Back to Journals » Drug Design, Development and Therapy » Volume 13
Astragaloside IV protects endothelial progenitor cells from the damage of ox-LDL via the LOX-1/NLRP3 inflammasome pathway
Authors Qian W, Cai X, Qian Q, Zhuang Q, Yang W, Zhang X, Zhao L
Received 6 March 2019
Accepted for publication 29 May 2019
Published 29 July 2019 Volume 2019:13 Pages 2579—2589
DOI https://doi.org/10.2147/DDDT.S207774
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Weibin Qian,1,2,* Xinrui Cai,2,3 Qiuhai Qian,4,* Qianzhu Zhuang,5 Wenjun Yang,4 Xinying Zhang,4 Lijie Zhao6
1Department of Lung Disease, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong 250011, People’s Republic of China; 2Postdoctoral Station, Shandong University of Traditional Chinese Medicine, Jinan, Shandong 250355, People’s Republic of China; 3Department of Traditional Chinese Medicine, Shandong Academy of Occupational Health and Occupational Medicine, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, Shandong 250062, People’s Republic of China; 4Department of Endocrinology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong 250011, People’s Republic of China; 5Academic Department, China Association of Chinese Medicine, Beijing 100029, People’s Republic of China; 6Preventive Treatment Department, Second Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong 250001, People’s Republic of China
*These authors contributed equally to this work
Purpose: Functional impairment of endothelial progenitor cells (EPCs) is frequently observed in patients with diabetic vascular complications. Astragaloside IV (ASV) has a significant protective effect against vascular endothelial dysfunction. Thus, this study aimed to investigate the role of ASV on oxidized low-density lipoprotein (ox-LDL)-induced EPCs dysfunction and its potential mechanisms.
Methods: EPCs were isolated from the peripheral blood of mice and treated with different concentration of ASV (10, 20, 40, 60, 80, 100 and 200 μM). ox-LDL was served as a stimulus for cell model. The proliferation and migration, and improved tube formation ability of EPCs were determined. Reactive oxygen species (ROS) production and the levels of inflammatory cytokines, including interleukin 1β (IL-1β), IL-6, IL-10 and tumor necrosis factor (TNF-α) were measured. The expression oflectin-like oxidized LDL receptor (LOX-1) andNod-like receptor nucleotide-binding domain leucine rich repeat containing protein 3 (NLRP3) inflammasome were detected by Western blot analysis.
Results: We found ASV treatment alleviated ox-LDL-induced cellular dysfunction, as evidenced by promoted proliferation and migration, and improved tube formation ability. Besides, ASV treatment significantly suppressed ox-LDL-induced ROS production and the levels of inflammatory cytokines. ASV inhibited ox-LDL-induced expression of LOX-1 in a concentration-dependent manner. Overexpression of LOX-1 in EPCs triggered NLRP3inflammasome activation, while inhibition of LOX-1 or treatment with ASV suppressed ox-LDL-induced NLRP3 inflammasome activation. Furthermore, overexpression of LOX-1 in ox-LDL-induced EPCs furtherly impaired cellular function, which could be ameliorated by ASV treatment.
Conclusion: Our study showed that ASV may protect EPCs against ox-LDL-induced dysfunction via LOX-1/NLRP3 pathway.
Keywords: endothelial progenitor cells, astragaloside IV, lectin-like oxidized LDL receptor, NLRP3 inflammasome
Introduction
Type 2 diabetes mellitus (T2DM), the major type of DM, has become a global emergency due to its rapidly rising morbidity and chronic microvascular and macrovascular complications. Patients with T2DM have risks of death and cardiovascular events that have 2–4 times the risks of the general population.1 Diabetic macrovascular disease morphologically and functionally takes after atherosclerotic lesion, but diabetic microvascular disease manifests as retinopathy, nephropathy and vascular abnormalities in the lower extremities,2 leading to visual impairment, kidney failure and lower extremity dysfunction.3 Endothelial dysfunction is a well-accepted central event that precedes the development of atherogenesis, which is the prominent pathophysiological disorder of diabetic cardiovascular disease.4 Endothelial dysfunction is commonly observed in T2DM patients, which is resulting from accumulation of advanced glycated end products and oxidized low-density lipoprotein (ox-LDL), and recent evidence suggesting endothelial dysfunction might be associated with endothelial progenitor cells (EPCs) dysfunction.5 EPCs, derived primarily from the bone marrow, are precursors to mature endothelial cells with distinctive characteristics. At present, ox-LDL plays a vital role in the development and progression of atherosclerosis, and it induces endothelial cells damage through increased oxidative stress, inflammatory response and secretion of adhesion molecules.6 Studies found ox-LDL also influences the growth and bioactivity of EPCs, with its binding to the lectin-like oxidized LDL receptor (LOX-1).7 It has been demonstrated that human EPCs express LOX-1 and its activation by ox-LDL induces cellular senescence8 and apoptosis.9 However, the exact mechanism through which LOX-1 exerts its role remains unclear.
