Back to Journals » Clinical Ophthalmology » Volume 15
Association Between the Degree of Inclusion of Components Identified on Fluorescein or Indocyanine Green Angiography in Target Spots and Relapse of Exudate in Eyes with Polypoidal Choroidal Vasculopathy and Typical Age-Related Macular Degeneration After Photodynamic Therapy
Authors Yoshida I , Taniguchi H, Sakamoto M, Maeno T
Received 11 February 2021
Accepted for publication 1 April 2021
Published 18 May 2021 Volume 2021:15 Pages 2063—2075
DOI https://doi.org/10.2147/OPTH.S305238
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Izumi Yoshida,1,2 Hikari Taniguchi,1 Masashi Sakamoto,1 Takatoshi Maeno1
1Sakura Medical Center, Toho University, Chiba, Japan; 2Toho-Kamagaya Hospital, Kamagaya-shi, Chiba, 273-0132, Japan
Correspondence: Izumi Yoshida
Sakura Medical Center, Toho University, Sakura-shi, Chiba, 285-8741, Japan
Tel +81-43-462-8811
Fax +81-43-462-8835
Email [email protected]
Purpose: To investigate the association between the inclusion of components identified on images in target spots of photodynamic therapy (PDT) and exudate relapse in eyes with age-related macular degeneration (AMD).
Methods: Forty-one eyes (39 patients) with polypoidal choroidal vasculopathy (PCV) and 32 eyes (31 patients) with typical AMD (tAMD) who underwent PDT were retrospectively investigated. Each component identified on fluorescein (FA) or indocyanine angiography (IA), optical coherence tomography (OCT), or color photography was graded as not depicted, covered with a margin ≥ 500 μm or < 500 μm, and protruding. Associations between these grades and the dry rate (proportion of subjects with continuous absence of exudate over following 12-month period) and the relapse index (2 × number of injections administered + accumulation of exudate for 12 months post-PDT) were investigated.
Results: In PCV, the association between worse coverage and decreasing dry rates for feeder vessels and polyps approached statistical significance. With coverage margins ≥ 500 μm, dry rate tended to be greater than with coverage margins < 500 μm for feeder vessels, classic lesions, and occult lesions on FA. In the tAMD group, coverage with margins ≥ 500 μm tended to yield a higher dry rate than coverage with margins < 500 μm for CNV on IA. Coverage with margins ≥ 500 μm for occult and classic lesions on FA yielded no dry subjects, and all subjects with classic lesions or staining had recurrence (P = 0.009 and 0.050). Worse coverage and worse dry rate in PCV and worse relapse index in tAMD were related to pigment epithelial detachment on OCT (P = 0.040 and 0.006).
Conclusion: Polyps in PCV and pigment epithelial detachment (PED) in tAMD were verified as appropriate targets, corresponding to the existing guidelines, and feeder vessels, classic lesion, occult lesion, and PED in PCV and CNV on IA in tAMD were suggested as further targets. OCT was superior to FA for evaluating PED.
Keywords: photodynamic therapy, age-related macular degeneration, spot size, angiography
Introduction
The current standard treatment for age-related macular degeneration (AMD) is the administration of intravitreal injections with anti- vascular endothelial growth factor (anti-VEGF) drugs. Though the EVEREST study had re-evaluated the efficacy of photodynamic therapy (PDT) with verteporfin in treating polypoidal choroidal vasculopathy (PCV),1 and infrequently, PDT has also been used for choroidal neovascularization (CNV) of typical AMD (tAMD),2 particularly for patients refractory to anti-VEGF drugs.3
PDT aims to avoid undertreating or overtreating the CNV membrane. If a laser spot is smaller than expected, the CNV margins, which are believed to be the most actively proliferating regions, may be left untreated, resulting in failure of the treatment. Conversely, an oversized treatment may destroy more healthy tissue than necessary.4
In the treatment of age-related macular degeneration with photodynamic therapy (TAP) study,5,6 it was considered necessary to include features that may obscure the boundaries or extent of either classic or occult CNV when deciding on treatment spot size. These features included hypofluorescence corresponding to blood, hypofluorescence not corresponding to blood, hyperfluorescence from fibrous tissue showing fluorescein staining, and hyperfluorescence from a serous pigment epithelial detachment (PED), identified on fluorescein angiography (FA). Infrequently, CNV membrane identified on indocyanine green angiography (IA) had been included in PDT.7 Moreover, for treatment of polypoidal choroidal vasculopathy (PCV), the results of both FA-guided8,9 and IA-guided10,11 PDT have been reported. In IA-guided PDT for PCV, the greatest linear dimension (GLD) was measured to ensure coverage of the entire area containing polyps and the branching vascular network (BVN) on IA, but adjacent PED or hemorrhage was not included with the lesion in the targeted region.12 Those guidelines have rarely been reconsidered till now, particularly in relation to tAMD.
