Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 12
Association between polymorphisms of LEP, LEPR, DRD2, HTR2A and HTR2C genes and risperidone- or clozapine-induced hyperglycemia
Authors Puangpetch A , Srisawasdi P , Unaharassamee W , Jiratjintana N, Vanavanan S , Punprasit S , Na Nakorn C, Sukasem C, Kroll MH
Received 2 April 2019
Accepted for publication 16 July 2019
Published 6 August 2019 Volume 2019:12 Pages 155—166
DOI https://doi.org/10.2147/PGPM.S210770
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin Bluth
Apichaya Puangpetch,1 Pornpen Srisawasdi,2 Weerapon Unaharassamee,3 Napa Jiratjintana,3 Somlak Vanavanan,2 Suweejuk Punprasit,2 Chalitpon Na Nakorn,1 Chonlaphat Sukasem,1 Martin H Kroll4
1Division of Pharmacogenomics and Personalized Medicine, Department of Pathology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 2Division of Clinical Chemistry, Department of Pathology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 3Department of Psychiatry, Somdet Chaopraya Institute of Psychiatry, Bangkok, Thailand; 4Quest Diagnostics, Secaucus, NJ 07094, USA
Objective: To determine whether genetic polymorphisms related to pharmacodynamics with metabolic adverse effects, namely leptin promoter (LEP) rs7799039, leptin receptor rs1137101, dopamine D2 rs4436578, serotonin 5-HT2A rs6313, and serotonin 5-HT2C rs518147 and rs12836771, are associated with hyperglycemia induced by risperidone or clozapine in adult Thai patients with psychosis.
Methods: A total of 180 patients treated with risperidone-based (n=130) or clozapine-based (n=50) regimens were included in this study. Blood samples were analyzed for genotyping of the candidate genes and biochemical testing. Genotyping was performed by conducting a TaqMan real-time polymerase chain reaction-based analysis.
Results: The prevalence of hyperglycemia was higher in patients receiving clozapine (64.0%) than in those receiving risperidone (30.8%). Among the candidate genes, only the LEP rs7799039 polymorphism demonstrated a significant association with hyperglycemia (χ2=9.879, P=0.008) in patients treated with risperidone; patients with the AA genotype had the highest risk (41.1%), followed by those with AG (20.8%) and GG (0%) genotypes. Using the recessive genetic model (AA vs AG + GG), the odds ratio and 95% CI were 3.28 and 1.44 −7.50, respectively. None of the genes were associated with hyperglycemia in patients treated with clozapine. A binary logistic regression revealed that the LEP rs7799039 polymorphism demonstrated a significant association with hyperglycemia, independent of body-mass index (BMI) in patients receiving risperidone; the odds ratio (95% CI) was 3.188 (1.399–7.262), P=0.006. By contrast, none of the pharmacodynamic genetic factors, except for BMI, were significantly associated with hyperglycemia in patients receiving clozapine.
Conclusion: The risk of type 2 diabetes mellitus is associated with the LEP rs7799039 polymorphism in Thai adults receiving risperidone but not in those receiving clozapine. Clarifying underlying mechanisms and risk of hyperglycemia provides an opportunity to prevent impaired glucose metabolism in patients receiving risperidone or clozapine.
Keywords: atypical antipsychotics, leptin promoter, leptin receptor, dopamine D2, serotonin 5-HT2A, serotonin 5-HT2C, diabetes mellitus
Introduction
Second-generation antipsychotic medications can considerably ameliorate the symptoms of psychotic disorders as well as prevent relapse and treatment discontinuation.1,2 However, metabolic adverse effects, particularly rapid weight gain and metabolic and endocrine abnormalities, are substantial problems for patients taking these medications.3–5 Patients with schizophrenia have a high risk of chronic diseases, such as type 2 diabetes mellitus (type 2 DM) and cardiovascular disease, or other obesity-related complications, independent of any antipsychotic effects.6–8 Consequently, patients with schizophrenia who are treated with these antipsychotic medications have a high risk of metabolic dysregulation.9,10
The prevalence of diabetes in individuals with psychotic disorders who receive treatment with antipsychotic drugs has been increasing over time; moreover, these individuals are 2–3 times more likely to develop diabetes compared with the general population.11,12 Published findings from clinical trials in patients with schizophrenia who undergo treatment with clozapine and olanzapine have indicated that these medications can disrupt glucose regulation.13,14 However, the results have been less conclusive for the disruption of glucose regulation for risperidone and type 2 DM; some studies have demonstrated a significantly positive association,11 whereas others have reported no association.13 In addition, a cross-sectional study conducted in large UK populations demonstrated that the prevalence of type 2 DM in patients with severe mental illness was higher in patients from ethnic minority groups (especially those who were Indian, Pakistani, or Bangladeshi) than in those who identified as white British.15 Thus, heterogeneity in the risk of diabetes has been observed not only among individual patients but also among ethnic groups.
Mechanisms underlying antipsychotic-induced diabetes are not well understood and possibly depend on multifactorial processes.16,17 Related genetic polymorphisms may explain the occurrence of hyperglycemia in some patients but not others. Different drugs interact with various neurotransmitter receptors, such as dopamine D2 and serotonin 5-HT2A and 5-HT2C; thus, a genetic susceptibility between these pharmocodynamics may contribute to glucose homeostasis.18,19 Other possibilities include the role of hormones involved in glucose regulation, such as the neuroendocrine leptin.20 The relationship among antipsychotic use, weight gain, metabolic disturbance, and the pharmacogenetics of leptin is still under investigation.