Ox-LDL induces inflammation by stimulating the production of plenty of inflammatory cytokines, including interleukin (IL)-1β.10 The level of IL-1β is remarkably increased in the serum of T2DM patients with macrovascular complications.11 The maturation of IL-1β is mediated by NLR family pyrin domain containing 3 (NLRP3) inflammasome. Once NLRP3 inflammasome is activated, NLRP3 oligomerizes with the adaptor protein ASC (apoptosis-associated speck-like protein containing a card), leading to the cleavage and activation of caspase-1. Caspase-1 then cleaves precursors IL-1β into IL-1β, which contribute to the pathogenesis and progression of vascular complications in T2DM.12 Recently, a study demonstrated that NLRP3 inflammasome can be activated by ox-LDL stimulation, subsequently causing the secretion of IL-1β in macrophage.13 However, the role of ox-LDL on NLRP3 inflammasome activation and its underlying mechanisms in EPCs remains poorly understood.
Astragaloside IV (ASV) is a traditional Chinese medicine extract with various pharmacological effects.14 It has also been suggested that ASV has protective effect on diabetic microvascular complications including diabetic retinopathy and diabetic nephropathy.15,16 ASV also improved aortic endothelial function induced by hyperglycemia.17 However, whether ASV has a protective effect on EPCs from ox-LDL-induced injury remains unclear. In the present study, we therefore studied the potential protective effects of ASV on ox-LDL induced EPCs dysfunction and investigated whether LOX-1 and NLRP3 inflammasome activation are involved.
Materials and methods
Isolation and characterization of EPCs
Mononuclear cells were isolated from peripheral blood of normal rats by density gradient centrifugation.18 Then, the cells were supplemented with endothelial growth medium-2 (EGM-2; Lonza, Basel, Switzerland) containing EGM-2 growth kit supplement (Lonza). After 7 days in the culture, EPCs were trypsinized and adjusted to 3×106/mL, followed by incubation for 30 mins at 4°C with fluorescent-labeled goat anti-rat antibodies against CD34 (1:50, ab81289, Abcam, Cambridge, USA), CD31 (1:50, ab216459, Abcam, Cambridge, USA), CD45 (1:20, ab214437, Abcam, Cambridge, USA) and CD133 (1:200, ab19898, Abcam, Cambridge, USA). After washed with PBS, the cells were measured using flow cytometry analysis. Immunophenotypic analysis was also performed by staining 5×105 cells with monoclonal antibodies against 1,1ʹ-dioctadecyl-3,3,3ʹ,3ʹ- tetramethylindocarbocyanine (DiI)-labelled Ac-LDL (10 μg/mL, Invitrogen, Carlsbad, USA) in cell medium for 4 hrs at 37°C. Cells were then incubated with FITC-labeled lectin (UEA-1, 10 μg/mL, Invitrogen) for another 1 hr at 37°C. EPCs were used at passages 7–9 for all experiments. All of the animal procedures were approved by the Animal Care and Use Committee of Shandong University of Traditional Chinese Medicine and the guidelines from the National Institutes of Health.
Treatments
EPCs were treated with different concentrations (0.1, 1, 5, 10, 25 and 50 μM) of ox-LDL (Sigma, Merck KGaA, Darmstadt, Germany) for 24 hrs and/or different concentrations (10, 20, 40, 60, 80, 100 and 200 μM) of ASV (C19193, purity ≥98%, Xiya Reagent, Shandong, China),19 as indicated. To identify whether LOX-1 is involved in the effects of ASV on EPCs, LOX-1 neutralizing antibody (R&D, 10 μg/mL), or the chemical inhibitor polyinosinic acid (Poly(I), Sigma, 250 μg/mL),20 were added to cultural medium 2 hrs prior to treatment with ox-LDL.
Cell proliferation assay
Cells were plated at a density of 2×103 cells/well in 96-well microplates and after 48 hrs culture, the number of viable cells was evaluated by a CCK8 regent (MedChem Express, Shanghai, China). Briefly, after the supernatants were discarded and CCK-8 solution was added to each culture well and incubated for 30 mins at 37°C. The number of viable cells was measured by evaluating absorbance at 450 nm on a microtiter plate reader (Bio-Rad Laboratories, Inc., Hercules, USA). The cell viability was calculated as the absorbance of treated cells/control cells×100%.