There are several unresolved problems related to determining the spot size. Distinct polyps and BVN in PCV, and distinct CNV membranes in typical AMD (tAMD) are always included as treatment targets. However, it has not been investigated whether omitting components outside of the CNV membrane, which could potentially obscure CNV, was related to treatment failure. Moreover, it has been suggested that an additional 1000 µm (500-µm margin on both sides) should be added to the GLD when determining spot size, to compensate for any slight movement of the patient,5 and most reports1,13 have followed this guideline. However, to minimize complications, such as choroidal infarction and ischemia, Eandi et al suggested inclusion of a 200-µm margin around the GLD when performing PDT for PCV patients.14 Other than their paper, a margin smaller than 500 µm and treatment failure have rarely been discussed. On the other hand, enlarging the spot size to include feeder vessels15 seen in the early phase by IA has been encouraged by Georgalas et al.16
The purpose of the present study was to investigate the association between omission of components from the target spot and recurrence of disease activity after PDT, and to evaluate the confounding margin of the spot size related to each component.
Materials and Methods
The study population consisted of patients with AMD, aged ≥50 years, who underwent PDT between January 2014 and November 2020 at Sakura Medical Center, Toho University. This retrospective study was approved by the Ethics Committee of Toho University Sakura Medical Center (No. S20062), and the study design adhered to the tenets of the Declaration of Helsinki. All patients provided written informed consent for treatment, and all private patient information was excluded from the database. The use of anonymous information was approved by the Ethics Committee of Toho University Sakura Medical Center and the patients were given an option to refuse entry into the study, as noted on the website of our hospital.
Medical records of all consecutive patients who visited the AMD clinic and underwent PDT in our hospital between January 2014 and November 2020 were evaluated retrospectively. Exclusion criteria were subjects who had undergone previous photocoagulation, a shorter than 12-month observation period, or lack of descriptions of PDT spots on photographs in medical records.
All patients underwent a comprehensive ocular examination, including fundus examinations, color fundus photography, optical coherence tomography (OCT), FA, and IA. Differential diagnosis of PCV and tAMD was made: if choroidal vessels with a polypoidal structure, with or without abnormal vascular networks, were observed on IA at a corresponding lesion on FA, the eyes were diagnosed as having PCV.
Interventions varied according to each clinician’s discretion. Patients who underwent initial PDT17 were administered intravitreal anti-VEGF injections once or twice before PDT. After PDT, pro re nata18 injections, the treat-and-extend19 approach, or observation was selected by each clinician if relapse of exudate was observed on OCT. The patients who had not undergone initial PDT were administered three consecutive intravitreal anti-VEGF injections initially, followed by pro re nata injections or the treat and extend method. When exudate was refractory, delayed PDT17 was performed for these patients. For the purpose of this study, both initial PDT and delayed PDT were evaluated. The number of injections performed before PDT and after PDT, over a 12-month period, and the best corrected visual acuity (BCVA), converted to log MAR units, both before and 12 months after PDT were also reviewed.
A Topcon Digital Imaging System with Imagenet 2000 software (Topcon TRC-50XT; Topcon Corporation, Tokyo, Japan), and the TRC-501A fundus camera (Topcon Corporation) was used to perform angiography. Each clinician determined the GLD from digital FA and IA images obtained with Imagenet, and decided the spot size according to the margins they chose. The conditions of the lens, phakia, or pseudophakia were also reviewed.
After PDT, patients visited the hospital every 1‒2 months and additional intravitreal anti-VEGF injections were administered if persistent fluid was seen on OCT. A Heidelberg Spectralis HRA+OCT (Heidelberg Engineering GmbH, Heidelberg, Germany) was used to evaluate exudates, intraretinal fluid, or subretinal fluid. In our hospital, further PDT was not added to either the initial PDT or the delayed PDT.
