Back to Journals » Psychology Research and Behavior Management » Volume 11
Associations of perceived stress with the present and subsequent cortisol levels in fingernails among medical students: a prospective pilot study
Authors Wu H , Zhou K, Xu P, Xue J, Xu X, Liu L
Received 31 July 2018
Accepted for publication 30 August 2018
Published 9 October 2018 Volume 2018:11 Pages 439—445
DOI https://doi.org/10.2147/PRBM.S181541
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Igor Elman
Hui Wu,1 Kexin Zhou,1 Peiyao Xu,1 Jiayu Xue,1 Xin Xu,2 Li Liu1
1Department of Social Medicine, School of Public Health, China Medical University, Shenyang, Liaoning, China; 2Department of Clinical Epidemiology, Shengjing Hospital of China Medical University, Shenyang, Liaoning, China
Purpose: Cortisol in fingernails could retrospectively reflect cumulative stress over a long period. However, the association between fingernail cortisol and perceived stress needs to be validated. This exploratory study aimed to investigate the associations of perceived stress with the present and subsequent cortisol levels in fingernails of the subjective stress measurement among medical students.
Methods: Students were recruited from a medical university in Shenyang, China. The final sample consisted of 51 students (16 men, 35 women). On the Day 30 of our data and fingernail collection procedure, the 10-item Perceived Stress Scale was used to measure perceived stress. Fingernail samples were collected twice, on Days 15 (denoted as FD15) and 45 (denoted as FD45) of the procedure, and participants were asked to grow fingernails for 15 days in each collection. Cortisol was determined by an enzyme immunoassay method using the ELISA kit. Multiple linear regression was performed to examine the association between perceived stress and cortisol level. The Bonferroni correction was made for multiple comparisons.
Results: The level of cortisol was 5.65 pg/mg (SD =1.88) for FD15 and 5.41 pg/mg (SD =1.63) for FD45. Perceived stress was not associated with the cortisol level of FD15 (β=−0.014, P=0.924), but it was significantly and positively associated with the cortisol level of FD45 (β=0.436, P=0.003), which remained significant after Bonferroni correction. The associations between fingernail cortisol and demographic variables (gender, age, BMI, and physical activity) were not significant.
Conclusion: This study was the first to investigate fingernail cortisol in China. Perceived stress was positively associated with the subsequent cortisol levels in fingernails, but not the present. The findings suggested that fingernail cortisol could indicate stress exposure in the past. Furthermore, a simple and easy self-reported measure could reflect cumulative stress as measured by fingernail cortisol.
Keywords: cortisol, fingernail, perceived stress, chronic stress, China
Introduction
Prolonged exposure to stress results in wide physical and psychological health issues through inducing a number of physiological changes in the neuroendocrine, immune, and cardiovascular systems.1 In particular, as one of the main stress response pathways, an activation of the hypothalamic–pituitary–adrenal (HPA) axis is involved in this process. As a result, cortisol, an adrenal cortex hormone secreted in response to stress, is released increasingly.2 Traditionally, the cortisol level of saliva, blood, or urine is considered as an indicator for acute stress.3 Use of saliva, blood, or urine as samples to assess chronic stress is a time-intensive and costly procedure.3,4 Obviously, the complexity of sample collection will greatly affect its acceptability and validity. Furthermore, the validity of self-reporting methods used to assess chronic stress level, such as perceived stress and experienced stressful life events, is often reduced by reporting bias and recall bias,5 which may result in some uncertainties in the study of health outcomes induced by chronic stress. Therefore, it seems of vital importance to conveniently and accurately measure a long-term cortisol level caused by chronic stress, especially the objective indicators. Also, the association between subjective and objective indicators of chronic stress needs to be further validated.6
Over the last decade, hair cortisol has received increasing attention by scientists engaged in stress research, and hair cortisol analysis has been used to assess long-term exposure to stress worldwide.3,7 Hair sampling has some advantages, such as it is a noninvasive sampling technique and relatively stable. So far, it has been found that hair cortisol level is associated with various types of chronic stress, such as lifetime trauma or discrimination,8,9 negative life events,10 neighborhood disadvantage,11 work stress (effort–reward imbalance),12,13 and wartime stress.14 Also, hair cortisol level has been demonstrated to be higher in long-term unemployed individuals,15 earthquake survivors,16 dementia caregivers,17 and women reporting intimate partner violence.18 Unfortunately, hair sample collection could exclude those individuals with short, less, or no hair, such as some men, the elderly, young infants, and patients, among whom some stress vulnerable populations could be included. Moreover, hair sampling could be unacceptable to some individuals for the reasons that it could affect their outward appearance or due to culture. For these reasons and more, the applicability of hair cortisol is limited to some specific populations to investigate chronic stress.19
