Back to Journals » Research and Reports in Urology » Volume 9
AT1 expression in human urethral stricture tissue
Authors Siregar S, Parardya A, Sibarani J , Romdan T , Adi K, Hernowo BS, Yantisetiasti A
Received 8 May 2017
Accepted for publication 7 August 2017
Published 12 September 2017 Volume 2017:9 Pages 181—186
DOI https://doi.org/10.2147/RRU.S141327
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Safendra Siregar,1 Aga Parardya,1 Jupiter Sibarani,1 Tjahjodjati Romdan,1 Kuncoro Adi,1 Bethy S Hernowo,2 Anglita Yantisetiasti2
1Department of Urology, 2Department of Pathological Anatomy, Hasan Sadikin Hospital, Faculty of Medicine University of Padjadjaran, Bandung, Indonesia
Background: Urethral stricture has a high recurrence rate. There is a common doctrine stating that “once a stricture, always a stricture”. This fibrotic disease pathophysiology, pathologically characterized by excessive production, deposition and contraction of extracellular matrix is unknown. Angiotensin II type 1 (AT1) receptor primarily induces angiogenesis, cellular proliferation and inflammatory responses. AT1 receptors are also expressed in the fibroblasts of hypertrophic scars, whereas angiotensin II (AngII) regulates DNA synthesis in hypertrophic scar fibroblasts through a negative cross talk between AT1 and angiotensin II type 2 (AT2) receptors, which might contribute to the formation and maturation of human hypertrophic scars.
Objective: This study was conducted to determine the expression of AT1 receptors in urethral stricture tissues.
Methods: Urethral stricture tissues were collected from patients during anastomotic urethroplasty surgery. There were 24 tissue samples collected in this study with 2 samples of normal urethra for the control group. Immunohistochemistry study was performed to detect the presence of AT1 receptor expression. Data were analyzed using Mann–Whitney U test, and statistical analysis was performed with SPSS version 20.
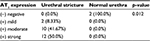
Results: This study showed that positive staining of AT1 receptor was found in all urethral stricture tissues (n=24). A total of 8.33% patients had low intensity, 41.67% had moderate intensity and 50% had high intensity of AT1 receptors, while in the control group, 100% patients had no intensity of AT1 receptors. Using the Mann–Whitney U test, it was found that urethral stricture tissue had a higher intensity of AT1 receptors than normal urethral tissue with a p-value = 0.012.
Conclusion: The results showed that AT1 receptor had a higher intensity in the urethral stricture tissue and that AT1 receptor may play an important role in the development of urethral stricture.
Keywords: angiotensin, AT1 receptor, urethral stricture
Introduction
Urethral stricture is defined as a narrowing of a segment of urethra that is enveloped by corpus spongiosum and specifically underwent spongiofibrosis process. Accumulation of fibrotic tissue will eventually lead to disturbance of voiding process and consequently affect the quality of life. The etiology of urethral stricture disease is traumatic, inflammatory, iatrogenic and idiopathic (Table 1). Every process that causes injury of urethral epithelium or corpus spongiosum could eventually lead to urethral stricture disease.1
  | Table 1 AT1 receptor expression in urethral tissue Abbreviation: AT1, angiotensin II type 1. |
Until now, urethral stricture disease is still a challenging problem, and its management has been still developing. From 16th century until now, the methods for overcoming urethral stricture have constantly changed from metal dilator, blind internal urethrotome in the 18th century, direct vision internal urethrotomy (DVIU) to urethroplasty surgery now.2
One challenging problem of the urethral stricture is its high recurrence rate. There is a common doctrine stating that “once a stricture, always a stricture”. A study by Zehri et al3 in 2009 revealed that the median duration between optical urethrotomy and recurrence was 4.5 months and the recurrence rate was 34%.
Another study by Han et al in 2015 found that 26% of all patients had a recurrence at a mean follow-up of 62 months. The recurrence rate after anastomotic urethroplasty was 18%, compared with 31% after substitution urethroplasty. The mean time to recurrence was 34 months.4
The mechanism of this fibrotic disease, pathologically characterized by excessive production, deposition and contraction of extracellular matrix, is still unclear. Recent studies showed the role of renin–angiotensin system (RAS) in fibroblast proliferation and extracellular matrix deposition. Shirazi et al5 in 2007 showed a decrease in urethral stricture recurrence after DVIU using intraurethral captopril gel compared to the placebo group.
Another study by Nababan et al (unpublished, 2013) observed an increasing expression of angiotensin II (AngII) receptor in injured urethra of Wistar mice.