Several studies investigating the relationship between genetic factors and metabolic outcomes in patients treated with antipsychotics have offered inconsistent conclusions.21–24 This conflict in results may be attributable to differences in study patients’ characteristics, such as treatment (enrolled single/multiple types of antipsychotics) and ethnicity, suggesting the influence of genetics. Furthermore, few studies have investigated pharmacodynamic genetic factors and hyperglycemia development in patients treated with risperidone or clozapine, 2 medications commonly prescribed for both acute and long-term schizophrenia.25,26 The aim of this study was to explore whether the genetic polymorphisms of the leptin promoter (LEP) rs7799039, leptin receptor (LEPR) rs1137101, dopamine D2 (DRD2) rs4436578, serotonin 5-HT2A (HTR2A) rs6313, serotonin 5-HT2C- (HTR2C) rs518147, and HTR2C rs12836771 are associated with hyperglycemia induced by risperidone or clozapine in ethnically Thai adult patients with psychosis disorders. Identifying polymorphisms of relevant genes in patients with psychosis may help individualize treatment to prevent impaired glucose regulation.
Materials and methods
Study patients
This cross-sectional observational study enrolled 202 Thai patients who had received treatment for psychosis at the Somdet Chaopraya Institute of Psychiatry in central Thailand. All patients were diagnosed by a psychiatrist. Patients were informed of the specific risks and benefits of participation, and written consent was obtained from patients. The study protocol was approved by the Ethics Committee of the Faculty of Medicine, Ramathibodi Hospital, Mahidol University, and the research center of the Somdet Chaopraya Institute of Psychiatry, Bangkok, Thailand. We excluded 22 patients who were treated with antipsychotic polypharmacy (n=10), quetiapine (n=4), or olanzapine (n=8). The study group consisted of 180 participants who had received either risperidone (n=130) or clozapine (n=50) for at least 1 month. Patient demographics and treatment history, including dosage and concomitant therapy, were extracted from medical and pharmacy records. Height, weight, and waist circumference measurements were also obtained at the time of enrollment. Exclusion criteria for this study were a known history of pervasive developmental disorders or conditions associated with convulsions, cancer, end-stage chronic kidney disease, or other serious physical conditions (ie, thyroid disorders). We excluded patients receiving lithium and sodium valproate or concomitant treatments that could potentially affect glucose and lipid metabolism.
Metabolic syndrome was defined using National Cholesterol Education Program-Third Adult Treatment Panel criteria modified for Asian populations.27
Single-nucleotide polymorphism selection and genotyping analysis
Candidate single-nucleotide polymorphisms (SNPs) were identified from three sources. Firstly, candidate SNPs were selected based on pathophysiological pathways in literature reviews of risperidone and clozapine-induced metabolic adverse effect such as weight gain and insulin resistance. Secondly, the minor allele frequency was greater than 0.05 in the Chinese Han in Beijing according to the International HapMap Project database (http://hapmap.ncbi.nlm.nih.gov). Thirdly, the significant association between the candidate gene and metabolic adverse effect in patients using antipsychotic medication has been reported in previous studies; LEP;20,21,24,28 LEPR,20,29 DRD230 HTR2A31 and HTR2C.22–24,32 In total, 6 SNPs related to pharmacodynamics genes were selected: LEP rs7799039 (−2548G/A), LEPR rs1137101 (668A/G), DRD2 rs4436578 (Tag-SNP T/C), HTR2A rs6313 (102T/C), HTR2C rs518147 (−697G/C), and HTR2C rs12836771 (Tag-SNP A/G). These SNPs were genotyped by performing a TaqMan-based analysis on an Applied Biosystems®ViiA™7 real-time PCR system (ABI, Foster City, CA, USA) according to the manufacturer’s protocol.
For genomic DNA extraction, blood samples were collected in EDTA tubes. DNA was isolated using the MagNA Pure automated extraction system (Roche Diagnostics), which is based on magnetic-bead technology with a lysis buffer and proteinase K. In this system, nucleic acids were bound to the surface of magnetic glass particles. Cellular debris was removed through several washing steps, and purified nucleic acids were eluted. From the 1-mL input volume of EDTA whole blood, a 200-µL output volume of extracted genomic DNA product was obtained. The quality and purity of genomic DNA were assessed using a Nano Drop ND-1000 (NanoDrop Technologies, Wilmington, DE, USA), with dynamic ranges of 220–750 nm. Wavelengths of 260 nm and 280 nm were considered to be suitable for measuring genomic DNA and evaluating contaminated proteins in the sample, respectively. In this study, 20 ng of the purified genomic DNA template was used, and the optical density ratio at 260/280 nm was >1.7. All DNA samples were aliquoted and stored at −20 °C until analysis.
Biochemical measurements
Blood samples were collected as serum or plasma in the fasting state. All samples were stored at 2 °C to 8 °C and analyzed within one day of collection for total cholesterol, triglyceride, low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C), glucose, insulin, adiponectin, leptin, and prolactin. Serum total cholesterol, triglyceride, LDL-C, HDL-C, and plasma glucose levels were tested using Siemens enzymatic methods (Siemens Medical Solution Diagnostics, Tarrytown, NY, USA). The high-sensitivity C-reactive protein level was measured on the Siemens BN Prospec (Siemens Medical Solution Diagnostics) by using the immune nephelometric method, and adiponectin and leptin levels were quantified using a sandwich ELISA system (Mediagnost Gesellschaft für Forschung and Herstellung von Diagnostika GmbH, D-72770 Reutlingen, Germany). Insulin and prolactin levels were determined using a chemiluminescent immunoassay on Immulite H2975 (Siemens Medical Solution Diagnostics) and Architect ci2000 (Abbott Laboratories Abbott Park, IL, USA), respectively. Insulin resistance was estimated using the homeostasis model assessment of insulin resistance (HOMA-IR). The HOMA-IR index was calculated using the following formula: HOMA-IR = fasting insulin (μU/mL) × fasting glucose (mmol/L)/22.5.