In contrast to hair, fingernails are available for a majority of populations. As a neutrally charged endogenous hormone, cortisol passively diffuses from capillaries into nail matrix, and it is incorporated into keratin during nail formation process.20 Therefore, measuring cortisol level in fingernails may be another objective method for addressing the limitations of hair sample. In 2010, Warnock et al firstly suggested that cortisol level in fingernails could indicate a cumulative stress exposure over a relatively long period.21 In general, fingernails grow at an average rate of 1 mm per 10 days.22 Buzalaf et al found that 3–4 months could be needed for fingernails to fully extend from the nail matrix through tracing fluoride in fingernails.23 In addition, once cortisol is incorporated into the nail matrix, it cannot be further metabolized. Therefore, fingernail samples may retrospectively reflect cortisol levels in the 3–4 months prior to clipping.21 In 2015, Izawa et al separately demonstrated a moderate association between fingernail cortisol and hair cortisol (58 middle-aged men) and salivary cortisol over the whole day (37 workers) in two studies.24 In 2016, Nejad et al reported that there was a moderate correlation between fingernail cortisol and facial hair cortisol, and pointed out that fingernail cortisol can be used to investigate the relationship between psychosocial stress and health.25 In the same year, Higashi et al confirmed that there was little difference in fingernail cortisol between the right and left hands, and fingernail analysis using either hand looked promising for the evaluation of cortisol level in the body.26 Currently, fingernail cortisol measurement has been applied in patients with euthymic bipolar I disorder and cancer,27,28 as well as in individuals experiencing a major depressive episode.29 In comparison with saliva, blood, or urine as samples to assess chronic stress, fingernail cortisol could be a convenient and accurate measure. In addition, as an alternative objective measurement of chronic stress, fingernail cortisol can be applied to those individuals with the inconvenience of providing their hair samples. However, there is very limited evidence of the relationship between fingernail cortisol and perceived stress, which is the fundamental aspect of fingernail cortisol measurement.19,30 Furthermore, if results from a self-reported measurement work to align with far more objective physiological measures, this would be of great practical value. However, one of the prerequisites for this value is to clarify the temporal relationship between subjective and objective indicators of chronic stress.
Therefore, this prospective pilot study aimed to investigate the associations of perceived stress with the present and subsequent cortisol levels in fingernails of the subjective stress measurement among medical students. We expected that perceived stress would be positively associated with the subsequent cortisol level in fingernails, but not the present, because fingernail samples reflect cumulative cortisol exposure in the past. Furthermore, we investigated the effects of some demographic variables (age, gender, body mass index, and physical activity) on fingernail cortisol level.
Materials and methods
Study design and sample
Students were recruited from a medical university in Shenyang, Liaoning Province, China, from June to September 2017. To be included, students were required to have good physiological and psychological health, with no fingernail diseases or use of any nail polish. “Healthy” was defined as “no acute or chronic diseases, trauma, and psychological disorders during the investigation.” Subjects were expected to not wear nail polish in the whole process of fingernail sample collection. Eligibility also required that students were on no medications in the last 3 months. Students were first screened for initial eligibility criteria during the recruitment process with an investigator. The screening was performed according to a structured participation qualification list, including health and fingernail conditions. Self-reported measurements were used to address the physiological and psychological health conditions of participants. Fingernail conditions and use of nail polish were checked by a direct observation. The sample initially comprised 57 participants; however, six were excluded for two reasons: three for missing questionnaire data and three for incomplete fingernail collection. Therefore, the final sample consisted of 51 students (16 men, 35 women). The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Committee on Human Experimentation of China Medical University. In this study, all of the participants were voluntary and anonymous, and written informed consent was provided by them.