All the study mentioned earlier showed us that RAS may have a role in urethral stricture formation and recurrence. To demonstrate the relationship of RAS with urethral stricture formation in human beings, we conducted this study.
Methods
Tissue samples
Tissue samples were obtained from urethral stricture segment that had been excised from the patients during anastomotic urethroplasty surgery for patients with anterior urethral stricture. The segment that was excised should also contain normal urethral tissue. In total, we obtained 24 urethral tissue samples from patients with urethral stricture and 2 normal urethra from cadaveric bodies. The urethral stricture tissue samples were obtained under written informed consent from the 24 patients who underwent anastomotic urethroplasty procedure for research purpose. This study was approved by the ethical committee of Hasan Sadikin Hospital, Bandung. The normal urethra tissue samples were obtained from cadaveric tissues that had deceased less than 24 hours. The cadaveric tissues were also obtained for education and research purpose and had been approved by the ethical committee of Hasan Sadikin Hospital, Bandung, and Department of Forensic Medicine of Hasan Sadikin Hospital, Bandung.
Immunohistochemical staining of AngII type 1
Immunohistochemistry (IHC) examination was done using angiotensin II type 1 (AT1) with 100 times dilution and ultra vision/HRP system with three-step anti-polyvalent detection system conjugated with secondary antibody.
The tissue samples were fixed with 10% formalin and embedded in paraffin. The samples then were cut as thick as 4–5 mm using microtome and stored in a 38–40°C incubator. Sections then were deparaffinized with xylol and sequentially rehydrated in a graded ethanol series for 5 min each.
The sections were dipped inside 90%, 80% and 70% alcohol solution and then washed using Aquadest and dipped inside boiled buffer citrate solution for 5 minutes and cooled at room temperature. After that, the sections were washed using phosphate-buffered saline (PBS) for 3 times, 5 minutes each. Blocking serum was added, and the sections were then incubated in a closed room for 10 minutes and washed using PBS for 3 times, 5 minutes each.
AngII was added to the sections, and the sections were then incubated in room temperature for 60 minutes and washed using PBS for 3 times, 5 minutes each. Biotinylated universal secondary antibody was added to the sections and then incubated in a closed room for 10 minutes and washed using PBS for 3 times, 5 minutes each. After that, diaminobenzylene chromogenic solution was added, incubated in the closed room for 5 minutes and washed using running water for 5 minutes. Counterstaining was done using Mayer’s hematoxylin, incubated for 2 minutes and then washed using running water.
Sequential dehydration was done by dipping the sections in 70%, 80% and 90% alcohol solutions for 5 minutes each and 100% ethanol solution for 2 times, 5 minutes each. The sections were then dried using filter paper, dipped into xylol for 3 minutes and observed and analyzed using light microscope.
AT1 receptor expression measurement procedure was done in Pathological Anatomy Department’s laboratory, Hasan Sadikin Hospital, Bandung. All sections were examined by experts in pathology. AT1 expression was measured by observing immunoexpression in the fibrotic tissue of the urethra.
Statistical analysis
To determine the expression of AT1 in urethral stricture tissue compared to normal urethral tissue, we used Mann–Whitney U test with 95% confidence level (a=5%). Statistical analysis was performed using SPSS version 20.0.
Result of the study
Descriptive analysis was done to d
etermine the AT1 receptor in urethral stricture tissue compared to normal urethral tissue. The AT1 expression was graded as negative, mild positive, moderate positive and strong positive (Figure 1).
  | Figure 1 AT1 expression in urethral stricture tissue: (A) mild positive, (B) moderate positive and (C) strong positive. |
From a total of 24 samples of urethral stricture tissues, 2 (8.33%) samples were categorized as mild positive, 10 (41.67%) samples were categorized as moderate positive, and 12 (50%) samples were categorized as strong positive. None of the samples were categorized as negative. From the normal urethra group, none of the samples expressed the AT1 receptor in their tissues.
Using Mann–Whitney U test, we concluded that AT1 receptor expression in urethral stricture tissue was significantly higher than normal urethral tissue (p=0.012; Table 1).
Discussion
Urethral stricture disease is a fibrotic condition of a segment of urethra due to spongiofibrotic process of the urethra and corpus spongiosum (Figures 2 and 3). Damage to urethral epithelium or corpus spongiosum would eventually lead to urethral stricture formation. Pathological process in urethral stricture formation showed a change in pseudostratified columnar epithelium that lines the urethra toward squamous metaplastic epithelium.7 Further damage to the metaplastic region can cause urine extravasation that leads to fibrotic process of the corpus spongiosum. This process will lead to narrowing of the urethral caliber and cause one to develop lower urinary tract symptoms and eventually urinary retention.