Lipoprotein subclass patterns were analyzed using polyacrylamide tube gel electrophoresis (Quantimetrix Lipoprint™, Redondo Beach, CA, USA), which electrophoretically separates plasma lipoproteins into the following bands: very low density lipoprotein; intermediate low density lipoprotein (midband-C, midband-B, and midband-A); large-buoyant LDL (LDL1 and LDL2); small-dense LDL (LDL3 to LDL7); and HDL. Relative areas for each lipoprotein band were determined through densitometry and multiplied by the total cholesterol concentration to yield the amount of cholesterol for each band. Mean LDL particle sizes were computed.
Statistical analyses
Continuous data for all clinical parameters and biochemical markers are reported as medians (minimum-maximum) and means (standard error of mean), and categorical variables are presented as numbers and percentages. Differences among groups were assessed using the χ2 test, Mann–Whitney U test, or t test, as appropriate. A χ2 test (Pearson chi-square or Fisher’s exact test) was used to assess differences in genotype frequencies among normal fasting plasma glucose (fasting glucose <5.55 mmol/L) and increased fasting plasma glucose (fasting glucose ≥5.55 mmol/L) groups. The recessive genetic model was used for LEP rs7799039: AA vs AG + GG and LEPR rs1137101: GG vs AG + GG and the dominant genetic model was used for DRD2 rs4436578: CC + CT vs TT; HTR2A rs6313: CC + CT vs TT; HTR2C rs518147: CC + CG vs.GG and HTR2C rs12836771: GG + AG vs AA. The association between the candidate gene polymorphisms and hyperglycemia was considered by the odds ratio (OR) and the corresponding 95% confidence interval (CI). A backward, stepwise multivariable logistic regression model was used to determine whether one or more pharmacodynamic genetic factors might predict hyperglycemia when non-pharmacodynamic genetic factors (sex, age, body-mass index [BMI], smoking status, and treatment duration) were also considered. The criteria of P=0.05 for a variable to enter and P>0.10 for a variable to be remove. P<0.05 (2 tailed) was considered statistically significant. All analyses were performed using SPSS, version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics and biomarkers of the study population
The study group consisted of 180 patients (86 men and 94 women) between the ages of 18 and 77 years. Schizophrenia was diagnosed in 85.5% of patients. The demographic and biochemical test results of participants stratified by the medication prescribed are summarized in Table 1. Overall, 130 (72.2%) and 50 (27.8%) patients were receiving risperidone and clozapine treatment, respectively. For patients in our study, the median dose equivalent to olanzapine33 for risperidone treatment (10.00 mg/d; interquartile range: 5.00–15.00 mg/d) was significantly higher than that for clozapine treatment (6.54 mg/d; interquartile range: 3.27–9.80 mg/d). By contrast, the duration of clozapine treatment was significantly longer than that of risperidone treatment; the median treatment durations for clozapine and risperidone were 81.5 (interquartile range: 25.0–110.8 months) and 24.6 months (interquartile range: 10.8–51.2 months), respectively. Patients treated with each antipsychotic medication were similar with respect to sex, age, weight, BMI, and prevalence of cigarette smoking and alcohol consumption. Although the median waist circumference and hip circumference did not differ between the 2 treatment groups, the waist to hip ratio was slightly higher in patients treated with clozapine than in those treated with risperidone. The results obtained for metabolic biomarkers were similar for participants in the 2 groups, with the exception of a higher mean fasting plasma glucose concentration and a lower mean prolactin concentration in patients receiving clozapine.
 |
Table 1 Clinical characteristics of patients treated with risperidone or clozapine |
As indicated in Figure 1, the prevalence of hyperglycemia was higher in patients receiving clozapine [32/50 (64.0%)] than in those receiving risperidone [40/130 (30.8%)] (χ2=16.615, P<0.001). By contrast, the prevalence of insulin resistance (HOMA-IR >3.0) in patients receiving clozapine [9/50 (18.0%)] was not significantly different from that in patients receiving risperidone [15/130 (11.5%)] (χ2=1.305, P=0.327). No difference was noted between the 2 treatment groups in terms of other clinical features of metabolic syndrome (all P>0.200).
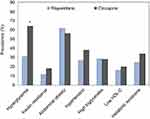 |
Figure 1 The prevalence of metabolic abnormality in patients receiving risperidone or clozapine. Metabolic syndrome and its major components are defined using the NCEP ATP III criteria modified for Asian population.27 *p-value<0.05. |
Associations of pharmacodynamic gene polymorphisms and hyperglycemia
Genotype distributions in patients with normal and increased fasting plasma glucose levels are presented in Table 2. Genotype frequencies were not significantly different between patients receiving risperidone or clozapine (all P>0.200). Among candidate genes, only the LEP rs7799039 polymorphism demonstrated a significant association with hyperglycemia (Pearson chi-square =9.879, P=0.008) in risperidone-treated patients; patients with the AA genotype had the highest risk [30/73 (41.1%)], followed by those with AG [10/48 (20.8%)] and GG [0/9 (0%)] genotypes. Using the recessive genetic model (AA vs AG + GG), the odds ratio (OR) and 95% CI were 3.28 and 1.44–7.50, respectively. Although the LEPR rs1137101 polymorphism was not significantly associated with hyperglycemia (Fisher’s exact =2.063, P=0.427), the GG genotype exhibited the highest risk of hyperglycemia [30/91 (33.0%)], followed by AG [10/35 (28.6%)] and AA [0/4 (0%)] genotypes. None of the study genes were associated with hyperglycemia in clozapine-treated patients.