Procedure
The day on which these participants were recruited was recorded as Day 0 of our data and fingernail collection procedure, and free fingernails were cut off from every digit by using nail clippers and then thrown away. On Day 30 of the collection procedure, participants were asked to complete a self-administered questionnaire of perceived stress, and free fingernails were cut off and thrown away again. Because the questionnaire of perceived stress inquires about one’s feelings and thoughts in the past month, as the first fingernail collection, participants were asked to provide their samples on Day 15 of the collection procedure (denoted as FD15) from every digit in order to indicate the present cortisol level of the perceived stress measurement. Then, as the second fingernail collection, participants were asked to provide their samples on Day 45 of the collection procedure (denoted as FD45) in order to indicate the subsequent cortisol level of the perceived stress measurement. Thus, fingernail samples were collected twice, and participants were asked to grow their fingernails for 15 days in each collection. Fingernail samples were transferred into a Ziploc bag and frozen at –20°C prior to the assay.
Measurement of perceived stress
The 10-item Perceived Stress Scale (PSS) was used to measure the perception of stress among the students.31 The PSS comprises six negative and four positive items that inquire about one’s feelings and thoughts in the last month, such as “In the last month, how often have you felt that you were unable to control the important things in your life?” and “In the last month, how often have you felt that things were going your way?” Each item in the PSS has a 5-point Likert scale ranging from “0” (never) to “4” (very often). The total PSS score ranges from 0 to 40, and a higher score indicates a higher level of perceived stress. The Chinese version of the PSS has been widely used in various Chinese populations and has shown adequate reliability and validity.32–34 In this study, the Cronbach’s alpha coefficient for the PSS was 0.81.
Demographic characteristics
Participants also completed a baseline questionnaire that included gender, age (years), height, weight, physical activity, and current smoking status. Body mass index (BMI) was calculated by dividing weight (in kg) by the square of height (in meters). Physical activity was obtained by the question “In a week, how often do you regularly do any physical activity long enough to make you sweat or your heart beat quickly during your leisure time?” Answer categories included “0” (never/rarely, 1 day, or less), “1” (sometimes, 2–3 days), and “2” (often, 4 days, or more).35,36 Current smoking status was measured by two questions: “Have you ever smoked more than 100 cigarettes in your lifetime?” and “Have you smoked a cigarette, even a puff in the past 30 days?” Respondent was coded as a current smoker if both questions were answered “yes” in this study.37,38
Fingernail cortisol extraction
Fingernail cortisol extraction method widely used in previous studies was adopted in this study.19,21,23,27,30 Each fingernail sample was transferred into a 15 mL Falcon tube (BD Biosciences, Franklin Lakes, NJ, USA). Then, 5 mL of isopropanol was added. As washing procedure, the tube was vortexed for 1 minute twice. Samples were air-dried overnight. Each dried sample was transferred to a 2 mL polypropylene tube with a steel ball (diameter, 2 mm) and ground for 10 minutes using a mixer mill (DHS TL2020; DHS Life Science and Technology, Beijing, China) at 25 Hz. Then, 30 mg of fingernail powder was transferred to another 2 mL tube, followed by the addition of 1.5 mL of HPLC-grade methanol. The tube was slowly rotated for 2 hours at room temperature for steroid extraction. After being centrifuged at 10,000 rpm for 5 minutes, 1.2 mL of the clear supernatant was transferred into a new tube, and subjected to evaporation until completely dry.
Enzyme immunoassay
Cortisol level was determined by an enzyme immunoassay method using the ELISA kit (Cortisol Competitive ELISA Kit; Thermo Fisher Scientific Inc., Waltham, MA, USA). The evaporated samples were resuspended in 400 µL of the assay diluent included in the ELISA kit, and the levels of cortisol were analyzed according to the manufacturer’s instructions. The inter-assay and intra-assay variations were 6.3% and 5.6%, respectively. The levels of cortisol are presented as pg cortisol/mg fingernail (pg/mg).
Statistical analysis
After the distribution of data was assessed using Kolmogorov–Smirnov test, the group differences in the levels of cortisol and perceived stress were analyzed by Student’s t-test or one-way ANOVA. Correlations among continuous variables were examined using Pearson’s correlation. Multiple linear regression analysis was performed to examine the associations of perceived stress with the cortisol levels of FD15 and FD45, respectively. Perceived stress was modeled as an independent variable, with cortisol level as an outcome, and demographic characteristics were adjusted. Statistical analysis was executed by SPSS 19.0 software and a two-tailed P<0.05 was considered as nominal significance. Moreover, the Bonferroni correction was made for multiple comparisons or tests in this study.
