Back to Journals » Clinical Ophthalmology » Volume 10
Astigmatism management in cataract surgery with Precizon® toric intraocular lens: a prospective study
Authors Vale C, Menezes C, Firmino-Machado J, Rodrigues P, Lume M , Tenedório P, Menéres P , Brochado MDC
Received 27 June 2015
Accepted for publication 21 October 2015
Published 19 January 2016 Volume 2016:10 Pages 151—159
DOI https://doi.org/10.2147/OPTH.S91298
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Carolina Vale,1 Carlos Menezes,2 J Firmino-Machado,3 Pedro Rodrigues,2 Miguel Lume,1 Paula Tenedório,2 Pedro Menéres,1 Maria do Céu Brochado1
1Department of Ophthalmology, Hospital Geral de Santo António – Centro Hospitalar do Porto, EPE, Porto, 2Department of Ophthalmology, Hospital de Pedro Hispano, Matosinhos, 3Public Health Unit, ACES – Porto Ocidental, Porto, Portugal
Purpose: The purpose of this study was to evaluate the visual and refractive outcomes and rotational stability of the new aspheric Precizon® toric intraocular lens (IOL) for the correction of corneal astigmatism in cataract surgery.
Setting: Department of Ophthalmology, Hospital Geral de Santo António – Centro Hospitalar do Porto, EPE and Hospital de Pedro Hispano, Matosinhos, Portugal.
Design: This was a prospective clinical study.
Patients and methods: A total of 40 eyes of 27 patients with corneal astigmatism greater than 1.0 diopter (D) underwent cataract surgery with implantation of Precizon® toric IOL. IOL power calculation was performed using optical coherence biometry (IOLMaster®). Outcomes of uncorrected (UDVA) and best-spectacle corrected distance visual acuities (BCDVA), refraction, and IOL rotation were analyzed at the 1st week, 1st, 3rd, and 6th month’s evaluations.
Results: The median postoperative UDVA was better than preoperative best-spectacle corrected distance visual acuity (0.02 [0.06] logMAR vs 0.19 [0.20] logMAR, P<0.001). At 6 months, postoperative UDVA was 0.1 logMAR or better in 95% of the eyes. At last follow-up, the mean spherical equivalent was reduced from -3.35±3.10 D to -0.02±0.30 D (P<0.001) with 97.5% of the eyes within ±0.50 D of emmetropia. The mean preoperative keratometric cylinder was 2.34±0.95 D and the mean postoperative refractive cylinder was 0.24±0.27 D (P<0.001). The mean IOL rotation was 2.43°±1.55°. None of the IOLs required realignment.
Conclusion: Precizon® toric IOL revealed very good rotational stability and performance regarding predictability, efficacy, and safety in the correction of preexisting regular corneal astigmatism associated with cataract surgery.
Keywords: astigmatism, cataract surgery, toric intraocular lens, stability, implantation outcomes
Introduction
Astigmatism is an extremely common refractive error whose incidence increases with age with a prevalence of astigmatism ≥1 diopter (D) of around 31% of the population older than 40 years old.1 Approximately 30% of eyes scheduled for cataract surgery have a high level of preexisting corneal astigmatism and as both cataract and astigmatism impair the quality of life of a patient, modern cataract surgery aims to treat both cataract and refractive errors with a single procedure.2–4
Various toric pseudophakic intraocular lenses (IOLs) are available for astigmatism correction during cataract surgery.5–9 Although new toric IOLs show good visual and refractive outcomes and rotational stability, misalignment keeps being the main factor for residual astigmatism and spectacle dependency after implantation of a toric IOL. It has been shown that every degree of misalignment results in a loss of up to 3.3% of the IOL’s cylindrical power.10 The success of a toric IOL lies not only on the IOL stability in the capsular bag over time, but also on its tolerance to misalignment. The aim of this study was to evaluate the visual and refractive outcomes and the rotational stability of the new aspheric Precizon® toric IOL after cataract surgery in patients with preexisting corneal astigmatism.
Patients and methods
This prospective noncomparative study included eyes that were implanted with Precizon® toric IOL after phacoemulsification surgery at Hospital Geral de Santo António – Centro Hospitalar do Porto, EPE in Oporto and Hospital de Pedro Hispano – Matosinhos Local Unity of Heath EPE in Matosinhos, between January 2014 and April 2014. This study followed the tenets of the Declaration of Helsinki and was approved by each local ethics committee of Centro Hospitalar do Porto, EPE and Hospital de Pedro Hispano. All patients provided written informed consent after receiving thorough explanation of the procedure.
