Back to Journals » Clinical Interventions in Aging » Volume 17
Association Between Large Numbers of Enlarged Perivascular Spaces in Basal Ganglia and Motor Performance in Elderly Individuals: A Cross-Sectional Study
Authors Yang S, Li X, Qin W , Yang L, Hu W
Received 3 March 2022
Accepted for publication 16 May 2022
Published 2 June 2022 Volume 2022:17 Pages 903—913
DOI https://doi.org/10.2147/CIA.S364794
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Maddalena Illario
Shuna Yang, Xuanting Li, Wei Qin, Lei Yang, Wenli Hu
Department of Neurology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Wenli Hu, Email [email protected]
Background and Objective: Motor dysfunction is common in the elderly, and is associated with adverse consequences. Enlarged perivascular spaces in basal ganglia (BG-EPVSs) are considered an MRI marker of cerebral small-vessel diseases. However, the consequences of BG-EPVSs are largely unknown. In the present study, we aimed to explore the association between large numbers of BG-EPVSs and motor performance.
Methods: We prospectively recruited elderly individuals in the Neurology Department of our hospital from December 1, 2020 to January 31, 2022. Participants with > 20 BG-EPVSs on the unilateral side of the slice containing the most EPVSs were classified as the BG-EPVS group (n=99) and the rest as controls (n=193). Motor performance was assessed by quantitative gait analysis, Tinetti test, timed up-and-go (TUG) test, and the Short Physical Performance Battery (SPPB). Spearman correlation analysis and multivariate linear regression analysis were performed to investigate the association between BG-EPVSs and motor performance.
Results: Compared with the control group, the BG-EPVS group had lower gait speed and cadence, shorter stride length, longer TUG duration, and lower Tinetti gait test, Tinetti balance test, and SPPB scores (P< 0.01). Spearman correlation analysis showed that BG-EPVSs were negatively related to gait speed, gait cadence, stride length, and Tinetti gait test, Tinetti balance test, and SPPB scores (ρ= – 0.539 to – 0.223, P< 0.001) and positively related to TUG duration (ρ=0.397, P< 0.001). Regression analysis indicated that BG-EPVSs were an independent risk factor of lower gait speed, shorter stride length, poor balance, and poor general physical performance after adjusting for confounders (β= – 0.313 to – 0.206, P< 0.01).
Conclusion: Large numbers of BG-EPVSs were independently related to poor gait, balance, and general physical performance in elderly individuals, which provides information about the consequences of BG-EPVSs and risk factors for motor dysfunction.
Keywords: cerebral small-vessel diseases, enlarged perivascular spaces, motor, gait, balance, physical performance
Introduction
Motor dysfunction is common in the elderly, and is related to adverse consequences, eg, falls, institutionalization, and death.1,2 There is important clinical significance in exploring the risk factors and mechanisms of motor dysfunction in elderly individuals. Normal motor function depends on the coordination of multiple brain regions.3,4 The basal ganglia (BG) are an important region related to the control of normal motor function, including gait, balance, and general physical performance.5
Perivascular spaces (PVSs), or Virchow–Robin spaces, are PV compartments surrounding small cerebral penetrating vessels serving as a protolymphatic system, and play an important role in interstitial fluid and solute clearance in brain.6 They dilate with the accumulation of interstitial fluids. Enlarged PVSs (EPVSs), visible on magnetic resonance imaging (MRI), appear as punctate or linear signal intensities similar to cerebrospinal fluid on all MRI sequences.7 EPVSs in BG (BG-EPVSs) are recognized as an MRI marker of cerebral small-vessel diseases (CSVDs).8,9
Many studies have demonstrated that CSVDs are associated with vascular dementia, depression, motor dysfunction, and increased risk of stroke.10–12 Some scholars have proposed that CSVDs deserve more attention for their relevance and impact on clinical practice.13 However, the consequences of BG-EPVSs are largely unknown. Several BG-EPVSs are generally considered clinically silent. Recent studies have shown a relationship between EPVSs and cognitive disturbances;14,15 but others have found EPVSs not to be related to cognitive impairment.16 Therefore, the association between EPVSs and cognitive impairment is attributed to a direct effect of EPVSs or accompanying CSVD markers, eg, white-matter hyperintensity (WMH), cerebral microbleeds (CMBs), and lacunae, and remains incompletely understood.