Coverage of Each Component
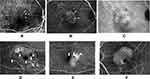
We classified angiographic components listed in previous reports of angiographic findings in AMD patients5,6,20–22 including the TAP and Verteporfin in Photodynamic Therapy (VIP) study. Gomi et al evaluated irregular choroidal vessels feeding the PCV lesion, seen during the early phase of IA, and a uniform hyperfluorescence region showing plaque, seen in the late-phase of IA, as part of the lesion in PCV patients. According to the TAP and VIP study,5,6,20 classic CNV was a bright area of well-demarcated choroidal fluorescence in the early phase of the angiogram. Occult CNV was an area of irregular elevation of the retinal pigment epithelium (RPE) without an intensely bright area in the early phase, and with stippled or granular hyperfluorescence. Moreover, these studies noted hyperfluorescent staining from fibrous tissue and serous detachments of the RPE, or PED. Additionally, window defects, which are granular fluorescence penetration throughout the damaged RPE, was often seen in AMD,23 and hemorrhage was included within the lesion in the TAP study. Moreover, existing reports had reported that IA could also improve the ability to detect tAMD, rather than only PCV.24,25 Therefore, we classified the following components on angiographic findings: 1) feeder vessel; 2) polyps (in PCV eyes) or CNV (in tAMD eyes) on IA; 3) BVN (in PCV eyes); 4) plaque; 5) classic lesion; 6) occult lesion; 7) window defect; 8) hypofluorescence not corresponding to blood; 9) staining; 10) serous PED; 11) blood. Components 1)–4) were identified on IA and components 5)–10) were identified on FA. Component 11 was identified using color photographs. Figure 1A–F shows these components.
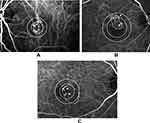
We retrospectively assessed the descriptions of spots on angiographic images and photographs taken immediately before PDT, using a ruler, and graded these components in each eye, according to four degrees of coverage, based on their relationships with the applied spot size: 1) The component was not depicted in any angiography image obtained immediately before PDT. 2) The component was covered with a margin ≥500 µm. 3) The component was covered with a margin <500 µm. 4) A part of the component protruded outside of the indicated spot size. Figure 2A–C shows these gradings. Moreover, we also evaluated the degree of coverage for PED, as identified on OCT images. Though OCT images were obtained with B-scan, it was difficult to determine whether they had a margin with ≥500 µm or not. Therefore, for PED, we assigned grades 3) or 4) only.
Dry Rate and the Relapse Index
To examine the relationships between the extent of coverage and relapse, we validated two outcomes of PDT. The first outcome was the proportion of subjects with continuous absence of exudates (dry subjects) among all subjects, or the dry rate, as evaluated on OCT during the 12 months after PDT. The second outcome was the degree of relapse related to further anti-VEGF injections. A continuous absence of relapse of exudate was ideal prognosis after PDT. However, relapses after PDT were common occurrences. The frequency of the injections administered after PDT would reflect the partial efficacy of PDT and enable its comparison, particularly in patients with relapses. However, this was somewhat problematic: as each clinician would choose injection or observation according to their discretion, the number of injections performed would not reflect the exact degree of relapse. Therefore, we calculated the accumulation of exudative periods26 during the 12 months after PDT. Figure 3 shows the method used in this study.
 |
Figure 3 The method to calculate the accumulation of exudative periods. Exudates present at the time point of the photodynamic therapy (PDT, visit A) and disappeared at visit D. It appeared at visit E and disappeared at visit F. The period from visit A to visit D calculated by optical coherence tomography date was considered an exudative period ①. The period from visit E to visit F was an exudative period ②. Period ① + period ② + ・・・added throughout 12 months was accumulated as a summary of the exudative periods (Revised figure from Yoshida26). Abbreviations: PDT, photodynamic therapy. |
Regarding the report of morphological changes after injection, as one injection could change exudates for 1‒3 months,27 we evaluated one injection as equal to 2 months’ exudate. We distinguished the relapse index as “2 × the number of injections performed + accumulation of exudative period over the 12-month period after PDT (both initial or delayed)''.
The relationship of the above outcomes with the degree of coverage was then evaluated.
Statistical Analysis
For statistical analysis, the baseline characteristics were analyzed using Fisher’s exact probability test. The numbers of injections, baseline and 12-month BCVA, GLD, and spot size were analyzed using the Mann‒Whitney U-test. For comparisons of dry rate, we applied Spearman correlation coefficient by rank test for multiple comparisons and applied Fisher’s exact probability test and Mann‒Whitney U-test with Bonferroni correction for comparisons between each group. In comparisons of the relapse index, we applied the Kruskal‒Wallis test for multiple comparisons and applied the Steel‒Dwass test with Bonferroni correction for comparisons between groups. Corrected P < 0.05 was considered to indicate a significant difference.
Results
Seventy-three consecutive eyes of 70 patients met the inclusion criteria. Table 1 shows the characteristics of both the PCV and tAMD groups. Age and sex did not differ between the groups. The PCV group had much more subjects who underwent initial PDT than the tAMD group (P = 0.001). Initial PDT in the tAMD group had been administered to only five patients and all of them had past cerebral or myocardial infarction. BCVAs were significantly better in the PCV group than in the tAMD group at 12 months after PDT (P = 0.060), while there was no significant difference between them at baseline.
 |
Table 1 Baseline Characteristics of the Study Population |
Table 2 shows the demographic characteristics of dry and wet subjects in each group. Eyes with initial PDT included more dry subjects than those with delayed PDT in the PCV group (P = 0.040). Dry subjects had better BCVAs at 12 months after PDT than did wet subjects in both groups, although the difference was not significant.