The inclusion criteria were cataract and preexisting keratometric astigmatism of at least 1.0 D. Exclusion criteria were glaucoma, irregular astigmatism, corneal disease, previous corneal or intraocular surgery, macular degeneration or retinopathy, and history of ocular inflammation.
Preoperative evaluation, IOL, and power calculation
All patients underwent an extensive evaluation that included medical history, refraction and monocular uncorrected (UDVA), and best-spectacle corrected (BCDVA) distance visual acuities measurements. The Early Treatment of Diabetic Retinopathy Study charts at 4 m and autokeratometry (KA-1000®) were used. In addition, slit-lamp examination, intraocular pressure (contact Goldmann tonometry), dilated fundoscopy (Goldmann 3 mirrors lens), macular evaluation using spectral-domain optical coherence tomography (Spectralis®), endothelial cell count (ECC) and morphology (ICONAN®), and corneal topography using Scheimpflug imaging (PentacamHR® [OCULUS Inc., WA, USA] or Sirius-CSO® [Scandicci, FI, Italy]) were performed. Keratometry (K) readings and Biometry measurements (eg, axial length and anterior chamber depth) used for IOL power calculation were obtained with optical coherence biometry (IOLMaster®, Carl Zeiss Meditec AG, Jena, Germany). The spherical power of the IOL was calculated using the SRK-T formula for IOL power calculation and the A-constant of 118.5 for the toric IOL. The target postoperative spherical equivalent (SE) was the closest possible to emmetropia. Calculations of the cylindrical power and axis placement were determined using the IOL manufacturer’s online calculator (PRECIZON™ Online Calculator, Ophtec BV, Groningen, the Netherlands, available from: http://calculator.ophtec.com/. Accessed May 20, 2015), taking into account the data obtained with optical coherence biometry, the incision location as well as the estimate of surgically-induced astigmatism (SIA) personalized for each surgeon at the incision axis.
Surgical technique
Before surgery, the 0°–180° axis was marked with the patient seated at the slit-lamp to avoid cyclotorsion using a gravity marker with a calibrated horizontal position (LRI Gravity marker, Rumex, Clearwater, FL, USA). Intraoperatively, the main incision location and the desired implantation axis were marked on the limbus after correctly aligning a Mendez ring to the primary marks to ascertain the intended angle of placement, according to preoperative plan. Phacoemulsification was performed through a 2.4-mm clear cornea incision. After a continuous curvilinear capsulorrhexis of approximately 5.5 mm and hydrodissection were performed, the cataract was removed using a phaco-chop technique (Infiniti, Alcon, Inc., Hünenberg, Switzerland; Bausch & Lomb Incorporated, Bridgewater, NJ, USA). The toric IOL was implanted in the capsular bag using a disposable injector and cartridge system Dualtec™ Kit (Ophtec BV) before ophthalmic viscosurgical device (OVD, sodium hyalorunate 1.0%, Provisc®) was removed. After OVD withdrawal, the IOL was rotated to its final position by exactly aligning the toric reference lines on the IOL with the limbal implantation marks. The postoperative treatment included antibiotic, corticosteroids, and nonsteroidal anti-inflammatory eye drops in all patients.
Intraocular lens
The IOL used in this study was Precizon® toric IOL Model 565 (Ophtec BV), a 1 piece hydrophilic acrylic, monofocal, aspheric IOL with a transitional conic toric surface (patent pending), and plate-loop design. It is a foldable IOL and has a supporting closed loop-haptic design with no angulation, a biconvex 360° square edged 6.0 mm optic, and an overall diameter of 12.5 mm. During IOL implantation, the available spherical power ranged from +10 to +30 D (0.5 D increments) and cylinder power from 1 to 6 D (0.5 D increments).