Cell migration assay
To determine cell migrative ability, 1×105 cells were plated on the top side of polycarbonate Transwell filter (Cell Biolabs, Inc. Santiago, USA). Cells were suspended in the upper chamber in medium without serum, and EGM-2 medium containing 10% serum was used as a chemoattractant in the lower chamber, and then incubated at 37°C for 24 hrs. The non-migrative cells in the upper chambers were gently removed and cells on the bottom membrane surface were fixed with 4% paraformaldehyde for 15 mins, followed by stained with 0.5% crystal violet solution (Beyotime), and counted under a microscope (Leica DMi8, Leica Company, Germany). Experiments were repeated three times independently.
Tube formation
Tube formation was evaluated with an Angiogenesis (Tube Formation) Assay Kit (BioVision, Inc. Milpitas, CA, USA). Extracellular Matrix Solution was mixed with ECMatrix diluent buffer and maintained in a μ-Slide plate at 37°C for 60 mins to allow the matrix solution to solidify. About 1×104 EPCs in 100 μL EGM-2 media were seeded into the wells. After 4 hrs, capillary-like structures were imaged under the microscope at ×100 magnification.
ROS staining
Intracellular ROS generation was determined using peroxidesensitive fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCFHDA, Molecular Probes). The cells were cultured into 6-well plates followed by exposure to the indicated experimental conditions, and the cells were incubated with 5 mM DCFH-DA for 20 mins at 37°C in the dark. Subsequently, the cells were rinsed with serum-free EGM-2 twice, and the representative images of ROS generation were captured using a Nikon Eclipse Ti-U epifluorescence microscope or a flow cytometry (FACS Calibur, Bio-Rad Laboratories, Inc., USA).
Enzyme-linked immunosorbent assay (ELISA)
After experimental treatments, cell membranes were disrupted and the cell lysates centrifuged at 10,000g at 4°C for 15 mins. The supernatant was separated from cell debris by a tissue tearor (Biospec, Bartlesville, OK, USA) and used for subsequent measurements. The concentrations of IL-1β, IL-6, IL-10 and TNF-a were measured using the corresponding ELISA kit (R&D Systems, Minneapolis, MN, USA) following the manufacturer’s instructions.
Western blot
Total protein was extracted from treated cells using 1% RIPA Lysis Buffer (Beyotime, Jiangsu, China) with a phosphorylation inhibitor (Roche, Indianapolis, USA). The total protein concentration for each sample was measured with a bicinchoninic acid (BCA) kit (Beyotime). Equal amounts of protein samples were then subjected to SDS-PAGE and proteins were then transferred to PVDF membranes (Beyotime). The membranes were blocked with 5% skim milk at room temperature for 1 hr and incubated with the primary antibodies against LOX-1 (1:1,000, ab60178, Abcam), NLRP3 (1:1,000, ab4207, Abcam), ASC (1:5,000, ab127537, Abcam), caspase1 (1:1,000, ab1872, Abcam), mature IL-1β (1:1,000, ab200478, Abcam) and β-actin (1:1,000, ab8226, Abcam). Subsequently, the membranes were incubated in anti-rabbit IgG horseradish peroxidase conjugated antibody (1:5,000; Abcam) for 1 hr at room temperature. The immune complexes were detected using the SuperSignal west pico kit (Shanghai solarbio Bioscience & Technology, China). The intensities of the signals were quantified using ImageJ software 6.0.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to detect the LOX-1 mRNA expression levels in EPCs. Firstly, RNA was reverse transcribed into cDNA and the resulting cDNA was used as templates for PCR amplification. The primer sequences used were: LOX-1 forward, 5ʹ‑AAAAAGTCGGGAGAATTGCCTATC‑3ʹ; reverse, 5ʹ‑CCGGGTTTTTGCTTCTGGTCTT‑3ʹ; β‑actin forward, 5ʹ-GAGGGGAGAGCGGGTAAGA−3ʹ reverse, 5ʹ-TCGGGGTCCGACAAAACCC−3ʹ. All reactions were performed in triplicates and each experiment was repeated three times in LightCycler480 PCR system (Roche Diagnostics, Rotkreuz, Switzerland), with the SYBR Green (Takara, Dalian, China) as fluorescent dye, and normalized towards β-actin mRNA levels. The conditions were as follows: at 94°C for 10 mins, followed by 40 cycles (94°C for 10 s; 60°C for 45 s; 72°C for 60 s). The gene expression level was calculated by 2−(ΔΔCT) methods.21
Statistical analysis
Data are presented as mean ± S.D. All statistical analyses were performed by SPSS statistics v20.0 (IBM Corp., Armonk, NY, USA). Multiple comparisons were performed using one-way ANOVA followed by Tukey–Kramer as a post-hoc test, as appropriate. P<0.05 indicated statistical significance. All experiments were conducted at least three separate times.
