elements that may contribute to the social acceptance of such protocol. In addition, factors contributing to the public unease toward providing blanket consent need to be addressed and mitigated through future research.
Data Sharing Statement
The survey questionnaire will be provided upon reasonable request from the first authors.
Ethics Approval
The study was verified by the Institutional Review Board (IRB) committee of Jordan University of Science and Technology (Ref# 38/117/2018) with no consent form requirement. A written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Data were kept anonymously with the lead author and the study followed the ethical standards in the Declaration of Helsinki guideline.
Acknowledgment
KMA and WSA are participants in the research ethics program in Jordan supported by grant # 5R25TW010026–02. The research ethics program in Jordan had no role in study design, data collection, and analysis, decision to publish, preparation of the manuscript, or funding of this publication. The publication of this article was funded by the Qatar National Library.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Al-Shami KM, Ahmed WS, Alzoubi KH. Motivators and barriers towards clinical research participation: a population-based survey from an Arab MENA country. PLoS One. 2022;17(6):e0270300. doi:10.1371/journal.pone.0270300
2. Emanuel E, Wendler D, Grady C. What makes clinical research ethical? JAMA. 2000;283(20):2701–2711. doi:10.1001/jama.283.20.2701
3. Sheridan R, Martin-Kerry J, Hudson J, et al. Why do patients take part in research? An overview of systematic reviews of psychosocial barriers and facilitators. Trials. 2020;21(1):259. doi:10.1186/s13063-020-4197-3
4. Ahmed WS, Nebeker C. Assessment of research ethics education offerings of pharmacy master programs in an Arab nation relative to top programs worldwide: a qualitative content analysis. PLoS One. 2021;16(2):e0238755. doi:10.1371/journal.pone.0238755
5. Ahmed W, Aburjai T, Hudaib M, et al. Chemical composition of essential oils hydrodistilled from aerial parts of Achillea fragrantissima (Forssk.) Sch. Bip. and Achillea santolina L. (Asteraceae) growing in Jordan. J Essential Oil Bearing Plants. 2020;23(1):15–25. doi:10.1080/0972060X.2020.1723442
6. Al-Omari A, Al-Hussaini M. Research ethics governance in the Arab region: jordan. In: Research Ethics in the Arab Region. Springer; 2017:221–228.
7. Ahmed WS, Ahmed A, Alzoubi KH, et al. Perceptions of pharmacy graduate students toward research ethics education: a cross-sectional study from a developing country. Sci Eng Ethics. 2022;28(6):47. doi:10.1007/s11948-022-00406-0
8. Nair SC, Ibrahim H, Celentano DD. Clinical trials in the Middle East and North Africa (MENA) region: grandstanding or grandeur? Contemp Clin Trials. 2013;36(2):704–710. doi:10.1016/j.cct.2013.05.009
9. Sweis RJ, Al-Ghawi HJ, AlSaleh NA, Al-Zu’bi ZB, Obeidat BY. Benchmarking of TQM: the case of Hikma Pharmaceuticals company. Benchmarking. 2015;22:488–504.