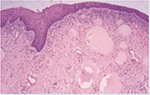  | Figure 2 Normal urethral tissue (100× magnification). |
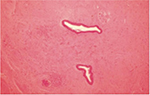  | Figure 3 Urethral stricture tissue (100× magnification). |
Baskin et al in 1993 showed us that there were no differences in the amount of collagens in urethral stricture tissue compared to that in normal urethra, but they have found a change in the ratio between collagen types that could explain the fibrotic and scar formation process. In corpus spongiosum of normal urethral tissue, the ratio between collagen type I and type III was 75%:25%, whereas in urethral stricture tissue, the ratio was 16%:84%.6
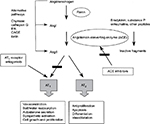
RAS is an endocrine system cascade, which consists of angiotensinogen (α-glycoprotein), that is released from the liver into the circulation and transformed by renin, which is produced by the juxtaglomerular apparatus of the kidney, into angiotensin I (AngI). AngI will further transform into more potent AngII by angiotensin-converting enzyme (ACE; Figure 4). AngII is the potent form that produces vasoconstriction of vascular wall or stimulates androgen production from adrenal cortex through angiotensin receptor.7–11
AngII receptor
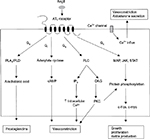
AngII has 2 kind of receptors, AT1 receptor and angiotensin II type 2 (AT2) receptor. AT1 is a G protein receptor that mediates several cascades, including phospholipase A, C and D activation, adenylate cyclase inhibition, tyrosine phosphorylation mediation and mediation of second messenger transduction, such as MAP kinase and phosphatidylinositol 3-kinase (PI3K).10 Most of AT1 mediate physiologic and pathophysiologic processes, including growth factors, vasoconstriction, aldosterone secretion and sympathetic outflow (Figure 5).13–17
The AT1 distribution and location in the tissues have been widely studied on human tissues or animal studies. Dominantly, AT1 has been found in the brain, adrenal glands, blood vessels and kidneys.12,18,19,20
Recent studies stated that there is expression of AT1 in human prostate, which is concentrated in the periurethral area and stromal tissue. This finding showed that AngII has its role in cellular growth and sympathetic activity of the prostate and is related to voiding stream.21
AngII stimulates the AT1 receptor in blood vessels that cause vasoconstriction. It also causes an increase in systemic vascular resistance. AT1 receptor in cardiac cells was known for its chronotropic and inotropic functions. AngII was also known to stimulate proliferation of cardiac cells and fibroblasts and has role in the formation of various growth factors such as fibroblast growth factor, transforming growth factor and platelet growth factor.
AT2 receptor is 30% homologous with AT1 receptor, even though its relation with G protein is still controversial. The most common AT2 expression was found in fetal tissue, and it downregulated after birth. AT2 receptor was believed to have a counterregulatory mechanism to protect kidney from damage that was caused by AngII.13,14,16,17,22,23
Some clinical studies have showed the nonhemodynamic effects of RAS.23 The involvement of AngII in fibrosis formation process was seen with the overexpression of renin and angiotensinogen which synergically induce extracellular matrix formation. AngII induces collagen and fibronectin expression.24 AngII also increases the proliferation of fibroblast and induces TGF-β1expression.25,26 Connective tissue growth factor (CTGF) presented as profibrotic cytokine and functioned as an important mediator of TGF-β1 and as a profibrotic component that stimulates proliferation and activation of fibroblast and induces epithelial cell transition to become fibroblast.27
In some other studies, there were a decrease in CTGF expression and fibrotic process in samples that give ACE inhibitor and AT1 blockers. Beside overexpression of extracellular matrix, disturbance of extracellular matrix degradation also leads to fibrosis process.28 AngII induces fibrosis not only by increasing extracellular matrix synthesis but also by decreasing the turnover of extracellular matrix. AngII, through AT1 receptor, induces PAI-1 and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) to inhibit metalloproteinase to slow the degradation of extracellular matrix and produce fibrosis.28
In this study, we found a significant difference in AT1 expression between urethral stricture tissue and normal urethra tissue. Despite some limitations in this study, such as lack of tissue samples and inequality between urethral stricture sample and normal urethra group, this study showed us another insight of fibrotic formation in urethral tissue and the probability of AT1 receptor involvement in fibrosis formation in human urethral tissue.
Study limitation
The cadaveric urethral tissue in this study was taken from the dead body less than 24 hours and was fixated using formaldehyde. We recognize the limitation of this sample that decomposing process might affect the AT1 expression in the urethral tissue. However, normal urethral tissue was impossible to collect unless we used cadaveric tissue. We excluded cadaver that decreased caused by urogenital cause, trauma or other conditions that may affect the urethra and collected the urethral tissue as soon as possible after the permission was granted from the ethical committee.