Results
ASV protected ox-LDL-induced cell damage on EPCs function
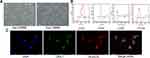
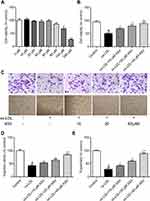
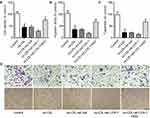
EPCs were isolated from rat peripheral blood cells, and the isolated cells displayed a characteristic spindle morphology 7 days later (Figure 1A). The surface markers of cells were additionally tested by flow cytometry and the percentage of CD31 was 95%±4%, the percentage of CD34 was 97%±6%, the percentage of CD133 was 95%±3%. The expression levels of CD45 were very low (Figure 1B). In addition, the characterized EPCs were proved by double staining with scavenger receptor of Dil-acLDL and the ligand of UEA-1, which two are hallmarks of EPCs (Figure 1C). To evaluate the effect of ASV on EPCs, cell viability was measured by CCK8 assay. As illustrated in Figure 2A, low concentration of (10, 20, 40, 60 and 80 μM) ASV exposure for 24 hrs had no influence on EPCs viability, indicating no toxicity on EPCs at this dose range. However, compared with normal control group, a high concentration of (100 and 200 μM) ASV presented statistical differences on cell viability of EPCs (P<0.05 or P<0.01). According to the above results, 10, 20, 40 μM ASV were chosen for the subsequent experiments. As depicted in Figure 2B, when compared with the control group, ASV with concentration range from 10 to 40 μM exerted protective effects on ox-LDL-induced cell viability in a dose-dependent manner. In addition, we found that ASV promoted cell migration and improved impaired tube formation upon stimulation of ox-LDL (Figure 2C–E). Taken together, above results revealed that ASV attenuates ox-LDL-induced dysfunction of EPCs.
ASV suppress oxidative stress and inflammation of EPCs induced by ox-LDL via inhibition of LOX-1
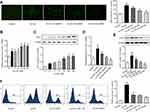
The intracellular ROS was detected by fluorescein-labeled dye, DCFH-DA. EPCs stimulated with ox-LDL led to a significant increase in intracellular ROS generation. Nevertheless, pretreatment with ASV for 24 hrs reduced the intracellular ROS levels induced by ox-LDL in a dose-dependent manner (Figure 3A). To reveal the mechanisms of the antioxidative effects of ASV, we investigated whether LOX-1, the receptor of ox-LDL, was involved in ox-LDL in induced cell damage. We found the expression levels of LOX-1 mRNA and protein were both enhanced by ox-LDL treatment in a concentration-dependent manner (Figure 3B and C). To the contrary, ASV treatment reduced LOX-1 mRNA and protein expression levels induced by 50 μM ox-LDL exposure in a dose-dependent manner (Figure 3D and E). Subsequently, a neutralizing antibody and chemical inhibitor to downregulate LOX-1 in EPCs were used to determine the role of LOX-1 in ROS production in response to ox-LDL. The intracellular ROS quantified by flow cytometer showed that similar with ASV, treatment with LOX-1 neutralizing antibody or inhibitor also reduced intracellular ROS levels (Figure 3F). Furthermore, our ELISA assay results showed that the level of inflammatory cytokines including IL-1β, IL-6, IL-10 and TNF-α were significantly increased in the ox-LDL-stimulated EPCs compared to that in the control EPCs. ASV, LOX-1 neutralizing antibody or inhibitor treatment all reduced above inflammatory cytokines significantly (Table 1). These results indicated that ASV inhibits ox-LDL-induced oxidative stress and inflammation through inhibition of LOX-1.
 |
Table 1 Effect of astragaloside IV on release of proinflammatory cytokines triggered by ox-LDL in EPCs (n=5, mean±SD) |
ASV inhibits ox-LDL-induced NLRP3 inflammasome activation via LOX-1
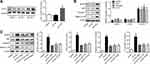
To reveal the further mechanisms of how LOX-1 regulates ox-LDL-induced inflammation in EPCs, we investigated the activation of NLRP3 inflammasome, which plays a key role in inflammation. As illustrated in Figure 4A, EPCs transfected with adenovirus containing the LOX-1 gene (ad LOX-1) showed higher LOX-1 expressions compared to the cells transfected with control adenovirus (as null). Interestingly, the results of Western blot showed that upregulation of LOX-1 in EPCs resulted in the activation of NLRP3 inflammasome, as evidenced by the great increase in expression of NLRP3, ASC, caspase1 and mature IL-1β proteins (Figure 4B). We then explored the effects of ASV on ox-LDL-induced NLRP3 inflammasome activation. As depicted in Figure 4C, ox-LDL stimulation caused an increase in NLRP3, ASC, caspase1 and mature IL-1β proteins. However, the stimulatory effect of ox-LDL on EPCs was partly abolished by pretreatment with ASV, LOX-1 neutralizing antibody or inhibitor, corroborating the notion that ASV inhibited ox-LDL-induced NLRP3 inflammasome activation via regulation of LOX-1 in EPCs.