 |
Table 2 Comparisons Between Dry Subjects and Wet Subjects in Each PCV and tAMD Group |
Extent of Coverage of Each Component and Dry Rate in the PCV Group
Table 3 shows the proportion of dry subjects according to the degree of coverage of each component in the PCV group. There were four subjects for whom no polyps were detected on IA performed immediately before PDT; these subjects had been diagnosed based on previous IA images that depicted polyps. There was no significant difference between any pairwise comparisons using either Fisher’s exact probability test or the Mann–Whitney U-test except for PED on OCT. For the multiple comparison, the association between the degree of coverage of feeder vessels and polyps and decreasing the number of dry subjects approached statistical significance (P = 0.06 and 0.09).
 |
Table 3 Dry Rate with PCV According to the Extent of Coverage |
There were two subjects with protruding polyp lesions: both subjects had relapse of exudates.
On the other hand, of 18 subjects with protrusion of a network, 9 subjects remained dry and nine had a relapse of exudates.
For both polyps and networks, dry rates were almost the same for use of margins ≥500 µm and margins <500 µm.
On the other hand, for the feeder vessel, classic lesion on FA, and occult lesion on FA, dry rate were slightly better with margins ≥500 µm than with margins <500 µm.
Window defects, hypofluorescence, and PED on FA showed a tendency towards a statistically significant association with dry rates (P = 0.08, 0.08, and 0.10). However, almost subjects lacked these components, or when present, they were protruded. There were rare subjects with these components present and covered.
There was no clear trends in plaque. Staining and hemorrhage showed no statistically significant association in the multiple comparison, and the use of margins <500 μm had more wet subjects than the other groups, although these differences were not significant and protruding with these components did not seem to affect worse outcomes. When PED lesions protruded on OCT, the dry rate was significantly lower than when PED was covered on OCT (P = 0.040).
Degree of Coverage for Each Component and the Relapse Index in the PCV Group
Table 4 shows the comparisons between the degree of coverage and the relapse index in the PCV group. There was no statistically significant difference in any pairwise comparison or in the multiple comparison.
 |
Table 4 Relapse Index with PCV According to the Extent of Coverage |
Regarding feeder vessels, polyps, and networks, protruding lesions tended to yield a worse relapse index than other coverage grades, although these differences were not statistically significant.
Moreover, for classic lesions on FA, occult lesions on FA, and hemorrhage, use of margins <500 μm yielded a worse relapse index than those with margins ≥500 μm, although these differences were not statistically significant. In terms of window defects, hypofluorescence, and PED on FA, most subjects lacked these components, or they were protruding, and the relapse index for the latter was worse than for the former, although these differences were not statistically significant.
There was no clear trends for plaque.
Protruding PED on OCT tended to yield a worse relapse index than when PED was covered on OCT, although the difference was not significant.
Degree of Coverage for Each Component and Dry Rate in the tAMD Group
Table 5 shows the dry rate and degree of coverage in the tAMD group. There was no statistically significant difference in any pairwise comparison using either Fisher’s exact probability test or the Mann–Whitney U-test. In multiple comparisons, classic lesions on FA and staining showed a significant relationship with relapse (P = 0.009 and 0.050).
 |
Table 5 Dry Rate with tAMD According to the Extent of Coverage |
It is worth consideration that all subjects with occult lesions who had PDT with margins ≥500 μm had recurring exudates.
Moreover, all subjects that had either classic lesions or staining had recurring exudates, irrespective of the grade of coverage.
Compared to these, regarding CNV on IA, 33% dry rate in coverage ≥500µm and 22% in coverage <500µm yielded slight better outcomes among all components, whereas these had only small numbers, and coverage ≥500 μm tended to yield a higher dry rate than coverage <500 μm, although the difference was not statistically significant.
In terms of window defects and hypofluorescence, most subjects lacked these components, or they were protruding. Subjects in whom these components protruded more often had recurrence, although the difference was not significant.
There were no significant differences or trends in terms of feeder vessels and plaque.
All four subjects with PED depicted on FA and all three subjects with hemorrhage had relapse of exudates.
Subjects with protruding PED lesions on OCT were less often dry than those in whom PED was covered on OCT; this showed a tendency for significance (p = 0.08).
Degree of Coverage of Each Component and the Relapse Index in the tAMD Group
Table 6 shows the comparisons between the degree of coverage and the relapse index in the tAMD group. There was no statistically significant difference between any pairwise comparison except for PED on OCT.
 |
Table 6 Relapse Index with tAMD According to the Extent of Coverage |
Regarding CNV on IA, subjects with margins ≥500 μm tended to have better relapse indexes than those with other grades of coverage, although the difference was not statistically significant.