 |
Table 2 Genotype frequency of LEP, LEPR, DRD2, HTR2A, and HTR2C polymorphisms among patients with and without impaired fasting glucose |
To assess the association between pharmacodynamic genetic factors and nonpharmacodynamic genetic factors with hyperglycemia in patients treated with risperidone or clozapine, we evaluated their contributions by performing a binary logistic regression, as shown in Table 3. In Model 1, all candidate genes and the clinical factors of sex, age, BMI, smoking status, and treatment duration were considered as independent variables. Individual variables that did not exhibit a statistically significant correlation (P>0.05) with hyperglycemia were removed using a backward stepwise logistic regression; the remaining variables are presented with Model 2. LEP rs7799039 was the only polymorphism that demonstrated a significant association with hyperglycemia, independent of BMI in patients on risperidone (OR, 3.188; 95% CI, 1.3990–7.262; P=0.006). In patients treated with clozapine, none of the candidate genes demonstrated an association with hyperglycemia. BMI was significantly associated with hyperglycemia; the OR (95% CI) was 1.6233 (1.170–2.252).
 |
Table 3 Logistic regression analysis between the pharmacodynamic genetic and clinical risk factors with impaired fasting glucose |
Discussions
Thai patients treated with clozapine demonstrated a higher rate of elevated fasting glucose (up to 64.0%), 2 times greater than that of patients treated with risperidone (30.8%). The pharmacoepidemiological–pharmacodynamic study performed using the VigiBase database revealed that among patients with schizophrenia who had diabetes, 53.4%, 30.6%, and 7.8% had received clozapine, olanzapine, and risperidone treatment, respectively.34 We noted that the rate of insulin resistance induced by clozapine and risperidone was not significantly different, suggesting that the diabetogenic effects of these drugs may operate under different mechanisms. The pharmacodynamic characteristics of each antipsychotic medication may provide further insights into mechanisms underlying the pathophysiology of glucose disturbances.
Most published studies on genetic polymorphisms have reported an association with metabolic syndrome, especially weight gain, in patients treated with antipsychotic agents.20–24,28–32,35–38 Because LEP, HTR2A, and HTR2C are involved in energy expenditure, polymorphisms in genes coding for these receptors might affect metabolic homeostasis in patients receiving long-term treatment with antipsychotic medications.20,29–32,37 However, less is known regarding their effect on type 2 DM in patients treated with antipsychotic medications. Our results indicated that LEP rs7799039 had a significant association with hyperglycemia in patients treated with risperidone. However, the association was independent of BMI, suggesting that risperidone-induced glycemic dysregulation occurs irrespective of changes in weight. Thus, risperidone may induce hyperglycemia through direct or indirect effects on leptin action and the leptin gene, rather than as a result of secondary weight gain. By contrast, none of the study gene polymorphisms, except for BMI, were significantly associated with hyperglycemia in patients receiving clozapine. These findings suggest that risperidone and clozapine possibility affect glucose homeostasis through different mechanisms, through a genetic polymorphism for risperidone-induced hyperglycemia and through weight gain for glucose intolerance induced by clozapine therapy.
Considering the distribution of genetic variants of LEP rs7799039, namely wild-type (AA), heterozygous (AG), and homozygous (GG), we discovered that on average, patients with the AA genotype had a 2-fold higher probability of developing glycemic abnormalities than did those with the AG genotype (as indicated by increased fasting glucose; 41.1% vs 20.8%). None of the patients with the GG genotype were noted to have risperidone-induced hyperglycemia. The carries of the AA genotype demonstrated an increased risk of type 2 DM after adjustment for sex, age, BMI, smoking status, and treatment duration (OR, 3.188; 95% CI, 1.3990–7.262). Therefore, the LEP AA genotype may be a key genetic predictor of hyperglycemia in patients treated with risperidone, whereas the LEP GG genotype may not or may even be protective.
The diabetogenic effect of risperidone may be explained, at least in part, by its action on leptin and insulin resistances as well as the functionality of the LEP gene. Leptin acts by directly regulating pancreatic β cells and insulin-sensitive tissues, independent of its effects on adiposity.39 In our previous study on children and adolescents with autistic spectrum disorders, we demonstrated that risperidone therapy directly and adversely affects glucose homeostasis by inhibiting the actions of leptin and insulin.40,41 Studies on mice conducted by Cheng et al revealed that palmitic acid and arachidonic acid can inhibit leptin signaling in the paraventricular nucleus of the hypothalamus, attenuating the central leptin regulation of glucose homeostasis.42,43 According to a study on metabolomic profiling in patients with schizophrenia, treatment with risperidone may contribute to increased palmitic acid and arachidonic acid levels.44 Thus, the occurrence of these metabolic disturbances after risperidone treatment is probably the cause of leptin resistance. In addition, risperidone may induce the leptin and insulin resistance in peripheral tissues, particularly skeletal muscle, through suppression of cytokine signaling (SOCS), a critical regulator of leptin and insulin signaling pathways.45 Increases in SOCS3 and SOCS6 mRNA expression levels result in the reduction of both insulin-induced protein kinase B activation and leptin-stimulated signal transducer and activates transcription 3 phosphorylation, which also mediates insulin and leptin resistance.