Studies have shown that WMH, CMBs, and lacunae are related to physical performance17 and gait disturbances.10,18,19 However, the relationship between BG-EPVSs and motor function is unclear. We speculated that a large number of BG-EPVSs might be associated with motor disturbances via disrupting the function of the BG. Therefore, we explored the association between large numbers of BG-EPVSs and motor performance in elderly individuals.
Methods
Figure 1 presents a flowchart of the research process.
 |
Figure 1 Flowchart of study process. |
Participants and Grouping
We prospectively reviewed medical records of elderly patients with CSVDs without acute cerebral infarction in the Neurology Department of Beijing Chaoyang Hospital affiliated with Capital Medical University from December 1, 2020 to January 31, 2022. CSVDs were defined as the presence of WMH and/or CMBs and/or lacunae of presumed vascular origin on brain MRI. Inclusion criteria were age 60 years or above and agreement to participate.
Exclusion criteria were: dementia, including Alzheimer’s disease, frontotemporal dementia, or dementia with Lewy bodies; Parkinson’s disease (PD), PD-plus syndrome, including multiple-system atrophy, corticobasal degeneration, and progressive supranuclear palsy; a history of severe stroke (largest lesion diameter >20 mm) due to difficulties and inaccurate assessments on CSVD MRI markers, or large-vessel cerebrovascular diseases, defined as internal carotid, middle cerebral, or basilar intracranial artery stenosis >50%; lacunar syndrome within 6 months after the event to avoid acute effects on outcomes, or patients with stroke sequelae 6 months after cerebral infarction onset; traumatic, toxic, or infectious brain injury, brain tumor, or brain metastases, non-CSVD–rated white-matter lesions, eg, multiple sclerosis and irradiation-induced gliosis; inability to walk for 30 m unaided; inability finish the tests because of prominent visual, hearing, language impairment, or psychiatric disease; conditions not related to CSVDs affecting motor function (eg, diabetic peripheral neuropathy, joint fusion, severe arthritis, joint replacement, or lumbar spondylopathy); heart failure, myocardial infarction, or angina pectoris disorders during the previous 3 months, severe nephrosis/liver diseases, and life expectancy <6 months; MRI contraindications/known claustrophobia/poor imaging quality affecting CSVD assessments because the patient’s head moved when performing MRI; and any problem in acquiring quantitative gait data.
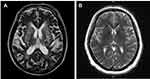
All enrolled patients were divided into two groups based on the number of BG-EPVSs on axial T2-weighted images. Patients with >20 BG-EPVSs on the unilateral side of the slice containing the most EPVSs comprised the BG-EPVS group (Figure 2A) and the rest were controls (Figure 2B). The cutoff of 20 BG-EPVSs has been used to represent “high grade” in previous studies.20
Demographic and Clinical Assessments
Age, sex, BMI, and history of hypertension, diabetes, hyperlipidemia, stroke, smoking, and alcohol consumption were collected. All blood samples were collected in the morning and sent to the clinical laboratory of our hospital for measurement of serum indices. Laboratory tests included total cholesterol (TC), triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), HbA1c, blood urea nitrogen (BUN), creatinine, and homocysteine.
MRI Examinations and Assessment of EPVSs, WMH, CMBs, and Lacunae
MRI was performed on a 3 T MRI scanner (Prisma; Siemens, Erlangen, Germany) in the Radiology Department of our hospital. The standardized MRI sequences used were axial T1-weighted, T2-weighted, and diffusion-weighted imaging, fluid-attenuated inversion recovery, and susceptibility-weighted imaging. Imaging markers of CSVDs, ie, EPVSs, WMH, CMBs, and lacunae, were defined as per the Standards for Reporting Vascular Changes on Neuroimaging criteria.8 BG-EPVSs were counted in the slice containing the most EPVSs. Periventricular and deep WMH were evaluated separately and combined as Fazekas scores. A detailed description of the Fazekas scale has been published.21 CMBs and lacunae were counted.