Regarding feeder vessels, plaque, staining, and hemorrhage, there were no significant associations or trends. Moreover, for occult lesions and window defects, there seemed to be no clear trends among the different coverage grades.
Subjects with any classic lesion tended to have a worse relapse index; nevertheless, their degree of coverage varied, compared to subjects without classic lesions, without statistical significance.
Subjects with protruding PED seemed to have a worse relapse index than those without PED on FA; although the difference was not significant in pairwise comparisons, it showed a tendency for significance in the multiple comparison (P=0.08). Subjects with a protruding PED lesion on OCT had a significantly worse relapse index than the subjects with covered PED on OCT (P = 0.006).
Discussion
In this study, we sought to determine the relationships between the coverage of various components identified on FA and IA with the recurrence of exudate after PDT in eyes with either PCV or tAMD types of AMD. In PCV, the association between worse coverage and decreasing dry rate for feeder vessels and polyps approached statistical significance. With coverage margins ≥500 µm, the dry rate tended to be greater than with coverage margins <500 µm for feeder vessels, classic lesions, and occult lesions on FA. Worse coverage and worse dry rates were related to PED on OCT (P=0.040). In the tAMD group, coverage with margins ≥500 µm tended to yield a higher dry rate than coverage with margins <500 µm for CNV on IA. Coverage with margins ≥ 500 µm for occult and classic lesions on FA yielded no dry subjects, and all subjects with classic lesions or staining had recurrence (P = 0.009 and 0.050). Worse coverage and a worse relapse index were related to PED on OCT (P = 0.006).
Our results showing superior visual prognosis in eyes with PCV than tAMD are similar to existing reports.12 Moreover, verified efficacy of PDT for PCV, particularly type 2 PCV without feeder vessel28 and with smaller lesions might have affected the clinician’s discretion related to the high dry rate in cases who underwent initial PDT.
Degree of Coverage in the PCV Group
In the present study, there was no significant difference in any pairwise comparison except for PED on OCT, which could be caused by the small sample size. The majority of the patients who visited our hospital had undergone anti-VEGF injection monotherapy and a small number of patients had undergone PDT.
Our study results presented some suggestions. Different degrees of coverage were more often related to dry rate in PCV cases, and more often to the relapse index in tAMD. This could be because the relapse index emphasized differences between wet subjects. In PCV patients, two subjects with protruding polyps involved disregarding some of multiple polyps. It seems clear that omitting to irradiate a polyp would lead to treatment failure, although this had not been assessed previously. These unintended failures confirmed this notion.
The presence of feeder vessels per se defined that the subject had type 1 PCV; therefore, our findings indicated differences in outcome between type 1 PCV and type 2 PCV, in agreement with existing reports.28,29 However, the use of margins ≥500 μm tended to yield better outcomes than using margins <500 μm. Moreover, a similar tendency was observed for classic lesions and occult lesions on FA.
On the other hand, the margins as related to polyps and networks did not seem to influence the outcome. It may be that, compared with polyps and networks that had relatively clear boundaries, feeder vessels, classic lesions, and occult lesions would require a more sufficient margin to cover all the components if they were presumed to contain any CNV membrane.
There were rare subjects with window defects or hypofluorescence that were present and included in the spot. Because these components were usually vague and broad when they were present, these components were classified in most subjects as not depicted or protruding. It was not known whether worse outcomes for subjects with these components were caused by disregarding the CNV membrane obscured by these components or broad and prolonged pathophysiology. Additional examinations revealed that subjects with protruding components had no larger GLD or spot size than subjects in whom these components were absent (data not shown).
Moreover, similar tendencies were seen for PED on FA, although the prevalence of PED on FA in this study was 17% in PCV and 13% in tAMD, markedly lower than previously reported.30,31 Small PED would be difficult to identify on FA, and only a relatively large PED would be depicted in the present study. We found any PED in essentially all the subjects on OCT, and PED on OCT that was covered significantly yield more dry subjects than when these lesions protruded from the spot. It suggested the presence of CNV membrane beneath the PED on OCT, though it should be noticed that comparing between two grades would yield significant difference more frequently than comparing between four grades.
Degree of Coverage in the tAMD Group
In terms of eyes with tAMD, feeder vessels and CNV on IA seemed to have a lower impact on outcomes than that of on feeder vessels and polyps in PCV eyes. Although use of margins ≥ 500 µm tended to yield better outcomes than use of those <500 µm for CNV on IA, the results were different from those obtained for polyps in PCV eyes.