The LEP rs7799039 (−2548G/A) is located in the promotor region of the LEP gene on chromosome 7q31.3.46 The gene polymorphism LEP rs7799039 has a strong influence on leptin gene transcription, expression, and adipose tissue secretion. Numerous studies have focused on the classic influence of leptin on obesity and obesity-related diseases, including antipsychotic-induced weight gain.28,47–50 Although a mutation in LEP rs7799039 has received recognition as a potential contributor to antipsychotic-associated weight gain, findings have yielded somewhat conflicting results.28,47,48 Lee and Bishop demonstrated that both G and A alleles have significant associations with weight gain that depend on the population and antipsychotic used.28 Their results indicated that a multifactorial network of pathways influence metabolic outcomes in patients treated with antipsychotic medications.
A meta-analysis study conducted by Shen et al indicated that genetic differences in the LEP rs7799039 polymorphism were discernible among ethnic populations.47 The frequencies of G allele were high in European populations, but low in Asian populations, with AA predominating. For the Thai population in our study, genetic frequencies were approximately 56% for AA, 37% for AG, and 7% for GG genotypes. Shen et al suggested that differences in ethnicity appear to correspond to inconsistencies in the relationship between the LEP rs7799039 polymorphism and antipsychotic-induced weight gain.47 The A allele was proposed as a genetic risk factor for weight gain in the Asian population, whereas it is considered to be a genetic protective factor in the European population. Consequently, the LEP rs7799039 polymorphism might increase the risk of type 2 DM in patients treated with risperidone, with effects that vary by ethnicity. As mentioned above, both the G and A alleles may differ in the function of the LEP gene. In regards to the gene frequencies, the recessive genetic model (AA vs AG +GG) dominated in our population, therefore the A allele may influence decreasing leptin transcription, expression and secretion. However, in our results, the mean of leptin level in the AA genotype carriers (13.54±12.97 ng/mL) were not significantly lower than that in the AG (11.98±9.9 ng/mL) or GG (18.28±13.09 ng/mL) genotype carriers. Hoffstedt et al studied in Swedish, they reported the LEP rs7799093 polymorphism appears to influence leptin transcription, expression and secretion such that AA genotype carriers have twice the rate of leptin secretion and 60% more leptin mRNA transcription than G allele carriers.51 Therefore, the dominant genetic model (AA + AG vs GG) usually dominates in European population, with the G allele influencing the function of the LEP gene and perhaps increasing the risk of type 2 DM in risperidone-treated patients.
Our results demonstrated that the LEP rs7799039 polymorphism was not associated with hyperglycemia in clozapine treated patients. The underling mechanisms to explain less association of clozapine with the polymorphisms than risperidone is not clear. However, we believe that it may be related to variety of other genes and various environmental factors. There were many reports describing both leptin and serotonin 5-HT2C that have strong affinity for and antagonize the serotonin 5-HT2C receptors.52,53 These neuropeptides act through the hypothalamic satiety mechanisms. Leptin has been shown to stimulate central serotonin turnover. Central leptin-induced anorexia is in part mediated by the 5-HT2C receptor as well. In addition, in antipsychotics treatment, the patients with the polymorphism on levels of 5-HT2C receptor expression tended to have higher baseline leptin levels and subsequent weight gain and metabolic syndrome.54 Thus, it is plausible that the multifactorial network of pathways has an interaction between both neurotransmitters.55 Because of linking HTR2C genotype with LEP genotype, the HTR2C polymorphism owing to inherent differences in gene expression and leptin signaling which may attenuate a role of leptin involved in the anorexic effect and the glucose regulatory during drug exposure.55 Due to clozapine have a higher affinity to 5-HT2C receptor compared to risperidone, the functionality of polymorphism on levels of 5-HT2C receptor expression may somehow more contribute to leptin dysregulation. Therefore, the relationship between leptin levels and levels of 5-HT2C receptor expression may explain why LEP rs7799039 polymorphism was not associated with hyperglycemia in clozapine treated patients. Future pharmacogenetic investigation will identify mechanistically and therapeutically informative of interactions or differential associations when HTR2C and LEP genetic variants are analyzed together.
A strong association between the LEPR rs1137101 polymorphism and obesity has been reported.20 In the present study, we did not identify an association between LEPR polymorphisms and the risk of diabetes. Our findings agree with those of a study published by Kuo and colleagues who demonstrated that the LEP gene, but not LEPR gene, was significantly associated with the insulin level and HOMA-IR in Taiwanese patients with schizophrenia.29 However, all patients who carried the wild-type gene, approximately 3% of our study population, demonstrated normal glucose homeostasis. Thus, the presence of the homozygous A allele may be associated with a low risk of glucose dysregulation in patients treated with risperidone.