Results
Participant characteristics
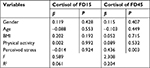
The means and SDs of fingernail cortisol and perceived stress, as well as demographic variables, are presented in Table 1. The value of Kolmogorov–Smirnov Z was 0.830, 0.564, and 0.523 for perceived stress, the cortisol levels of FD15 and FD45, respectively. The data showed normal distribution in this study. The mean age of our sample was 20.02 years (SD =1.24), and the mean score of perceived stress was 15.19 (SD=5.93). The level of cortisol in fingernails was 5.65 pg/mg (SD =1.88) for FD15 and 5.41 pg/mg (SD =1.63) for FD45. Gender and physical activity were not significantly related to the levels of perceived stress and fingernail cortisol. Only one subject was identified with current smoking status.
Correlations
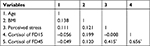
Correlations among study variables are presented in Table 2. Perceived stress was positively correlated with the cortisol level of FD45 (r=0.415, P<0.001), but not FD15 (r=−0.008, P=0.956). In addition, the cortisol level of FD15 had a significantly positive correlation with that of FD45 (r=0.656, P<0.001). For the Bonferroni correction, a two-sided P(corrected) value <0.005 (0.05 divided by 10) was considered as statistical significance. Thus, these correlations remained significant after Bonferroni correction.
Association between perceived stress and fingernail cortisol
The results of multiple linear regression analyses are shown in Table 3. After adjusting for gender, age, BMI and physical activity, perceived stress was not associated with the fingernail cortisol level of FD15 (β=−0.014, P=0.924), but it was significantly and positively associated with the fingernail cortisol level of FD45 (β=0.436, P=0.003). For the Bonferroni correction, a two-sided P(corrected) value <0.005 (0.05 divided by 10 for five independent variables and two models) was considered as statistical significance. Thus, the association of perceived stress with the fingernail cortisol level of FD45 remained significant after Bonferroni correction.
Discussion
This exploratory study investigated the association between perceived stress and fingernail cortisol level in a sample of medical students. We found that perceived stress was not associated with the present (FD15) cortisol level, whereas it was positively associated with the subsequent (FD45) cortisol level in fingernails after adjusting for gender, age, BMI, and physical activity. Fingernail samples could reflect cumulative hormonal exposure in the past.19,30 Therefore, it could be interpreted that fingernail cortisol is associated with perceived stress in the past, rather than with the present. To the best of our knowledge, this is the first study of cortisol in fingernails in China.
In fact, the results of two existing studies could provide some indirect evidence for our findings. In a sample of middle-aged workers, Izawa et al found that the experience of stressful life events in the workplace in the previous year, but not job strain and perceived stress in the present, was associated with higher cortisol level in fingernails.30 In a sample of high school students, Doan et al found a significant relationship between fingernail cortisol and stressful events. However, there was no relationship between cortisol and perceived subjective stress.19 Consistent with research in hair cortisol, no correlation between cortisol level and self-reports of perceived stress emerged in a meta-analysis study.39 Concordantly, in research studies using experiencing stressful or negative life events to indicate chronic stress, hair cortisol had significant relationships with them.8–14 Lifetime exposure to trauma was associated with the elevated level of hair cortisol; however, hair cortisol level was not associated with current depression symptoms in a community-based children sample.8 The patterns of worrying and thinking about these stressful situations pervade individuals’ lives, which could result in cumulative burdens that impair the regulatory capacities of the HPA axis.39,40 Moreover, some,41–43 but not all,12,13 investigators have reported that there is no relationship between work stress and hair cortisol level. However, a significantly higher hair cortisol level was found in workers with symptoms of depression compared with controls.42 Similarly, workers with high burnout scores exhibited elevated hair cortisol level compared to those with no or moderate burnout.44 These findings imply that a certain stress threshold should be reached to detect a significant relationship between self-reported stress and cortisol level.13 In general, symptoms of depression and burnout are considered to be the psychological outcomes of long-term work stress in the workplace.