Moreover, all four subjects with occult lesions on FA treated with margins ≥ 500 µm had recurrent exudates. These results suggest that it is difficult to determine the boundary of components in tAMD eyes and that determining the spot size for CNV on IA might be slightly better than or equal to that for determining this on FA. Additionally, IA was reported to improve the ability to detect CNV as compared to FA alone.24,25
It was unknown whether the low impact of feeder vessel in tAMD in the present study resulted from identification problems or needlessness to radiate.32,33
Additionally, for window defects, hypofluorescence, and PED, the existence of these components tended to result in worse outcomes, as similar in PCV. Additional examinations revealed that subjects with protruding window defects had a larger GLD than subjects in whom these components were absent (P = 0.030, data not shown). Moreover, there was a significant association between the degree of coverage of PED on OCT and the relapse index.
All three subjects with hemorrhage had relapse of exudates, although we could not certify whether hemorrhage should be included in legions with tAMD as stated in the existing guidelines, due to the very small numbers.
Moreover, all subjects with classic lesions or staining on FA had recurrent exudates, irrespective of the degree of coverage. Four subjects with classic lesions had predominantly classic AMD. These outcomes seemed to contradict the results of the TAP study5 and other pivotal assessments34 that mentioned the favorable clinical benefit of PDT for predominantly classic AMD, as compared to occult AMD, although these studies compared subjects of each subtype who underwent PDT and placebo treatment. Moreover, a mean of 3.4 treatments in the TAP study5 and 2.8 treatments in the Japanese age-related macular degeneration trial (JAT)34 study were performed during the subsequent 12 months; thereafter, no leakage was obtained in only 19% and 50% of classic AMD, whereas it was obtained in 77% of occult AMD cases in the JAT study. Therefore, it could be considered that the classic lesion itself might be refractory to PDT.
The current examination yielded some insights about determining the spot size in PDT. In PCV eyes, first, essentially all polyps should be included in spot size, in accordance with existing reports. On the other hand, our results did not certify the necessity to radiate network. However, regarding that network and polyps were inseparably organized, this issue should be carefully examined in future research. Second, we should consider including feeder vessels and classic lesions with margins ≥500 µm. Moreover, we must carefully decide on the spot size for subjects with window defects, hypofluorescence, and PED on FA. Additionally, it may be better to include part of the occult lesion and PED on OCT in the target spot. In tAMD eyes, CNV on IA might be a target for irradiation and would be best irradiated with margins ≥500 µm, more so than polyps in PCV eyes. Identification of the boundary of CNV is difficult, and it is even more difficult to determine the spot size of occult lesions on FA. Moreover, care should be taken for tAMD subjects with window defects, hypofluorescence, and PED on FA, similar to that in PCV eyes. If possible, it is better to irradiate the entire PED lesion seen on OCT, in agreement with existing reports, although OCT is superior to FA for evaluating PED. Additionally, we should be aware that the outcome of PDT in tAMD would not be favorable and that classic lesions or staining might disturb its efficacy, even if they are included in a spot size with a sufficient margin.
Nevertheless, the study had some limitations. First, the present study had a small sample size, and thus, we are only able to make suggestions. This was a retrospective study, and calculation of margin and spot size were performed based on medical records. Additionally, intervals of specific visits after PDT varied, which would affect the duration of exudate seen on OCT. Furthermore, this examination included both initial and delayed PDT. Moreover, interventions varied according to each clinician’s discretion. If more frequent injections with treat-and-extend approach had been administered after PDT for all patients, their visual prognosis might have been better. Further prospective investigations are therefore needed.
Conclusion
Our retrospective assessment of the association between the degree of coverage of components identified on various image types and relapse of exudates verified the validity of appropriateness of polyps in PCV eyes and PED in tAMD eyes as target components, corresponding with the existing guidelines, although OCT was superior to FA for evaluating PED, and the validity of feeder vessels, classic lesion, occult lesion, and PED on OCT in PCV eyes and CNV on IA in tAMD eyes as additionally provided further insights into determining spot size.
Abbreviations
AMD, age-related macular degeneration; BCVA, best corrected visual acuity; BVN, branching vascular network; CNV, choroidal neovascularization; FA, fluorescein angiography; GLD, greatest linear dimension; IA, indocyanine angiography; IRB, Institutional Review Board; OCT, optical coherence tomography; PCV, polypoidal choroidal vasculopathy; PDT, photodynamic therapy; PED, pigment epithelial detachment; RPE, retinal pigment epithelium; tAMD, typical age-related macular degeneration.
Data Sharing Statement
The datasets generated and analyzed during the current study are not publicly available because we are not able to permit any possibility of identifying persons from treatment history regardless of data anonymity, but data are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank Editage for English language editing.
Author Contributions
I.Y, H.T, and M.S conceived the study. Data acquisition and interpretation were performed by I.Y and H.T. The manuscript was drafted by I.Y and H.T. Critical revision was made by M.S and T.M. All authors read and approved the final version of the paper for submission. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There was no sponsorship for this study.