Disclosure
The authors report no conflicts of interest in this work.
References
Stein MJ, DeSouza RA. Anterior urethral stricture review. Transl Androl Urol. 2013;2(1):32–38. | ||
Smith TG. Current management of urethral stricture disease. Indian J Urol. 2016;32(1):27–33. | ||
Zehri AA, Ather MH, Afshan Q. Predictors of recurrence of urethral stricture disease following optical urethrotomy. Int J Surg. 2009;7(4):361–364. | ||
Han JS, Liu J, Hofer MD, et al. Risk of urethral stricture recurrence increases over time after urethroplasty. Int J Urol. 2015;22(7):695–699. | ||
Shirazi M, Khezri A, Samani SM, Monabbati A, Kojoori J, Hassanpour A. Effect of intraurethral captopril gel on the recurrence of urethral stricture after direct vision internal urethrotomy: phase II clinical trial. Int J Urol. 2007;14(3):203–208. | ||
Baskin LS, Constantinescu SC, Howard PS, et al. Biochemical characterization and quantitation of the collagenous components of urethral stricture tissue. J Urol. 1993;150(2 pt 2):642–647. | ||
Deschepper CF. Angiotensinogen: hormonal regulation and relative importance in the generation of angiotensin II. Kidney Int. 1994;46(6):1561–1563. | ||
Hall JE. Historical perspective of the renin-angiotensin system. Mol Biotechnol. 2003;24(1):27–39. | ||
Menard J, Bouhnik J, Clauser E, Richoux JP, Corvol P. Biochemistry and regulation of angiotensinogen. Clin Exp Hypertens. 1983;5(7–8):1005–1019. | ||
Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990;70(4):1067–1116. | ||
de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52(3):415–472. | ||
Kohlstedt K, Brandes RP, Muller-Esterl W, Busse R, Fleming I. Angiotensin-converting enzyme is involved in outside-in signaling in endothelial cells. Circ Res. 2004;94(1):60–67. | ||
Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press. 2003;12(2):70–88. | ||
Unger T. The angiotensin type 2 receptor: variations on an enigmatic theme. J Hypertens. 1999;17:1775–1786. | ||
Unger T, Chung O, Csikos T, et al. Angiotensin receptors. J Hypertens Suppl. 1996;14(5):S95–S103. | ||
Timmermans PB, Wong PC, Chiu AT, et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45(2):205–251. | ||
Stoll M, Steckelings UM, Paul M, Bottari SP, Metzger R, Unger T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest. 1995;95(2):651–657. | ||
Ferrario CM, Chappell MC. Novel angiotensin peptides. Cell Mol Life Sci. 2004;61(21):2720–2727. | ||
Kohlstedt K, Busse R, Fleming I. Signaling via the angiotensin-converting enzyme enhances the expression of cyclooxygenase-2 in endothelial cells. Hypertension. 2005;45(1):126–132. | ||
Kohlstedt K, Shoghi F, Muller-Esterl W, Busse R, Fleming L. CK2 phosphorylates the angiotensin-converting enzyme and regulates its retention in the endothelial cell plasma membrane. Circ Res. 2002;91(8):749–756. | ||
Dinh DT, Frauman AG, Somers GR, et al. Evidence for activation of the renin-angiotensin system in the human prostate: increased angiotensin II and reduced AT(1) receptor expression in benign prostatic hyperplasia. J Pathol. 2002;196(2):213–219. | ||
Unger T, Chung O, Csikos T, et al. Angiotensin receptors. J Hypertens Suppl. 1996;14(5):S95–S103. | ||
Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3):251–287. | ||
Wolf G. Renal injury due to renin-angiotensin-aldosterone system activation of the transforming growth factor-beta pathway. Kidney Int. 2006;70(11):1914–1919. | ||
Zhang C, Meng X, Zhu Z, Liu J, Deng A. Connective tissue growth factor regulates the key events in tubular epithelial to myofibroblast transition in vitro. Cell Biol Int. 2004;28(12):863–873. | ||
Ruiz-Ortega M, Ruperez M, Esteban V, et al. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant. 2006;21(1):16–20. | ||
Zhang C, Meng X, Zhu Z, Yang X, Deng A. Role of connective tissue growth factor in renal tubular epithelial-myofibroblast transdifferentiation and extracellular matrix accumulation in vitro. Life Sci. 2004; 75(3):367–379. | ||
Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110(3):341–350. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.