In this study, we did not find significant associations between fingernail cortisol and these demographic variables (gender, age, BMI, and physical activity). These results were consistent with the findings from two previous studies, which showed that gender, age, and BMI were not associated with the level of cortisol in fingernails.19,30 However, as indexed by hair, findings on the relationships of gender, age, BMI, and physical activity with cortisol level in hair are inconsistent. Taking into account gender differences, in the meta-analysis study mentioned earlier, men exhibited 21.4% higher hair cortisol level than women.39 The findings of age–hair cortisol correlations from many previous studies may have been affected by the restricted age range of their study samples. In 2012, Dettenborn et al firstly reported elevated hair cortisol levels in young children and older adults.45 However, no significant non-linear relationship between age and hair cortisol level was revealed in the meta-analysis study.39 Recently, two studies have comparably reported this non-linear age–hair cortisol relationship.46,47 Therefore, study sample with a broader age spectrum is needed to confirm the relationship between age and cortisol in hair or fingernail in future.3 BMI has been frequently reported to be positively associated with hair cortisol level,47–50 and the meta-regression model also suggested that samples with a higher BMI exhibited an elevated hair cortisol level.39 The comparatively low mean BMI and limited BMI range of our student sample could result in an insignificant association in this study. Additionally, Izawa et al found that smokers had higher fingernail cortisol levels.30 However, a correlation-based meta-analysis showed that hair cortisol was unrelated to smoking.39 In this study, the effect of smoking on fingernail cortisol level was not investigated because there was only one participant having current smoking status. Moreover, it has been found that both objectively and subjectively assessed vigorous physical activity is associated with increased hair cortisol in young or older adults.49,51 However, consistent with the finding of the present study, Stalder et al found no association between hair cortisol and physical activity level.52
The present study has several limitations, which warrant careful interpretation of our exploratory findings. First, this study was only conducted in healthy medical students, and the sample size was small. Therefore, the associations of perceived stress and demographic variables with fingernail cortisol level could be influenced, and stratification analyses based on these demographic variables cannot be investigated. Based on the regression result of FD45 cortisol level in fingernails, given effect size was 0.26 (it was calculated according to R2=0.204), α=0.05, sample size was 51, and the number of predictors was 5. Thus, statistical power was 0.76. Also, caution should be taken concerning generalizability due to convenience sampling. Thus, a larger and more hetereogenous sampling will be needed to confirm our findings in future studies. Second, we only measured the fingernail cortisol level of FD45, and there was a relatively short follow-up. Although chronic stress is a persistent state, the short follow-up will affect the intensity of the relationship between perceived stress and fingernail cortisol level. Third, as one of the potential factors of cortisol level, fingernail growth rate was not investigated in this study. Fortunately, our highly homogenous sample could contribute to reducing individual differences in fingernail growth rates. Further, fingernails were clipped by participants in dormitory, and the conditions could not be accurately controlled.
Conclusion
This study is the first to investigate fingernail cortisol in China. Perceived stress was not associated with the present cortisol level, whereas it was positively associated with the subsequent cortisol level in fingernails. The findings suggested that fingernail cortisol could indicate stress exposure in the past. Furthermore, a simple and easy self-reported measure could reflect cumulative stress as measured by fingernail cortisol. Further research with rigorous design based on the hypothetical theory of fingernail cortisol as an indicator of chronic stress is needed.