Disclosure
The authors have no conflicting interests to disclose.
References
1. Koh A, Lee WK, Chen L-J, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32(8):1453–1464. doi:10.1097/IAE.0b013e31824f91e8
2. Tsuchihashi T, Mori K, Ueyama K, Yoneya S. Five-year results of photodynamic therapy with verteporfin for Japanese patients with neovascular age-related macular degeneration. Clin Ophthalmol. 2013;7:615–620. doi:10.2147/OPTH.S43566
3. Teper S, Nowinska A, Pilat J, Wylegala E. Photodynamic therapy in VEGF inhibition non-responders-Pharmacogenetic study in age-related macular degeneration assessed with swept source optical coherence. Photodiagnosis Photodyn Ther. 2013;13:108–113. doi:10.1016/j.pdpdt.2016.01.006
4. Ranchod TM, Brucker AJ, Liu C, Cukras CA, Hopkins TB, Ying GS. Evaluation of actual vs expected photodynamic therapy spot size. Am J Ophthalmol. 2008;147(5):859–864. doi:10.1016/j.ajo.2008.11.010
5. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials–TAP report. Arch Ophthalmol. 1999;117(10):1329–1345. doi:10.1001/archopht.117.10.1329
6. Barbazetto I, Burdan A, Bressler NM, et al. Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin: fluorescein angiographic guidelines for evaluation and treatment–TAP and VIP report No. 2. Arch Ophthalmol. 2003;121(9):1253–1268.
7. Wygnanski-Jaffe T, Desatnik H, Alhalel A, et al. ICG angiography-guided photodynamic therapy for large pigment epithelial detachments in age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2006;37(5):358–363. doi:10.3928/15428877-20060901-01
8. Spaide RF, Donsoff I, Lam DL, et al. Treatment of polypoidal choroidal vasculopathy with photodynamic therapy. Retina. 2002;22(5):529–535. doi:10.1097/00006982-200210000-00001
9. Lee SC, Seong YS, Kim SS, Koh HJ, Kwon OW. Photodynamic therapy with verteporfin for polypoidal choroidal vasculopathy of the macula. Ophthalmologica. 2004;218(3):193–201. doi:10.1159/000076844
10. Quaranta M, Maget-Faysse M, Coscas G. Exudative idiopathic polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Am J Ophthalmol. 2002;134(2):277–280. doi:10.1016/S0002-9394(02)01516-7
11. Chan WM, Lam DS, Lai TY, et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy. One-year results of a prospective case series. Ophthalmology. 2004;111(8):1576–1784. doi:10.1016/j.ophtha.2003.12.056
12. Gomi F, Ohji M, Sayanagi K, et al. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology. 2008;115(1):141–146. doi:10.1016/j.ophtha.2007.02.031
13. Yoshida Y, Kohno T, Yamamoto M, et al. Two-year results of reduced-fluence photodynamic therapy combined with intravitreal ranibizumab for typical age-related macular degeneration and polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2013;57(3):283–293. doi:10.1007/s10384-013-0234-z
14. Eandi C, Ober M, Freund K, Slakter JS, Yannuzzi LA. Selective photodynamic therapy for neovascular age-related macular degeneration with polypoidal choroidal neovascularization. Retina. 2007;27(7):825–831. doi:10.1097/IAE.0b013e31804b3f70
15. Sheth J, Anantharaman G, Changra S, Gopalakrishnan M. Management of recalcitrant polypoidal choroidal vasculopathy by feeder vessel photocoagulation. Am J Ophthalmol Case Rep. 2018;9:112–115. doi:10.1016/j.ajoc.2018.01.031
16. Georgalas I, Rouvas AA, Karagiannis DA, Kotsolis AI, Ladas ID. Photodynamic therapy of choroidal neovascularization with enlargement of the spot size to include the feeding complex. Clin Ophthalmol. 2009;3:13–16.