Postoperative assessment
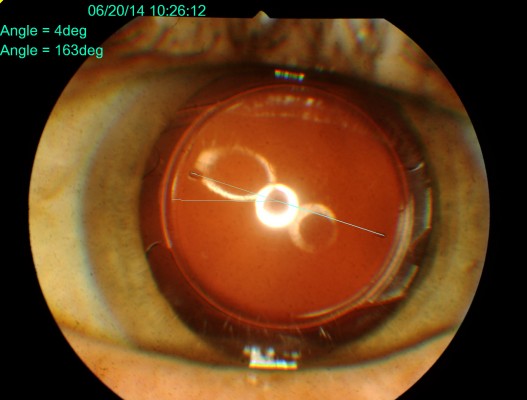
Postoperative examinations were performed at 1 week and 1, 3, and 6 months. The examinations included UDVA, BCDVA, subjective refraction, and slit-lamp examination with IOP measurement. At 6 months, postoperative corneal astigmatism was assessed using the same device used for IOL calculation (IOLMaster®, Carl Zeiss Meditec AG) to calculate the surgically-induced corneal astigmatism (SICA). Rotation of the IOL was assessed as follows: slit-lamp digital photographs in retroillumination of the IOL were obtained after full mydriasis, as the IOL marks are located at the periphery of the IOL optic, and digital image analysis was performed (Figure 1). Postoperative photographs were compared between them and also with the picture indicating the torus position at the end of the surgery. Clockwise (CW) rotation was counted as negative rotation and counterclockwise (CCW) as positive rotation. Absolute rotation was used to compare the observation periods. Patient satisfaction was rated as very poor, poor, moderate, good, or very good at 3rd month. ECC and morphology were analyzed at 6th month. Complications during follow-up were recorded.
  | Figure 1 Postoperative digital analysis of Precizon® toric IOL rotation. |
Vector analysis of astigmatism changes
At 6 months of follow-up, the overall accuracy of the astigmatism correction was calculated by using a vector analysis according to Alpins and Goggin.11 The Alpins method uses three astigmatism parameters: preoperative, target, and achieved astigmatism. The postoperative refractive astigmatism was compared with the preoperative keratometric astigmatism (IOLMaster®, Carl Zeiss Meditec AG). The target astigmatism was 0, because emmetropia was the goal in all patients. Refractive astigmatism data were calculated to the corneal plane for a back vertex distance of 12.0 mm. Three fundamental vectors were determined and evaluated: target-induced astigmatism (TIA) vector, which represents the change (by magnitude and axis) the surgery was intended to induce for each treatment; the SIA vector, which is the astigmatic change the surgery actually achieved; and the difference vector, which represents the astigmatism change between the achieved and the target astigmatism outcome, and is an absolute measure of success and is preferably 0. The following parameters derived from the relationship between these vectors were calculated: the magnitude of error, defined as the arithmetic difference between the magnitudes of the SIA and the TIA, that is positive for overcorrection and negative for undercorrection; the angle of error, which is the angle between the SIA and TIA vectors, that is positive if the achieved correction is CCW to the intended axis, and negative if the achieved correction is CW to the intended axis; the flattening effect, which is the amount of astigmatism reduction achieved at the intended (TIA) meridian; the flattening index is calculated by dividing the flattening effect of the TIA and is preferably 1.0; the correction index was calculated by the ratio of the magnitude of the SIA to the magnitude of the TIA, and is preferably 1.0. If an overcorrection occurred it is greater than 1.0 and if an undercorrection was found it is less than 1.0; the index of success was calculated by dividing the difference vector by the TIA and is a relative measure of success which is preferably 0.12,13 In this study, the TIA was the corneal astigmatism measured by optical biometry.
Statistical analysis
Categorical variables are presented as frequencies and percentages, and continuous variables as means and standard deviations, or medians and interquartile ranges for variables with skewed distributions. Normal distribution was checked using Shapiro–Wilk test or skewness and kurtosis.
Paired sample t-test was used to compare the number of endothelial cells between preoperative examinations and 6 months postoperative examinations. Cylinder and SE values were compared using one-way analysis of variance for repeated measures. Sphericity could not be assumed, so Greenhouse-Geisser was used as a correction factor. Post hoc comparisons were performed using Bonferroni test. UDVA and BCDVA examinations were compared using Friedman’s analysis of variance and post hoc analysis were performed using Wilcoxon signed-rank test, considering Bonferroni correction (α/number of comparisons).
All reported P-values are two-tailed, with a P-value of 0.05 indicating statistical significance. Analyses were performed using SPSS, version 22.0.
Results
This study comprised 40 eyes of 27 consecutive patients submitted to cataract surgery and Precizon® toric IOL implantation. Table 1 shows the demographic and preoperative data of our sample.
Visual acuity and refraction
Visual and refractive outcomes are shown in Table 2. The UDVA and BCDVA improved significantly after surgery.