Acknowledgments
This work was supported by the Doctoral Scientific Research Startup Foundation of Liaoning Province (201601124, Li Liu). We would like to thank all the students who voluntarily participated in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Mcewen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22(2):108–124. | ||
Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–391. | ||
Wosu AC, Valdimarsdóttir U, Shields AE, Williams DR, Williams MA. Correlates of cortisol in human hair: implications for epidemiologic studies on health effects of chronic stress. Ann Epidemiol. 2013;23(12):797–811. | ||
Schreier HM, Chen E. Low-grade inflammation and ambulatory cortisol in adolescents: interaction between interviewer-rated versus self-rated acute stress and chronic stress. Psychosom Med. 2017;79(2):133–142. | ||
Gow R, Thomson S, Rieder M, van Uum S, Koren G. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci Int. 2010;196(1–3):32–37. | ||
O’Brien KM, Tronick EZ, Moore CL. Relationship between hair cortisol and perceived chronic stress in a diverse sample. Stress Health. 2013;29(4):337–344. | ||
Russell E, Koren G, Rieder M, van Uum S. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37(5):589–601. | ||
Simmons JG, Badcock PB, Whittle SL, et al. The lifetime experience of traumatic events is associated with hair cortisol concentrations in community-based children. Psychoneuroendocrinology. 2016;63:276–281. | ||
O’Brien KM, Meyer J, Tronick E, Moore CL. Hair cortisol and lifetime discrimination: Moderation by subjective social status. Health Psychol Open. 2017;4(1):2055102917695176. | ||
Schreier HM, Enlow MB, Ritz T, et al. Lifetime exposure to traumatic and other stressful life events and hair cortisol in a multi-racial/ethnic sample of pregnant women. Stress. 2016;19(1):45–52. | ||
Zilioli S, Slatcher RB, Fritz H, Booza JC, Cutchin MP. Brief report: Neighborhood disadvantage and hair cortisol among older urban African Americans. Psychoneuroendocrinology. 2017;80:36–38. | ||
Qi X, Zhang J, Liu Y, et al. Relationship between effort-reward imbalance and hair cortisol concentration in female kindergarten teachers. J Psychosom Res. 2014;76(4):329–332. | ||
van der Meij L, Gubbels N, Schaveling J, Almela M, van Vugt M. Hair cortisol and work stress: Importance of workload and stress model (JDCS or ERI). Psychoneuroendocrinology. 2018;89:78–85. | ||
Etwel F, Russell E, Rieder MJ, van Uum SH, Koren G. Hair cortisol as a biomarker of stress in the 2011 Libyan war. Clin Invest Med. 2014;37(6):E403–E408. | ||
Dettenborn L, Tietze A, Bruckner F, Kirschbaum C. Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology. 2010;35(9):1404–1409. | ||
Gao W, Zhong P, Xie Q, et al. Temporal features of elevated hair cortisol among earthquake survivors. Psychophysiology. 2014;51(4):319–326. | ||
Stalder T, Tietze A, Steudte S, Alexander N, Dettenborn L, Kirschbaum C. Elevated hair cortisol levels in chronically stressed dementia caregivers. Psychoneuroendocrinology. 2014;47:26–30. | ||
Boeckel MG, Viola TW, Daruy-Filho L, Martinez M, Grassi-Oliveira R. Intimate partner violence is associated with increased maternal hair cortisol in mother-child dyads. Compr Psychiatry. 2017;72:18–24. | ||
Doan SN, Deyoung G, Fuller-Rowell TE, Liu C, Meyer J. Investigating relations among stress, sleep and nail cortisol and DHEA. Stress. 2018;21(2):188–193. | ||
de Berker DA, André J, Baran R. Nail biology and nail science. Int J Cosmet Sci. 2007;29(4):241–275. | ||
Warnock F, Mcelwee K, Seo RJ, et al. Measuring cortisol and DHEA in fingernails: a pilot study. Neuropsychiatr Dis Treat. 2010;6:1–7. | ||
Gupta GR, Dhruw VK, Athawal BK, et al. Human nail growth pattern and medicolegal aspect. JIAFM. 2005;27(2):971–973. | ||
Buzalaf MA, Pessan JP, Alves KM. Influence of growth rate and length on fluoride detection in human nails. Caries Res. 2006;40(3):231–238. | ||
Izawa S, Miki K, Tsuchiya M, et al. Cortisol level measurements in fingernails as a retrospective index of hormone production. Psychoneuroendocrinology. 2015;54:24–30. | ||
Nejad JG, Ghaseminezhad M. A cortisol study; facial hair and nails. J Steroids Horm Sci. 2016;7(2):177. | ||
Higashi T, Yamagata K, Kato Y, et al. Methods for determination of fingernail steroids by LC/MS/MS and differences in their contents between right and left hands. Steroids. 2016;109:60–65. | ||
Herane-Vives A, Cleare AJ, Chang CK, et al. Cortisol levels in fingernails, neurocognitive performance and clinical variables in euthymic bipolar I disorder. World J Biol Psychiatry. 2017:1–12. | ||