10. Alrabadi NN, Mukattash TL, Alzoubi KH, et al. Awareness of pharmacy researchers about the national research code of ethics: a study from Jordan. Heliyon. 2021;7(6):e07180. doi:10.1016/j.heliyon.2021.e07180
11. Barr M, Souan L, MacGabhann P, et al. The establishment of an ISO compliant cancer biobank for Jordan and its neighboring countries through knowledge transfer and training. Biopreserv Biobank. 2014;12(1):3–12. doi:10.1089/bio.2013.0072
12. Wendler D. Broad versus blanket consent for research with human biological samples. Hastings Cent Rep. 2013;43(5):3–4. doi:10.1002/hast.200
13. Köngeter A, Schickhardt C, Jungkunz M, et al. Patient’s willingness to provide their clinical data for research purposes and acceptance of different consent models: findings from a representative survey of patients with cancer. J Med Internet Res. 2022;24(8):e37665. doi:10.2196/37665
14. Dankar FK, Gergely M, Malin B, et al. Dynamic-informed consent: a potential solution for ethical dilemmas in population sequencing initiatives. Comput Struct Biotechnol J. 2020;18:913–921. doi:10.1016/j.csbj.2020.03.027
15. Ploug T, Holm S. The “Expiry problem” of broad consent for biobank research-and why a meta consent model solves it. J Med Ethics. 2020;46(9):629–631. doi:10.1136/medethics-2020-106117
16. Grady C, Eckstein L, Berkman B, et al. Broad consent for research with biological samples: workshop conclusions. Am J Bioethics. 2015;15(9):34–42. doi:10.1080/15265161.2015.1062162
17. Stoeklé H-C, Hulier-Ammar E, Hervé C. Data Medicine:‘Broad’or “Dynamic” Consent? Public Health Ethics. 2022;15:181–185.
18. Tohid H, Choudhury SM, Agouba S, et al. Perceptions and attitudes to clinical research participation in Qatar. Contemporary Clin Trials Commun. 2017;8:241–247. doi:10.1016/j.conctc.2017.10.010
19. Al-Tannir MA, El-Bakri N, Abu-Shaheen AK. Knowledge, attitudes and perceptions of Saudis towards participating in clinical trials. PLoS One. 2016;11(2):e0143893. doi:10.1371/journal.pone.0143893
20. Willison DJ, Richards DP, Orth A, et al. Survey of awareness and perceptions of canadians on the benefits and risks of clinical trials. Therap Innov Regulat Sci. 2019;53(5):669–677. doi:10.1177/2168479018805433
21. Salem R, Matar C, Assi R, et al. Perceptions and attitudes of cancer patients and caregivers towards enrollment in clinical trials in Lebanon. J Cancer Educ. 2017. 34:334–338.
22. Owens OL, Jackson DD, Thomas TL, et al. African American men’s and women’s perceptions of clinical trials research: focusing on prostate cancer among a high-risk population in the South. J Health Care Poor Underserved. 2013;24(4):1784–1800. doi:10.1353/hpu.2013.0187
23. Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22(17):R741–R752. doi:10.1016/j.cub.2012.07.024
24. Harman D. The aging process: major risk factor for disease and death. Proc Natl Acad Sci USA. 1991;88(12):5360–5363. doi:10.1073/pnas.88.12.5360
25. Costello JT, Bieuzen F, Bleakley CM. Where are all the female participants in sports and exercise medicine research? Eur J Sport Sci. 2014;14(8):847–851. doi:10.1080/17461391.2014.911354
26. Peck TC, Sockol LE, Hancock SM. Mind the gap: the underrepresentation of female participants and authors in virtual reality research. IEEE Transact. 2020;26:1945–1954.