At last follow-up, 6 months after surgery, the median UDVA was 0.02 (0.06) logMAR (range 0.16 to −0.10 logMAR), significantly better than the median preoperative BCDVA that was 0.19 (0.20) logMAR (range 0.70–0.0 logMAR) (P<0.001). The UDVA was equal or better than preoperative BCDVA in all the eyes. The final UDVA was 0.1 logMAR or better in 95% of the eyes (n=38) and 0.0 logMAR or better in 42.5% (n=17) (Figure 2).
  | Figure 2 Cumulative postoperative distance visual acuities (UDVA – uncorrected; BCDVA – best-spectacle corrected) at 6 months evaluation (6 m) (n=40 eyes). |
The mean SE significantly decreases from −3.35±3.10 D (range −11.5 to +1.88 D) preoperatively to −0.02±0.30 D (range −0.75 to +0.75 D) at last follow-up (P<0.001). The mean SE remained stable after the 1st week evaluation (Table 2). After 6 months, 97.5% of the eyes (n=39) were within ±0.50 D of the target emmetropia and 100% (n=40) within ±0.75 D.
The mean corneal astigmatism targeted to be corrected was 2.34±0.95 D (range 1.12-4.81) and the mean residual refractive astigmatism was 0.24±0.27 D (range 0.0-1.0 D) (P<0.001). The mean refractive astigmatism remained stable after the 1st week evaluation (Table 2). At last follow-up, the mean refractive astigmatism was ≤0.50 D in 95% of the eyes (n=38), and ≤1.00 D in 100% of the eyes (n=40) (Figure 3).
  | Figure 3 Astigmatism shift during the follow-up in all 40 eyes implanted with Precizon® toric IOL during cataract surgery. |
At 6 months, the mean postoperative corneal cylinder was 2.32±1.03 D (range 0.87-5.07), with no statistical difference when compared with the mean preoperative corneal cylinder (P=0.56), being the mean SICA that was not incorporated in IOL power calculation of 0.02±0.24 D, that was not significantly different from 0.
Table 3 and Figures 4 and 5 show the results of the vectorial astigmatism analysis at 6 months of follow-up. The mean angle of error indicated that the mean angle of SIA vector was −0.70°±3.62° CW to the TIA vector. The mean flattening effect was 2.23±0.85 D.
  | Table 3 Astigmatism analysis by Alpins method (40 eyes) |
  | Figure 4 Single-angle polar plots for the target-induced astigmatism (TIA) vector at 6 months follow-up. |
  | Figure 5 Single-angle polar plots for the surgically-induced astigmatism (SIA) vector at 6 months follow-up. |
IOL rotation
Table 4 shows the absolute misalignment of the toric IOL between the observation periods. IOL rotation occurred mainly within the 1st week after surgery (P<0.0125) and minimal rotation was observed afterwards, with just one IOL rotation between the 3rd- and 6th-month evaluations (P<0.0125) (Figure 6). A mean rotation relative to the intended axis of 2.43°±1.55° (range 0°–6°) was recorded at the final visit (P<0.001). During follow-up, IOL rotation was ≤4° in 90% of the eyes (n=36) with no IOL rotation more than 6°. At 6 months, rotation was CW in 16 eyes, CCW in 17 eyes, and 7 IOLs were in the intended position.
  | Figure 6 Absolute IOL rotation between observation periods in all 40 eyes implanted with Precizon® toric IOL during cataract surgery. |
Patient satisfaction
Satisfaction with visual acuity and quality of vision was rated as very good by all the patients.
ECC and complications
The mean ECC decreased from 2,458±381 cells/mm2 to 2,423±389 cells/mm2 (range 1,693-3,188 cells/mm2) (P<0.05), which amounts to a 1.42% decrease in ECC, as shown in Table 2.
All surgeries were uneventful. No complication occurred during the follow-up. No patient required IOL repositioning due to misalignment. No posterior capsule opacification was observed.