ASV alleviates ox-LDL-induced EPCs dysfunction via LOX-1 dependent NLRP3 signaling pathway
To validate the role of LOX-1/NLRP3 pathway in the protective effects of ASV on EPCs proliferation, migration and tube formation were detected. We found that overexpression of LOX-1 in ox-LDL-induced EPCs showed decreased cell viability than the control, which could be revised by ASV treatment (Figure 5A). Similarly, the results of cell migration and tube formation assay showed that LOX-1-upregulated EPCs had worse migrative and angiogenic ability than the control. And as expected, ASV treatment could improve LOX-1-upregulation-induced EPCs dysfunction (Figure 5B–D). The data suggested that ASV protects EPCs from ox-LDL-induced damage through LOX-1/NLRP3 pathway.
Discussion
The results of the present study revealed that ox-LDL stimulation increased the expression of LOX-1 and subsequent NLRP3 inflammasome activation, resulted in reduced cell proliferation and migration, and impaired tube formation in EPCs. Moreover, we found that ASV could attenuate ox-LDL-induced EPCs dysfunction and the protective effect of ASV on EPCs function is dependent on the LOX-1/NLRP3 pathway (Figure 6).
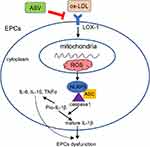 |
Figure 6 Schematic diagram showing the protective effects of ASV in ox-LDL-induced EPCs dysfunction.Abbreviations: EPCs, endothelial progenitor cells; ASV, astragaloside IV. |
EPCs are a population of cells with the inherent capacity to differentiate into mature endothelial cells. They are under investigation due to their association with vascular injury in response to trauma or ischemia.22 A variety of disease conditions including T2DM negatively affect EPCs.23 Ox-LDL induced reduction and dysfunction of EPCs has been shown in many studies. Hamed et al found ox-LDL exerted a deleterious effect on migrative ability of EPCs.24 Similarly, other researchers identified that ox-LDL-affected EPC tube formation by activating eNOS mechanisms.25 Consistently, we also found that ox-LDL reduced proliferation and migration, and impaired tube formation of EPCs. Therefore, the number and function of both cultured and circulating EPCs are profoundly altered in patients with T2DM, contributing to both the microvascular and macrovascular complications of diabetic disease.26
Plenty of studies were taken to find solutions to improve EPCs function. Anti-inflammatory agents, anti-hypertensive agents, as well as vitamin D supplementation are all considered as therapeutic strategies.22 Recently, an increasing number of studies focused on traditional Chinese medicine.27 Yang et al demonstrated that the expression of VCAM-1/ICAM-1 in EPCs were promoted by TNF-α, which were reduced by Tanshinone IIA.28 Salvianolic acid and Salidroside B were also found to promote cell migration and capillary tube formation of EPCs.29 In addition, icariin could exert proangiogenic effects by promoting cell viability, migration, cell-matrix adhesion and enhancing capillary tube formation of EPCs.30 In our previous published papers, we have demonstrated that ASV inhibited TGFβ1-induced epithelial-mesenchymal transition of alveolar epithelial cells.31 And studies have found that ASV has certain endothelial cell protective function.32 However, whether ASV could protect EPCs from ox-LDL-induced injury is unclear. Here, we showed for the first time that ASV prevented ox-LDL-induced EPCs dysfunction, which was evidenced by enhanced cell proliferation, migration and improved tube formation capacity.
To figure out how ASV exerts its protective effects in EPCs, we tested the influence of ASV on LOX-1 expression in EPCs under ox-LDL stimulation. Interestingly, we found ASV significantly inhibited ox-LDL-induced upregulation of LOX-1 expression in EPCs in a concentration-dependent manner. LOX-1 is the major receptor for binding and uptake of ox-LDL in endothelial cells. Activation of LOX-1 promotes secretion of pro-inflammatory cytokines like IL-6, IL-8 and TNFα connecting LOX-1 to the development of atherosclerosis.33 Knockdown of LOX-1 prevented ox-LDL-induced endothelial dysfunction and blocking the LOX-1 receptor preserved nitric oxide synthase and decreased superoxide anion radical formation.34,35 We also found that inhibition of LOX-1 attenuated ox-LDL-induced EPCs dysfunction in the current study. Therefore, LOX-1 is a potential target for prevention of ox-LDL-mediated EPCs dysfunction and ASV could be served as an agent targeting LOX-1 in EPCs.