Many studies have reported that serotonin 5-HT2C receptors, which are known to be associated with antipsychotic-induced weight gain due to a high degree of occupancy with antipsychotic medications (particularly clozapine), have a strong influence on the development of glucose intolerance and diabetes32,33,56 Hypothalamic 5-HT2C receptors can mediate glucose homeostasis through the cholinergic neurons of the sympathetic nervous system, which subsequently activate systems controlling plasma glucose in adipose, muscle, and liver tissues.57 In the current study, we did not identify statistically significant associations between HTR2C rs518147 or HTR2C rs12836771 (Tag-SNP of rs3813929) and hyperglycemia development in patients treated with clozapine; this finding is consistent with those of 2 studies on Caucasian and Korean patients.36,58 However, BMI demonstrated a strong association with hyperglycemia development in patients treated with clozapine. This may confirm that obesity-related insulin resistance may be the cause of the diabetogenic effect seen with clozapine.11,20,59
This study has some limitations. First, this study adopted a cross-sectional design, which may not be capable of adequately confirming associations between risperidone or clozapine therapy and changes in glucose metabolism. Second, our sample size was relatively small, particularly for patients treated with clozapine. The small sample for clozapine treatment was reflected the clinical practice of psychotic patients in our population. A relatively small sample size increases the risk of a type I error with false-positive findings. Our results require validation with a larger independent sample of patients, which can provide sufficient statistical power to infer the involvement of the LEP gene in risperidone-induced hyperglycemia. Third, all patients in the present study were ethnically Thai. The genotype frequency of the LEP rs7799039 polymorphism differ noticeably among ethnic populations. Thus, the results of this study may not be applicable to people of other ethnicities until they receive validation from a prospective study involving other non-Asian patients. Fourth, age, sex, BMI, smoking status and duration of treatment are known to be risk factors associated with developing hyperglycemia; although these factors were accounted for and adjusted in our analysis, other factors were not accounted for, despite being are known to be risk factors in developing hyperglycemia in patients with schizophrenia such as the duration of untreated psychosis or duration of illness, the duration and type of previous medication, dietary intake, level of education, physical activity and family history.
In conclusion, in adult Thai patients with psychosis, we identified substantially different mechanisms underlying the effects of risperidone and clozapine on hyperglycemia. The high interindividual variability in the risk of hyperglycemia suggests that genetics may play a crucial role in an individual’s susceptibility to hyperglycemia, making it a target for future research in pharmacogenetics. The polymorphism LEP rs7799039 was associated with risperidone-induced hyperglycemia. The LEP AA genotype may be a key genetic predictor of hyperglycemia, whereas the LEP GG genotype may be a genetic protective factor. Testing for LEP rs7799039 in patients with psychosis may help to individualize treatment and prevent impaired glucose metabolism in patients receiving risperidone. However, we did not observe an association between clozapine and polymorphisms assessed in this study. Clozapine-induced weight gain appeared to have a primary effect on the development of glucose intolerance and diabetes. Understanding underlying mechanisms and risks may lead to effective interventions for preventing impaired glucose metabolism in patients receiving risperidone or clozapine.
Ethics approval and consent to participate
The study protocol was reviewed and approved by the ethics committee of Ramathibodi Hospital (Approval reference number ID MURA2015/23). All methods were carried out in accordance with the Declaration of Helsinki.
Acknowledgments
The authors gratefully acknowledge the staff of Somdet Chaopraya Institute of Psychiatry in central Thailand and Jirapa Kerdmongkol at the Department of Pathology, Ramathibodi Hospital, Mahidol University for their assistance. The authors wish to thank all the patients who took part in this study. This work was supported by Mahidol University.
Author contributions
All the authors participated in the interpretation and the review of the data. PS, AP and WU designed the study. PS, AP, WU, NJ, SP and CNN conducted the data retrieval and analyzed the data. PS, AP, SV and MHK wrote the manuscript. PS, AP, CS and MHK gave constructive suggestions during the preparation of the manuscript. All the authors also participated in the revision of the manuscript and read and approved the final manuscript. All authors agree to be accountable for all aspects of the work.
Disclosure
The abstract of this paper was presented at the 70th AACC Annual Scientific Meeting as a poster presentation with interim findings. The poster’s abstract was published in “Abstract Guide” in poster sessions: Endocrinology/Hormones. All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Zhang J, Gallego J, Robinson D, et al. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2013;16:1205–1218. doi:10.1017/S1461145712001277
2. Chakos M, Lieberman J, Hoffman E, et al. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am J Psychiatry. 2001;158:518–526. doi:10.1176/appi.ajp.158.4.518
3. Daumit GL, Goff DC, Meyer JM, et al. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res. 2008;105:175–187. doi:10.1016/j.schres.2008.07.006
4. Nasrallah HA. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008;13:27–35. doi:10.1038/sj.mp.4002066
5. Tschoner A, Engl J, Laimer M, et al. Metabolic side effects of antipsychotic medication. Int J Clin Pract. 2007;61:1356–1370. doi:10.1111/j.1742-1241.2007.01416.x
6. Enez Darcin A, Yalcin Cavus S, Dilbaz N, et al. Metabolic syndrome in drug-naïve and drug-free patients with schizophrenia and in their siblings. Schizophr Res. 2015;166:201–206. doi:10.1016/j.schres.2015.05.004
7. Pillinger T, Beck K, Gobjila C, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74:261–269. doi:10.1001/jamapsychiatry.2016.3803
8. Voruganti LP, Punthakee Z, Van Lieshout RJ, et al. Dysglycemia in a community sample of people treated for schizophrenia: the Diabetes in Schizophrenia in Central-South Ontario (DiSCO) study. Schizophr Res. 2007;96:215–222. doi:10.1016/j.schres.2007.07.016
9. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–962. doi:10.1016/S0140-6736(13)60733-3
10. Nielsen J, Skadhede S, Correll CU. Antipsychotics associated with the development of type 2 diabetes in antipsychotic-naïve schizophrenia patients. Neuropsychopharmacology. 2010;35:1997–2004. doi:10.1038/npp.2010.78
11. Vancampfort D, Correll CU, Galling B, et al. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry. 2016;15:166–174. doi:10.1002/wps.20309
12. Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophr Bull. 2013;39:306–318. doi:10.1093/schbul/sbr148
13. Hirsch L, Yang J, Bresee L, et al. Second-generation antipsychotics and metabolic side effects: a systematic review of population-based studies. Drug Saf. 2017;40:771–781. doi:10.1007/s40264-017-0543-0