Discussion
High levels of corneal astigmatism are prevalent in a significant proportion of the population, and its correction along with cataract surgery can allow higher rates of spectacle independence. Limbal relaxing incisions or opposite corneal incisions may be performed during cataract surgery; however, they depend on the corneal healing response that is relatively unpredictable.14,15 Laser refractive surgery can be used when not contraindicated in the correction of residual refractive errors but may be complicated with dry eye, wound healing problems, and infections and is an expensive and not widely available tool.16 Toric IOL is the correction of choice of high levels of astigmatism during cataract surgery toric IOL implantation is a predictable method with minimal impact to the cornea; however, careful patient selection, correct measurement of corneal astigmatism, IOL calculation, IOL alignment during surgery, rotational stability, and tolerance to misalignment of the IOL are crucial factors in its efficacy. Patient with regular bowtie astigmatism benefits the most with toric IOL implantation, and irregular astigmatism is a relative contraindication although in selected cases with mild to moderate amounts of irregular astigmatism toric IOLs have achieved good functional results.17,18 In our study, all patients had corneal tomography using Scheimpflug imaging; and if irregular astigmatism was detected, then the patient was not included.
As manual and automated keratometry and corneal topography have been shown to measure comparable astigmatism values, we preferred to use IOL Master automated keratometry for accurate IOL calculation, which is the customized choice in our practice for nontoric IOL spherical power calculation.19,20
Calculations of the IOL spherical and cylindrical power and axis placement were determined using the valuable tool of IOL manufacturer’s online calculator taking into account the estimate of SICA and incision location. However, several factors influence SICA and make it difficult to predict and the most accurate method in practice is to use the surgeon’s personalized amount of SICA and was the one used in this study.5,21,22
Performance of toric IOLs is extremely dependent on correct positioning at the time of the surgery and on the early postoperative rotation stability of the IOL.
Rotation of the IOL occurs mainly in the early postoperative period before the capsular bag healing process is completed and several mechanisms such as OVD clearance, IOP fluctuations, capsulorrhexis size and centration, and IOL design and material influence early rotation stability.5,23,24 Late rotation due to capsule shrinkage and compression of the IOL haptics may occur in certain IOL designs and materials. Closed loop-haptics of the IOL used in this study are longer than plate-haptics, which should gave good initial friction and the loops have a second insertion on the IOL that might resist later capsular compression and subsequent rotation.23–25 In our study, rotation occurred mainly within 1st week after surgery with a median of 1°, with negligible rotation afterwards. This result might confirm that capsule bag had fused by the 1st week, and is apparent that most of IOL misalignment is mainly due to factors other than IOL rotation such as errors with marking and implantation procedures, incomplete clearance of OVD trapped behind the IOL and postoperative axis measurement as mentioned in the literature.5,12,23–29
The very low mean rotation at 6 months from the intended axis in our study of 2.43°±1.55°, with IOL rotation ≤4° in 90% of the eyes and with no IOL rotation more than 6° was excellent and in accordance with very good rotational stability reported with other loop-haptic acrylic IOLs and slightly superior to plate-loop IOLs.5–9,27–30
Following this IOL implantation, the magnitude of error was close to 0 and the correction index was close to 1 but with a slight tendency toward undercorrection. In our study, the absolute angle of error was 1.90°±0.69°, which seems to be a mean misalignment slightly better than reported with other types of IOLs.5,12,31–33 The angle of error obtained is not directly comparable to the level of rotation of 2.43°±1.55° measured because of the subjective component of the refractive outcome, the influence of incision and possibly the effect of other refractive surfaces of the eye (posterior corneal surface, vitreous).12,26,27 The low amount of residual refractive astigmatism obtained at 6 months follow-up is in accordance with the mean index of success obtained that was close to 0 and the mean flattening index that was close to 1 indicating that Precizon® toric IOL was very effective in reducing astigmatism at the intended meridian of treatment. The relationship between toric misalignment of a fully-correcting IOL and residual refractive astigmatism is known to be sinusoidal with small deviations resulting in a proportional greater loss of cylinder effect.10,29,30 The Precizon® toric IOL Model 565 has a transitional conic toric surface (patent pending), where the diopter power is calculated per meridian in a constant diopter power from the center to the edge of the IOL, resulting in a broader toric meridian that might be more tolerant for misalignment, tilt, and decentration than previous standard toric IOL. Comparative large-scale studies are needed to elucidate the potential advantage of the transitional conic toric surface of the IOL regarding tolerance to misalignment.