27. Sadiqi F, Ennaji M. Women in the Middle East and North Africa. Routledge; 2013.
28. Oster E, Dorsey ER, Bausch J, et al. Fear of health insurance loss among individuals at risk for Huntington disease. Am J Med Genet A. 2008;146(16):2070–2077. doi:10.1002/ajmg.a.32422
29. Moyce SC, Schenker M. Migrant workers and their occupational health and safety. Annu Rev Public Health. 2018;39:351–365. doi:10.1146/annurev-publhealth-040617-013714
30. Jansen W. Gender and the expansion of university education in Jordan. Gend Educ. 2006;18(5):473–490. doi:10.1080/09540250600881600
31. Amr M. Teacher education for inclusive education in the Arab world: the case of Jordan. Prospects. 2011;41(3):399. doi:10.1007/s11125-011-9203-9
32. Sanderson SC, Brothers KB, Mercaldo ND, et al. Public attitudes toward consent and data sharing in biobank research: a large multi-site experimental survey in the US. Am J Human Genet. 2017;100:414–427. doi:10.1016/j.ajhg.2017.01.021
33. Joly Y, Dalpé G, So D, et al. Fair shares and sharing fairly: a survey of public views on open science, informed consent and participatory research in biobanking. PLoS One. 2015;10(7):e0129893. doi:10.1371/journal.pone.0129893
34. Trauth J, Musa D, Siminoff L, et al. Public attitudes regarding willingness to participate in medical research studies. J Health Soc Policy. 2000;12(2):23–43. doi:10.1300/J045v12n02_02
35. Gilliss C, Lee KA, Gutierrez Y, et al. Recruitment and retention of healthy minority women into community-based longitudinal research. J Womens Health Gender Based Med. 2001;10(1):77–85. doi:10.1089/152460901750067142
36. Treweek S, Pitkethly M, Cook J, et al. Strategies to improve recruitment to randomised trials. Cochrane Database Syst Rev. 2018;2(2):MR000013. doi:10.1002/14651858.MR000013.pub6
37. Beskow LM, Dean E. Informed consent for biorepositories: assessing prospective participants’ understanding and opinions. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1440–1451. doi:10.1158/1055-9965.EPI-08-0086
38. Ahram M, Othman A, Shahrouri M. Public perception towards biobanking in Jordan. Biopreserv Biobank. 2012;10(4):361–365. doi:10.1089/bio.2012.0010
39. Alrabadi N, Makhlouf H, Khabour OF, et al. Jordanian’s perspectives on open consent in biomedical research. Risk Manag Healthc Policy. 2019;12:265–273. doi:10.2147/RMHP.S217209
40. Alemayehu C, Mitchell G, Nikles J. Barriers for conducting clinical trials in developing countries-a systematic review. Int J Equity Health. 2018;17(1):37. doi:10.1186/s12939-018-0748-6
41. Dee-Price B-JM. Social researchers and participants with intellectual disabilities and complex communication (access) needs. Whose capacity? Whose competence? Res Pract Intellect Dev Disabil. 2020;7(2):132–143. doi:10.1080/23297018.2020.1788418
42. Kraft S, Duenas DM, Lewis H, et al. Bridging the researcher-participant gap: a research agenda to build effective research relationships. Am J Bioethics. 2020;20(5):31. doi:10.1080/15265161.2020.1745936
43. Parkin RT. Communications with research participants and communities: foundations for best practices. J Expo Anal Environ Epidemiol. 2004;14(7):516–523. doi:10.1038/sj.jea.7500393
44. Moorcraft SY, Marriott C, Peckitt C, et al. Patients’ willingness to participate in clinical trials and their views on aspects of cancer research: results of a prospective patient survey. Trials. 2016;17. doi:10.1186/s13063-015-1105-3
45. Millum J, Bromwich D. Informed consent: what must be disclosed and what must be understood? Am J Bioethics. 2021;21(5):46–58. doi:10.1080/15265161.2020.1863511
46. Yusof MY, Teo CH, Ng CJ. Electronic informed consent criteria for research ethics review: a scoping review. BMC Med Ethics. 2022;23(1):117. doi:10.1186/s12910-022-00849-x
47. Ahmed WS, Abu Farha R, Halboup AM, et al. Knowledge, attitudes, perceptions and practice towards seasonal influenza and its vaccine: a cross-sectional study from a country of conflict. Front Public Health. 2023;11:271. doi:10.3389/fpubh.2023.1030391
48. Garrison NA, Sathe NA, Antommaria AHM, et al. A systematic literature review of individual’s perspectives on broad consent and data sharing in the United States. Genet Med. 2016;18(7):663–671. doi:10.1038/gim.2015.138
 © 2023 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2023 The Author(s). This work is published by Dove Medical Press Limited, and licensed under a Creative Commons Attribution License.
The full terms of the License are available at http://creativecommons.org/licenses/by/4.0/.
The license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