14. Newcomer J. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2015;19(Suppl. 1):1–93.
15. Das-Munshi J, Ashworth M, Dewey ME, et al. Type 2 diabetes mellitus in people with severe mental illness: inequalities by ethnicity and age. Cross-sectional analysis of 588 408 records from the UK. Diabet Med. 2017;34:916–924. doi:10.1111/dme.13298
16. Ward M, Druss B. The epidemiology of diabetes in psychotic disorders. Lancet Psychiatry. 2015;2:431–451. doi:10.1016/S2215-0366(15)00007-3
17. Whicher CA, Price HC, Holt RIG. Mechanisms in endocrinology: antipsychotic medication and type 2 diabetes and impaired glucose regulation. Eur J Endocrinol. 2018;78:R245–R258. doi:10.1530/EJE-18-0022
18. Scigliano G, Ronchetti G. Antipsychotic-induced metabolic and cardiovascular side effects in schizophrenia: a novel mechanistic hypothesis. CNS Drugs. 2013;27:249–257. doi:10.1007/s40263-013-0054-1
19. Reynolds GP, Kirk SL. Metabolic side effects of antipsychotic drug treatment–pharmacological mechanisms. Pharmacol Ther. 2010;125:169–179. doi:10.1016/j.pharmthera.2009.10.010
20. Gregoor JG, van der Weide J, Mulder H, et al. Polymorphisms of the LEP- and LEPR gene and obesity in patients using antipsychotic medication. J Clin Psychopharmacol. 2009;29:21–25. doi:10.1097/JCP.0b013e31819359be
21. Zhang XY, Tan YL, Zhou DF, et al. Association of clozapine-induced weight gain with a polymorphism in the leptin promoter region in patients with chronic schizophrenia in a Chinese population. J Clin Psychopharmacol. 2007;27:246–251. doi:10.1097/jcp.0b013e3180582412
22. Templeman LA, Reynolds GP, Arranz B, et al. Polymorphisms of the 5-HT2C receptor and leptin genes are associated with antipsychotic drug-induced weight gain in Caucasian subjects with a first-episode psychosis. Pharmacogenet Genomics. 2005;15:195–200.
23. Reynolds GP, Zhang Z, Zhang X. Polymorphism of the promoter region of the serotonin 5-HT(2C) receptor gene and clozapine-induced weight gain. Am J Psychiatry. 2003;160:677–679. doi:10.1176/appi.ajp.160.4.677
24. Kang SH, Lee JI, Han HR, et al. Polymorphisms of the leptin and HTR2C genes and clozapine-induced weight change and baseline BMI in patients with chronic schizophrenia. Psychiatr Genet. 2014;24:249–256. doi:10.1097/YPG.0000000000000053
25. Madaan V, Bestha DP, Kolli V, et al. Clinical utility of the risperidone formulations in the management of schizophrenia. Neuropsychiatr Dis Treat. 2011;7:611–620. doi:10.2147/NDT.S14385
26. Samara MT, Dold M, Gianatsi M, et al. Efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia: a network meta-analysis. JAMA Psychiatry. 2016;73:199–210. doi:10.1001/jamapsychiatry.2015.2955
27. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. doi:10.1161/CIRCULATIONAHA.109.192644
28. Lee AK, Bishop JR. Pharmacogenetics of leptin in antipsychotic associated weight gain and obesity-related complications. Pharmacogenomics. 2011;12:999–1016. doi:10.2217/pgs.11.45
29. Kuo PH, Kao CF, Chen PY, et al. Polymorphisms of INSIG2, MC4R, and LEP are associated with obesity- and metabolic-related traits in schizophrenic patients. J Clin Psychopharmacol. 2011;31:705–711. doi:10.1097/JCP.0b013e318234ee84
30. Barnard ND, Noble EP, Ritchie T, et al. D2 dopamine receptor Taq1A polymorphism, body weight, and dietary intake in type 2 diabetes. Nutrition. 2009;25(1):58–65. doi:10.1016/j.nut.2008.07.012
31. Lane HY, Liu YC, Huang CL, et al. Risperidone-related weight gain: genetic and nongenetic predictors. J Clin Psychopharmacol. 2006;26:128–134. doi:10.1097/01.jcp.0000203196.65710.2b
32. Kroeze WK, Hufeisen SJ, Popadak BA, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28:519–526. doi:10.1038/sj.npp.1300027
33. Leucht S, Samara M, Heres S, et al. Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr Bull. 2015;41:1397–1402. doi:10.1093/schbul/sbv037
34. Montastruc F, Palmaro A, Bagheri H, et al. Role of serotonin 5-HT2C and histamine H1receptors in antipsychotic-induced diabetes: a pharmacoepidemiological-pharmacodynamic study in VigiBase. Eur Neuropsychopharmacol. 2005;25:1556–1565. doi:10.1016/j.euroneuro.2015.07.010
35. Boumaiza I, Omezzine A, Rejeb J, et al. Relationship between leptin G2548A and leptin receptor Q223R gene polymorphisms and obesity and metabolic syndrome risk in Tunisian volunteers. Genet Test Mol Biomarkers. 2012;16:726–733. doi:10.1089/gtmb.2011.0324
36. Hong CJ, Liou YJ, Bai YM, et al. Dopamine receptor D2 gene is associated with weight gain in schizophrenic patients under long-term atypical antipsychotic treatment. Pharmacogenet Genomics. 2010;20:359–366. doi:10.1097/FPC.0b013e3283397d06