It was reported that ASV could decrease the release of IL-1β, TNFα and IL-6 in diabetic rats.36 ASV treatment also reduced the level of ROS and apoptosis of renal tubular epithelial cells under free fatty acids stimulation.37 Furthermore, administration of ASV ameliorates diabetic nephropathy by inhibition of ERK1/2 signaling,15 indicating that ASV has the ability to prevent diabetic vascular complications. In this study, we found that the levels of IL-1β, IL-6, IL-10 and TNFα were inhibited by ASV treatment. We also identified that ASV treatment suppressed LOX-1 expression in ox-LDL induced EPCs. Previous studies have demonstrated that NLRP3 inflammasome played an important role in inflammatory disease, including diabetes and diabetic complications.38 NLRP3 inflammasome has been reported to be related to LOX-1.39 Knockdown of LOX-1 decreased ox-LDL or xanthine oxidase-induced NLRP3 inflammasome in endothelial cells40 and vascular smooth muscle cells.41 Blockade LOX-1 with neutralizing antibody also inhibited electronegative LDL-induced activation of caspase-1 and NF-κB in human macrophages.42 Consistent with these studies, we found depletion of LOX-1 using chemical inhibitor or LOX-1 neutralizing antibody in EPCs resulted in significant inhibition of NLRP3 inflammasome activation. All these data suggested that the LOX-1 and NLRP3 inflammasome pathways formed a signaling axis and this axis is a novel mechanism through which ASV exerts its anti-inflammation and antioxidant property in EPCs.
In summary, our results demonstrated that ASV may suppress EPCs dysfunction, which was relating to LOX-1/NLRP3 signaling pathway disturbed by ox-LDL. The data suggested that ASV may be considered as a strategy for T2DM vascular complications treatment.
Abbreviation list
EPCs, endothelial progenitor cells; T2DM, Type 2 diabetes mellitus; ASV, astragaloside IV; ox-LDL, oxidized low-density lipoprotein; ROS, reactive oxygen species; TNF-α, tumor necrosis factor; LOX-1, lectin-like oxidized LDL receptor; NLRP3, Nod-like receptor nucleotide-binding domain leucine-rich repeat containing protein 3.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant no. 81704071), Key Research and Development Plan of Shandong province (Grant no. 2018GSF119027), the Taishan Scholars Youth Expert Program of Shandong Province in China (tsqn201812146), Young Elite Scientists Sponsorship Program by CAST (CACM-2018-QNRC2-B01), the Natural Science Foundation of Shandong Province (Grant nos. ZR2017BH027, ZR2016HB19 and ZR2012HM093), the Project of Scientific and Technological Development Program of Shandong Province (Grant no. 2010GSF10242), Project of Scientific and Technological Development Program of Traditional Chinese Medicine of Shandong Province (Grant nos. 2017-180, 2011-038, 2009Z004-1), the Project of Scientific and Technological Development Program of Jinan (Grant nos. 201805081, 201805009).
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rawshani A, Rawshani A, Franzen S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633–644. doi:10.1056/NEJMoa1800256
2. Gilbert RE. Endothelial loss and repair in the vascular complications of diabetes: pathogenetic mechanisms and therapeutic implications. Circ J. 2013;77(4):849–856.
3. Shi Y, Vanhoutte PM. Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes. 2017;9(5):434–449. doi:10.1111/1753-0407.12521
4. Kim JA, Montagnani M, Koh KK, et al. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113(15):1888–1904. doi:10.1161/CIRCULATIONAHA.105.563213
5. van Sloten TT, Henry RM, Dekker JM, et al. Endothelial dysfunction plays a key role in increasing cardiovascular risk in type 2 diabetes: the Hoorn study. Hypertension. 2014;64(6):1299–1305. doi:10.1161/HYPERTENSIONAHA.114.04221
6. Di Pietro N, Formoso G, Pandolfi A. Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vascul Pharmacol. 2016;84(1–7):1–7. doi:10.1016/j.vph.2016.05.013
7. Ma FX, Zhou B, Chen Z, et al. Oxidized low density lipoprotein impairs endothelial progenitor cells by regulation of endothelial nitric oxide synthase. J Lipid Res. 2006;47(6):1227–1237. doi:10.1194/jlr.M500507-JLR200
8. Imanishi T, Hano T, Sawamura T, Nishio I. Oxidized low-density lipoprotein induces endothelial progenitor cell senescence, leading to cellular dysfunction. Clin Exp Pharmacol Physiol. 2004;31(7):407–413. doi:10.1111/j.1440-1681.2004.04022.x
9. Cheng J, Cui R, Chen CH, et al. Oxidized low-density lipoprotein stimulates p53-dependent activation of proapoptotic Bax leading to apoptosis of differentiated endothelial progenitor cells. Endocrinology. 2007;148(5):2085–2094. doi:10.1210/en.2006-1709
10. Chen X, Lin J, Hu T, et al. Galectin-3 exacerbates ox-LDL-mediated endothelial injury by inducing inflammation via integrin beta1-RhoA-JNK signaling activation. J Cell Physiol. 2018;234(7):10990–11000. doi:10.1002/jcp.27910.