Frugé AD, Cases MG, Howell CR, et al. Fingernail and toenail clippings as a non-invasive measure of chronic cortisol levels in adult cancer survivors. Cancer Causes Control. 2018;29(1):185–191. | ||
Herane-Vives A, Fischer S, de Angel V, et al. Elevated fingernail cortisol levels in major depressive episodes. Psychoneuroendocrinology. 2018;88:17–23. | ||
Izawa S, Matsudaira K, Miki K, Arisaka M, Tsuchiya M. Psychosocial correlates of cortisol levels in fingernails among middle-aged workers. Stress. 2017;20(4):386–389. | ||
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. | ||
Shi M, Wang X, Bian Y, Wang L. The mediating role of resilience in the relationship between stress and life satisfaction among Chinese medical students: a cross-sectional study. BMC Med Educ. 2015;15:16. | ||
Li S, Li L, Zhu X, et al. Comparison of characteristics of anxiety sensitivity across career stages and its relationship with nursing stress among female nurses in Hunan, China. BMJ Open. 2016;6(5): e010829. | ||
Liu CL, Liu L, Zhang Y, Dai XZ, Wu H. Prevalence and its associated psychological variables of symptoms of depression and anxiety among ovarian cancer patients in China: a cross-sectional study. Health Qual Life Outcomes. 2017;15(1):161. | ||
Greenlee TA, Greene DR, Ward NJ, et al. Effectiveness of a 16-week high-intensity cardioresistance training program in adults. J Strength Cond Res. 2017;31(9):2528–2541. | ||
Shen B, Mccaughtry N, Martin J. The influence of self-determination in physical education on leisure-time physical activity behavior. Res Q Exerc Sport. 2007;78(4):328–338. | ||
Liu L, Xu X, Wu H, Yang Y, Wang L. Associations of psychological capital, demographic and occupational factors with cigarette smoking among Chinese underground coal miners. BMC Public Health. 2015;15:20. | ||
Azagba S, Baskerville NB, Minaker L. A comparison of adolescent smoking initiation measures on predicting future smoking behavior. Prev Med Rep. 2015;2:174–177. | ||
Stalder T, Steudte-Schmiedgen S, Alexander N, et al. Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology. 2017;77:261–274. | ||
Mcewen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–179. | ||
Herr RM, Barrech A, Gündel H, et al. Effects of psychosocial work characteristics on hair cortisol - findings from a post-trial study. Stress. 2017;20(4):363–370. | ||
Janssens H, Clays E, Fiers T, Verstraete AG, de Bacquer D, Braeckman L. Hair cortisol in relation to job stress and depressive symptoms. Occup Med. 2017;67(2):114–120. | ||
Steinisch M, Yusuf R, Li J, et al. Work stress and hair cortisol levels among workers in a Bangladeshi ready-made garment factory - Results from a cross-sectional study. Psychoneuroendocrinology. 2014;50:20–27. | ||
Penz M, Stalder T, Miller R, Ludwig VM, Kanthak MK, Kirschbaum C. Hair cortisol as a biological marker for burnout symptomatology. Psychoneuroendocrinology. 2018;87:218–221. | ||
Dettenborn L, Tietze A, Kirschbaum C, Stalder T. The assessment of cortisol in human hair: associations with sociodemographic variables and potential confounders. Stress. 2012;15(6):578–588. | ||
Binz TM, Rietschel L, Streit F, et al. Endogenous cortisol in keratinized matrices: Systematic determination of baseline cortisol levels in hair and the influence of sex, age and hair color. Forensic Sci Int. 2018;284:33–38. | ||
Smy L, Shaw K, Amstutz U, et al. Assessment of hair cortisol as a potential biomarker for possible adrenal suppression due to inhaled corticosteroid use in children with asthma: A retrospective observational study. Clin Biochem. 2018;56:26–32. | ||
Abell JG, Stalder T, Ferrie JE, et al. Assessing cortisol from hair samples in a large observational cohort: The Whitehall II study. Psychoneuroendocrinology. 2016;73:148–156. | ||
Steptoe A, Easterlin E, Kirschbaum C. Conscientiousness, hair cortisol concentration, and health behaviour in older men and women. Psychoneuroendocrinology. 2017;86:122–127. | ||
Jackson SE, Kirschbaum C, Steptoe A. Hair cortisol and adiposity in a population-based sample of 2,527 men and women aged 54 to 87 years. Obesity. 2017;25(3):539–544. | ||
Gerber M, Jonsdottir IH, Kalak N, et al. Objectively assessed physical activity is associated with increased hair cortisol content in young adults. Stress. 2013;16(6):593–599. | ||
Stalder T, Kirschbaum C, Alexander N, et al. Cortisol in hair and the metabolic syndrome. J Clin Endocrinol Metab. 2013;98(6):2573–2580. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.