37. Gunes A, Melkersson KI, Scordo MG, et al. Association between HTR2C and HTR2A polymorphisms and metabolic abnormalities in patients treated with olanzapine or clozapine. J Clin Psychopharmacol. 2009;29:65–68. doi:10.1097/JCP.0b013e31819302c3
38. Hill MJ, Reynolds GP. Functional consequences of two HTR2C polymorphisms associated withantipsychotic-induced weight gain. Pharmacogenomics. 2011;5:727–734. doi:10.2217/pgs.11.16
39. Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005;2:411–420. doi:10.1016/j.cmet.2005.10.009
40. Srisawasdi P, Vanwong N, Hongkaew Y, et al. Impact of risperidone on leptin and insulin in children and adolescents with autistic spectrum disorders. Clin Biochem. 2017;50:678–685. doi:10.1016/j.clinbiochem.2017.02.003
41. Sukasem C, Vanwong N, Srisawasdi P, et al. Pharmacogenetics of risperidone induced insulin resistance in children and adolescents with autism spectrum disorder. Basic Clin Pharmacol Toxicol. 2018;12:42–50. doi:10.1111/bcpt.12970
42. Cheng L, Yu Y, Szabo A, et al. Palmitic acid induces central leptin resistance and impairs hepatic glucose and lipid metabolism in male mice. J Nutr Biochem. 2015;26:541–548. doi:10.1016/j.jnutbio.2014.12.011
43. Cheng L, Yu Y, Zhang Q, et al. Arachidonic acid impairs hypothalamic leptin signaling and hepatic energy homeostasis in mice. Mol Cell Endocrinol. 2015;412:12–18. doi:10.1016/j.mce.2015.04.025
44. Xuan J, Pan G, Qiu Y, et al. Metabolomic profiling to identify potential serum biomarkers for schizophrenia and risperidone action. J Proteome Res. 2011;10:5433–5443. doi:10.1021/pr2006796
45. Piao L, Park J, Li Y, et al. SOCS3 and SOCS6 are required for the risperidone-mediated inhibition of insulin and leptin signaling in neuroblastoma cells. Int J Mol Med. 2014;33:1364–1370. doi:10.3892/ijmm.2014.1693
46. Le Stunff C, Le Bihan C, Schork NJ, et al. A common promoter variant of the leptin gene is associated with changes in the relationship between serum leptin and fat mass in obese girls. Diabetes. 2000;49:2196–2200. doi:10.2337/diabetes.49.12.2196
47. Shen J, Ge W, Zhang J, et al. Leptin −2548G/A gene polymorphism in association with antipsychotic-induced weight gain: a meta-analysis study. Psychiatr Danub. 2014;26:145–151.
48. Brandl EJ, Frydrychowicz C, Tiwari AK, et al. Association study of polymorphisms in leptin and leptin receptor genes with antipsychotic induced body weight gain. Prog. Neuropsychopharmacol. Biol Psychiatry. 2012;38:134–141.
49. Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247–E1259. doi:10.1152/ajpendo.00274.2009
50. Moon HS, Dalamaga M, Kim SY, et al. Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev. 2013;34:377–412. doi:10.1210/er.2012-1053
51. Hoffstedt J, Eriksson P, Mottagui-Tabar S, et al. A polymorphism in the leptin promoter region (−2548 G/A) influences gene expression and adipose tissue secretion of leptin. Horm Metab Res. 2002;34:355–359. doi:10.1055/s-2002-33466
52. Calapai G, Corica F, Corsonello A, et al. Leptin increases serotonin turnover by inhibition of brain nitric oxide synthesis. J Clin Invest. 1999;104:975–982. doi:10.1172/JCI5867
53. von Meyenburg C, Langhans W, Hrupka BJ. Evidence for a role of the 5-HT2C receptor in central lipopolysaccharide-, interleukin-1 beta-, and leptin-induced anorexia. Pharmacol Biochem Behav. 2003;74:1025–1031. doi:10.1016/S0091-3057(03)00030-3
54. Klemettilä JP, Kampman O, Seppälä N, et al. Association study of the HTR2C, leptin and adiponectin genes and serum marker analyses in clozapine treated long-term patients with schizophrenia. Psychiatry. 2015;30:296–302.
55. Gregoor JG, Mulder H, Cohen D, et al. Combined HTR2C-LEP genotype as a determinant of obesity in patients using antipsychotic medication. J Clin Psychopharmacol. 2010;30:702–705. doi:10.1097/JCP.0b013e3181fa05a2
56. Correll CU, Lencz T, Malhotra AK. Antipsychotic drugs and obesity. Trends Mol Med. 2011;17:97–107. doi:10.1016/j.molmed.2010.10.010
57. Zhou L, Sutton GM, Rochford JJ, et al. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab. 2007;6:398−405. doi:10.1016/j.cmet.2007.10.008
58. Kang SH, Lee JI, Chang AK, et al. Genetic polymorphisms in the HTR2C and peroxisome proliferator-activated receptors are not associated with metabolic syndrome in patients with schizophrenia taking clozapine. Psychiatry Investig. 2011;8:262–268. doi:10.4306/pi.2011.8.3.262
59. Manu P, Correll CU, Van Winkel R, et al. Prediabetes in patients treated with antipsychotic drugs. J Clin Psychiatry. 2012;73:460–466. doi:10.4088/JCP.10m06822
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