11. Yang M, Tian M, Zhang X, et al. Role of the JAK2/STAT3 signaling pathway in the pathogenesis of type 2 diabetes mellitus with macrovascular complications. Oncotarget. 2017;8(57):96958–96969. doi:10.18632/oncotarget.18555
12. Sharma A, Tate M, Mathew G, Vince JE, Ritchie RH, de Haan JB. Oxidative stress and NLRP3-inflammasome activity as significant drivers of diabetic cardiovascular complications: therapeutic implications. Front Physiol. 2018;9(114). doi:10.3389/fphys.2018.00114
13. Jiang Y, Wang M, Huang K, et al. Oxidized low-density lipoprotein induces secretion of interleukin-1beta by macrophages via reactive oxygen species-dependent NLRP3 inflammasome activation. Biochem Biophys Res Commun. 2012;425(2):121–126. doi:10.1016/j.bbrc.2012.07.011
14. Li L, Hou X, Xu R, Liu C, Tu M. Research review on the pharmacological effects of astragaloside IV. Fundam Clin Pharmacol. 2017;31(1):17–36. doi:10.1111/fcp.12232
15. Song G, Han P, Sun H, et al. Astragaloside IV ameliorates early diabetic nephropathy by inhibition of MEK1/2-ERK1/2-RSK2 signaling in streptozotocin-induced diabetic mice. J Int Med Res. 2018;46(7):2883–2897. doi:10.1177/0300060518778711
16. Ding Y, Yuan S, Liu X, et al. Protective effects of astragaloside IV on db/db mice with diabetic retinopathy. PLoS One. 2014;9(11):e112207. doi:10.1371/journal.pone.0112207
17. Leng B, Tang F, Lu M, et al. Astragaloside IV improves vascular endothelial dysfunction by inhibiting the TLR4/NF-kappaB signaling pathway. Life Sci. 2018;209:111–121. doi:10.1016/j.lfs.2018.07.053
18. Zhu J, Cheng X, Wang Q, Zhou Y, Wang F, Zou L. Transplantation of endothelial progenitor cells for improving placental perfusion in preeclamptic rats. Arch Gynecol Obstet. 2015;291(5):1113–1119. doi:10.1007/s00404-014-3522-z
19. Li X, Lin Y, Zhou H, et al. Puerarin protects against endothelial dysfunction and end-organ damage in Ang II-induced hypertension. Clin Exp Hypertens. 2017;39(1):58–64. doi:10.1080/10641963.2016.1200603
20. Li D, Mehta JL. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of human coronary artery endothelial cells: evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler Thromb Vasc Biol. 2000;20(4):1116–1122.
21. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
22. Edwards N, Langford-Smith AWW, Wilkinson FL, et al. Endothelial progenitor cells: new targets for therapeutics for inflammatory conditions with high cardiovascular risk. Front Med (Lausanne). 2018;(5):200. doi:10.3389/fmed.2018.00200
23. Medina RJ, Barber CL, Sabatier F, et al. Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl Med. 2017;6(5):1316–1320. doi:10.1002/sctm.16-0360
24. Hamed S, Brenner B, Abassi Z, et al. Hyperglycemia and oxidized-LDL exert a deleterious effect on endothelial progenitor cell migration in type 2 diabetes mellitus. Thromb Res. 2010;126(3):166–174. doi:10.1016/j.thromres.2010.03.002
25. Lin FY, Tsao NW, Shih CM, et al. The biphasic effects of oxidized-low density lipoprotein on the vasculogenic function of endothelial progenitor cells. PLoS One. 2015;10(5):e0123971. doi:10.1371/journal.pone.0123971
26. Pysna A, Bem R, Nemcova A, et al. Endothelial progenitor cells biology in diabetes mellitus and peripheral arterial disease and their therapeutic potential. Stem Cell Rev. 2018;15(2):157–165. doi:10.1007/s12015-018-9863-4.