In our study, the UDVA was 0.1 logMAR or better in 95% of the eyes at 6 months follow-up and was equal or better than preoperative BCDVA in all the eyes, so this IOL seems to have excellent efficacy and safety and resulted in a very high level of patient satisfaction. Other studies also reported good UDVA with different IOLs.5–9,28–30
In accordance with functional results in our study, after 6 months, 97.5% of the eyes were within ±0.50 D of the target emmetropia and the mean refractive cylinder was 0.24±0.27 D, being ≤0.50 D in 95% of the eyes and ≤1.00 D in 100% of the eyes, which is slightly better than observed in previous studies.5,7–9,28
The reduction in ECC is expected after phacoemulsification technique, ranging from 4% to 18% according to the literature.34 The endothelial cell loss of 1.42% observed in our study was not regarded as an IOL-related complication. No complication occurred during the 6-month follow-up. Long-term follow-up is desirable to assess for long-term complications such as posterior capsule opacification and possible future misalignments.
Conclusion
Precizon® toric IOL appears to have very good rotational stability and performance regarding predictability and efficacy in the correction of preexisting corneal astigmatism during cataract surgery. As long as patients and IOL are carefully selected, there are no major safety-concerned complications. Patients reported a very high level of satisfaction with this new IOL. The toric IOL implantation can allow spectacle-independence for distance vision and will play an increasing role in modern cataract surgery. Techniques to optimize intraoperative alignment seem to now play the key role in achieving even better results. Further studies with this new IOL are desirable to confirm our results.
Acknowledgments
We thank Study Group – Orthoptists: 1) Paulo Sousa from Department of Ophthalmology, Hospital Geral de Santo António – Centro Hospitalar do Porto, EPE, Porto, Portugal and 2) Ana Duarte from Department of Ophthalmology, Hospital de Pedro Hispano, Matosinhos, Portugal.
Disclosure
The authors report no conflicts of interest in this work.
References
Vitale S, Ellwein L, Cotch MF, Ferris FL III, Sperduto R. Prevalence of refractive errors in the United States, 1999–2004. Arch Ophthalmol. 2008;126:1111–1119. | ||
Hoffer KJ. Biometry of 7,500 cataractous eyes. Am J Ophthalmol. 1980;90:360–368. | ||
Desai P, Reidy A, Minassian DC, Vafidis G, Bolger J. Gains from cataract surgery: visual function and quality of life. Br J Ophthamol. 1996;80:868–873. | ||
Savage H, Rothstein M, Davuluri G, El GL, Zaetta DM. Myopic astigmatism and presbyopia trial. Am J Ophthalmol. 2003;135:628–632. | ||
Visser N, Bauer NJC, Nuijts RMMA. Toric intraocular lenses: historical overview, patient selection, IOL calculation, surgical techniques, clinical outcomes, and complications. J Cataract Refract Surg. 2013;39:624–637. | ||
Bauer NJ, de Vries NE, Webers CA, Hendrikse F, Nuijts RM. Astigmatism management in cataract surgery with AcrySof toric intraocular lens. J Cataract Refract Surg. 2008;34:1483–1488. | ||
Hayashi K, Masumoto M, Takimoto M. Comparison of visual and refractive outcomes after bilateral implantation of toric intraocular lenses with or without a multifocal component. J Cataract Refract Surg. 2015;41:73–83. | ||
Mencucci R, Favuzza E, Guerra F, Giacomelli G, Menchini U. Clinical outcomes and rotational stability of a 4-haptic toric intraocular lens in myopic eyes. J Cataract Refract Surg. 2014;40:1479–1487. | ||
Bachernegg A, Rückl T, Riha W, Grabner G, Dexl A. Rotational stability and visual outcome after implantation of a new toric lens for the correction of corneal astigmatism during cataract surgery. J Cataract Refract Surg. 2013;39:1390–1398. | ||
Novis C. Astigmatism and toric intraocular lenses. Curr Opin Ophthalmol. 2000;11:47–50. | ||
Alpins NA, Goggin M. Practical astigmatism analysis for refractive outcomes in cataract and refractive surgery. Surv Ophthalmol. 2004;49:109–122. | ||
Visser N, Berendschot TTJM, Bauer NJC, Jurich J, Kersting O, Nuijts RMMA. Accuracy of toric intraocular lenses implantation in cataract and refractive surgery. J Cataract Refract Surg. 2011;37:1394–1402. | ||