17. Gomi F, Oshima Y, Mori R, et al. Initial versus delayed photodynamic therapy in combination with ranibizumab for treatment of polypoidal choroidal vasculopathy: the Fujisan Study. Retina. 2014;35(8):1569–1576. doi:10.1097/IAE.0000000000000526
18. Hatz K, Prunte C. Changing from a pro re nata regimen to a treat and extend regimen with ranibizumab in neovascular age-related macular degeneration. Br J Ophthalmol. 2016;100(10):1341–1345. doi:10.1136/bjophthalmol-2015-307299
19. Adrean D, Chaili S, Ramkumar A, Pirouz A, Grant S. Consistent long-term therapy of neovascular age-related macular degeneration managed by 50 or more anti-VEGF injections using a treat-extend stop protocol. Ophthalmology. 2018;125(7):1047–1053. doi:10.1016/j.ophtha.2018.01.012
20. Blinder KJ, Bradley S, Bressler NM, et al.; Treatment of Age-related Macular Degeneration with Photodynamic Therapy study group; Verteporfin in Photodynamic Therapy study group. Effect of lesion size, visual acuity, and lesion composition on visual acuity change with and without verteporfin therapy for choroidal neovascularization secondary to age-related macular degeneration: TAP and VIP report no. 1. Am J Ophthalmol. 2003;136(3):407–418. doi:10.1016/s0002-9394(03)00223-x
21. Gomi F, Sawa M, Mitarai K, Tsujikawa M, Tano Y. Angiographic lesion of polypoidal choroidal vasculopathy on indocyanine green and fluorescein angiography. Graefes Arch Clin Exp Ophthalmol. 2007;245(10):1421–1427. doi:10.1007/s00417-007-0564-y
22. Cheung CMG, Lai TYY, Chen SJ, et al. Understanding indocyanine green angiography in polypoidal choroidal vasculopathy. Retina. 2014;34(12):2397–2406. doi:10.1097/IAE.0000000000000255
23. Miets H, Merrill PT, Lambert HM, Font RL. Combined subretinal and subretinal pigment epithelium neovascular membranes in age-related macular degeneration: a clinicopathologic study of six cases. Ophthalmic Surg Lasers. 1997;28(8):645–652.
24. Yanuzzi LA, Slakter JS, Sorenson RA, Guyer DR, Orlock DA. Digital indocyanine green videoangiography and choroidal neovascularization. Retina. 1992;12(3):191–223. doi:10.1097/00006982-199212030-00003
25. Guyer DR, Yannuzzi LA, Ladas I, Slakter JS, Sorenson JA, Orlock D. Indocyanine green-guided laser photocoagulation of focal spots at the edge of plaques of choroidal neovascularization. Arch Ophthalmol. 1996;114(6):693–697. doi:10.1001/archopht.1996.01100130685008
26. Yoshida I, Sakamoto M, Sakai A, Maeno T. Effect of the duration of intraretinal or subretinal fluid on the response to treatment in undertreated age-related macular degeneration. J Ophthalmol. 2020;2020:5308597. doi:10.1155/2020/5308597
27. Ahlers C, Golbaz I, Stock G, et al. Time course of morphologic effects on different retinal compartments after ranibizumab therapy in age-related macular degeneration. Ophthalmology. 2008;115(8):e39–e46. doi:10.1016/j.ophtha.2008.05.017
28. Kawamura A, Yuzawa M, Mori R, Haruyama M, Tanaka K. Indocyanine green angiographic and optical coherence tomographic findings support classification of polypoidal choroidal vasculopathy into two types. Acta Ophthalmol. 2013;91(6):e474–e481. doi:10.1111/aos.12110
29. Yang J, Yuan M, Wang E, Xia S, Chen Y. Five-year real-world outcomes of anti-vascular endothelial growth factor monotherapy versus combination therapy for polypoidal choroidal vasculopathy in a Chinese population: a retrospective study. BMC Ophthalmol. 2019;19(1):237. doi:10.1186/s12886-019-1245-4
30. Yannuzzi LA, Hope-Ross M, Slakter JS, et al. Analysis of vascularized pigment epithelial detachments using indocyanine green videoangiography. Retina. 1994;14(2):99–113. doi:10.1097/00006982-199414020-00003
31. Meredith TA, Braley RE, Aaberg TM. Natural history of serous detachments of the retinal pigment epithelium. Am J Ophthalmol. 1979;88(4):643–651. doi:10.1016/0002-9394(79)90659-7
32. Kuehlewein L, Bansal M, Lenis TL, et al. Optical coherence tomography angiography of type 1 neovascularization in age-related macular degeneration. Am J Ophthalmol. 2015;160(4):739–48.e2. doi:10.1016/j.ajo.2015.06.030
33. Youssfi-Rich A, Clement-Fernández F, García-Urtueta E, Clement-Corral A. Feeder vessel detection by means of dynamic indocyanine green angiography of subfoveal choroidal neovascularization secondary to age-related macular degeneration. Arch Soc Esp Ophthalmol. 2006;81(2):79–84. doi:10.4321/s0365-66912006000200007
34. The Japanese age-related macular degeneration trial (JAT) study group. Japanese age-related macular degeneration trial: 1-year results of photodynamic therapy with verteporfin in Japanese patients with subfoveal choroidal neovascularization secondary to age-related macular degeneration. Am J Ophthalmol. 2003;136(6):1049–1061. doi:10.1016/S0002-9394(03)00576-2
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.