27. Zhao QT, Li BF, Kong H. Roles of Chinese medicine bioactive ingredients in the regulation of cellular function of endothelial progenitor cells. Chin J Nat Med. 2014;12(7):481–487. doi:10.1016/S1875-5364(14)60075-3
28. Yang JX, Pan YY, Ge JH, et al. Tanshinone II A attenuates TNF-alpha-induced expression of VCAM-1 and ICAM-1 in endothelial progenitor cells by blocking activation of NF-kappaB. Cell Physiol Biochem. 2016;40(1–2):195–206. doi:10.1159/000452537
29. Tang Y, Vater C, Jacobi A, Liebers C, Zou X, Stiehler M. Salidroside exerts angiogenic and cytoprotective effects on human bone marrow-derived endothelial progenitor cells via Akt/mTOR/p70S6K and MAPK signalling pathways. Br J Pharmacol. 2014;171(9):2440–2456. doi:10.1111/bph.12611
30. Tang Y, Jacobi A, Vater C, Zou L, Zou X, Stiehler M. Icariin promotes angiogenic differentiation and prevents oxidative stress-induced autophagy in endothelial progenitor cells. Stem Cells. 2015;33(6):1863–1877. doi:10.1002/stem.2005
31. Qian W, Cai X, Qian Q, Zhang W, Wang D. Astragaloside IV modulates TGF-beta1-dependent epithelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. J Cell Mol Med. 2018;22:4354–4365. doi:10.1111/jcmm.2018.22.issue-9
32. Lin XP, Cui HJ, Yang AL, Luo J-K, Tang T. Astragaloside IV improves vasodilatation function by regulating the PI3K/Akt/eNOS signaling pathway in rat aorta endothelial cells. J Vasc Res. 2018;55(3):169–176. doi:10.1159/000489958
33. Hofmann A, Brunssen C, Morawietz H. Contribution of lectin-like oxidized low-density lipoprotein receptor-1 and LOX-1 modulating compounds to vascular diseases. Vascul Pharmacol. 2017;107–11. doi:10.1016/j.vph.2017.10.002.
34. Xu X, Gao X, Potter BJ, Cao J-M, Zhang C. Anti-LOX-1 rescues endothelial function in coronary arterioles in atherosclerotic ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2007;27(4):871–877. doi:10.1161/01.ATV.0000259358.31234.37
35. Mehta JL, Sanada N, Hu CP, et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res. 2007;100(11):1634–1642. doi:10.1161/CIRCRESAHA.107.149724
36. Wang Z, Li Q, Xiang M, et al. Astragaloside alleviates hepatic fibrosis function via PAR2 signaling pathway in diabetic rats. Cell Physiol Biochem. 2017;41(3):1156–1166. doi:10.1159/000464122
37. Chen Q, Su Y, Ju Y, et al. Astragalosides IV protected the renal tubular epithelial cells from free fatty acids-induced injury by reducing oxidative stress and apoptosis. Biomed Pharmacother. 2018;108(679–686). doi:10.1016/j.biopha.2018.09.049
38. Wu M, Han W, Song S, et al. NLRP3 deficiency ameliorates renal inflammation and fibrosis in diabetic mice. Mol Cell Endocrinol. 2018;478:115–125. doi:10.1016/j.mce.2018.08.002
39. Ding Z, Liu S, Wang X, et al. LOX-1 - dependent mitochondrial DNA damage and NLRP3 activation during systemic inflammation in mice. Biochem Biophys Res Commun. 2014;451(4):637–643. doi:10.1016/j.bbrc.2014.08.034
40. Wang S, Xie X, Lei T, et al. Statins attenuate activation of the NLRP3 inflammasome by oxidized LDL or TNFalpha in vascular endothelial cells through a PXR-dependent mechanism. Mol Pharmacol. 2017;92(3):256–264. doi:10.1124/mol.116.108100
41. Dai Y, Cao Y, Zhang Z, Vallurupalli S, Mehta JL. Xanthine oxidase induces foam cell formation through LOX-1 and NLRP3 activation. Cardiovasc Drugs Ther. 2017;31(1):19–27. doi:10.1007/s10557-016-6706-x
42. Yang TC, Chang PY, Lu SC. L5-LDL from ST-elevation myocardial infarction patients induces IL-1beta production via LOX-1 and NLRP3 inflammasome activation in macrophages. Am J Physiol Heart Circ Physiol. 2017;312(2):H265–H274. doi:10.1152/ajpheart.00509.2016
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.