Reinstein DZ, Archer TJ, Randleman JB. JRS standard for reporting astigmatism outcomes of refractive surgery. J Refract Surg. 2014;40:654–659. | ||
Khokhar S, Lohiya P, Murugiesan V, Panda A. Corneal astigmatism correction with opposite clear cornea incisions or single corneal incisions: comparative analysis. J Cataract Refract Surg. 2006;32:1432–1437. | ||
Kaufmann C, Peter J, Ooi K, Phipps S, Cooper P, Goggin M. Limbal relaxing incisions versus on-axis incisions to reduce corneal astigmatism at the time of cataract surgery. J Cataract Refract Surg. 2005;31:2261–2265. | ||
de Oliveira GC, Solari HP, Ciola FB, Lima AL, Campos MS. Corneal infiltrates after excimer laser photorefractive keratectomy and LASIK. J Refract Surg. 2006;22(2):159–165. | ||
Visser N, Gast STJM, Bauer NJC, Nuijts RMMA. Cataract surgery with toric intraocular lens implantation in keratoconus: a case report. Cornea. 2011;30:720–723. | ||
Luck J. Costumized ultra-high power toric intraocular lens implantation for pellucid marginal degeneration and cataract. J Cataract Refract Surg. 2010;36:1235–1238. | ||
Shirayama M, Wang L, Weikert MP, Koch DD. Comparison of corneal powers obtained from 4 different devices. Am J Ophthalmol. 2009;148:528–535. | ||
Visser N, Berendschot TTJM, Verbakel F, de Brabander J, Nuijts RMMA. Comparability and repeatability of corneal astigmatism measurements using different measurements technologies. J Cataract Refract Surg. 2012;38:1764–1770. | ||
Masket S, Wang L, Belani S. Induced astigmatism with 2.2- and 3.0-mm coaxial phacoemulsifications incisions. J Refract Surg. 2009;25:21–24. | ||
Storr-paulsen A, Madsen H, Perried A. Possible factors modifying the surgically induced astigmatism in cataract surgery. Acta Ophthalmol Scand. 1999;77:548–551. | ||
Buckhurst JP, Wolffshon JS, Davies LN, Naroo SA. Surgical correction of astigmatism during cataract surgery. Clin Exp Optom. 2010;93(6):409–418. | ||
Patel CK, Ormonde S, Rosen PH, Bron AJ. Postoperative intraocular rotation; a randomized comparison of plate and loop haptics implants. Ophthalmology. 1999;106:2190–2195. | ||
Prinz A, Neumayer T, Buehl W, et al. Rotational stability and posterior capsule opacification of a plate-loop and an open-loop-haptic intraocular lens. J Cataract Refract Surg. 2011;37:251–257. | ||
Ma JJK, Tseng SS. Simple method for accurate alignment in toric phakic and aphakic intraocular lens implantation. J Cataract Refract Surg. 2008;34:1631–1634. | ||
Shimizu K, Misawa A, Suzuki Y. Toric intraocular lenses: correcting astigmatism while controlling axis shift. J Cataract Refract Surg. 1994;20:523–524. | ||
Bascaran L, Mendicute J, Macias-Murelaga B, Arbelaitz N, Martinez-Soroa I. Efficacy and stability of AT Torbi 709M toric IOL. J Refract Surg. 2013;29:194–199. | ||
Chuan W-H, Yuen LH, Chua J, The G, Hill WE. Matched comparison of rotational stability of 1-piece acrylic and plate-haptic silicon toric intraocular lens in Asian eyes. J Cataract Refract Surg. 2012;38:620–624. | ||
Entabi M, Harman F, Lee N, Bloom PA. Injectable 1-piece hydrophilic acrylic toric intraocular lens for cataract surgery: efficacy and stability. J Cataract Refract Surg. 2011;37:235–240. | ||
Visser N, Berendschot TTJM, Bauer NJC, Jurich J, Nuijts RMMA. Vector analysis of corneal and refractive astigmatism changes following toric pseudophakic and toric phakic IOL implantation. Invest Ophthalmol Vis Sci. 2012;53:1865–1873. | ||
Alió JL, Pinero DP, Tomás J, Alesón Alicia. Vector analysis of astigmatism changes after cataract surgery with toric intraocular lens implantation. J Cataract Refract Surg. 2011;37:1038–1049. | ||
Alió JL, Agdepa MCC, Pongo VC, El Kaby B. Microincision cataract surgery with toric intraocular lens for the correction of moderate to high astigmatism: pilot study. J Cataract Refract Surg. 2010;36:44–52. | ||
Wilczynski M, Supady E, Loba P, Synder A, Palenga-Pydyn D, Omulecki W. Comparison of early corneal endothelial cell loss after coaxial phacoemulsification through 1.8 mm microincision and bimanual phacoemulsification through 1.7 mm microincision. J Cataract Refract Surg. 2009;35:1570–1574. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