Assessments of EPVSs, WMH, CMBs, and lacunae were performed by two experienced neurologists blinded to clinical data. Random scans of 50 individuals were independently examined by the two neurologists blinded to each other’s readings. Intrarater agreement for EPVSs, WMH, CMBs, and lacunae was assessed on a random sample of 50 individuals with a month’s interval between the first and second readings. Intrarater and interrater agreement had values of κ=0.80–0.98, indicating good reliability. Disagreement was resolved through discussion with the other authors.
Motor-Function Assessments
Motor-function assessments covered gait, balance, and general physical performance based on quantitative gait analysis, timed up-and-go (TUG) test and clinical scales consisting of the Tinetti test and Short Physical Performance Battery (SPPB). Quantitative gait parameters were acquired by a MiniSun IDEEA, which has an excellent test–retest reliability and validity.22,23 The IDEEA is equipped with Windows-based software that analyzes gait data and provides parameters of gait speed, gait cadence (number of steps per minute), and stride length (distance between the heel points of two consecutive footprints from the same foot). The participants were instructed to walk 8 m wearing the device at their usual gait speed in low-heeled shoes before the formal test. Then, the formal 8 m test was performed.
The TUG test was performed and the duration recorded using a 3 m walk. The Tinetti test contains 17 items — nine for balance (score 0–16) and eight for gait (score 0–12) — with a maximum score of 28.24 The Tinetti test was independently scored by the two neurologists blinded to clinical data, and then the scores were averaged. Interrater reliability was good (κ=0.9). General physical performance was measured with the SPPB. This scale consists of three measurements of gait speed (score 0–4), standing balance (score 0–4), and chair stand (score 0–4). The gait speed subscale uses a 4 m walk. Total SPPB scores range from 0 to 12 points. Measurement and scoring methods of the SPPB have been described previously.17
Statistical Analysis
Continuous variables are presented as means ± SD or medians (interquartile range) according to conformation with normal distribution. Categorical variables are presented as numbers and percentages. Continuous variables with both normal distribution and homogeneity of variance were compared with Student’s t test, and otherwise with Wilcoxon’s rank-sum test. For comparison of categorical variables, χ2 tests were used. Spearman correlation analysis was performed to analyze correlations between large numbers of BG-EPVSs and motor function. Partial correlation analysis and multivariate linear regression analysis were performed to analyze if BG-EPVSs were independently related to motor function after adjusting for confounding factors. Analysis was performed with SPSS 21.0, and statistical significance was accepted at P<0.05.
Results
Participants’ General Clinical Characteristics
During the study period, 442 elderly patients with CSVDs and without acute cerebral infarction were identified. In sum, 52 patients were excluded because of a history of severe stroke and large-vessel diseases, 16 because of stroke sequelae, seven because of a diagnosis of PD. Another 40 were excluded because they had severe arthritis, joint fusion, lumbar spondylopathy, or visual impairment affecting walking, four because of poor imaging quality not clear enough to assess CSVD markers and 28 patients did not agree to participate. Three patients were excluded because of problems in acquiring quantitative gait parameters. Finally, 292 elderly patients were enrolled and finished the tests, of which 99 were classified into BG-EPVS group and 193 served as controls according to the number of BG-EPVSs.
The general clinical characteristics of all participants are presented in Table 1. Mean age was 72±7.7 years, and 171 (58.56%) were men. The BG-EPVS group was older than the controls (75±8 vs 70±7, P<0.001). Male sex, hypertension, diabetes, and stroke in the BG-EPVS group were more predominant than the control group (P<0.05). The BG-EPVS group had higher levels of BUN (6.2±1.86 vs 5.8±2.00, P=0.047), creatinine (76±23.1 vs 68±20.9, P=0.010), and homocysteine (16±6.40 vs 13±5.63, P=0.004). There were no statistical differences in BMI, hyperlipidemia, smoking, alcohol consumption, TC, triglycerides, HDL, LDL, or HbA1c between the groups. In terms of other CSVD markers, the BG-EPVS group had more serious WMH and more lacunae and CMBs (P<0.001).
 |
Table 1 General clinical characteristics of participants |
Association Between Motor Function and BG-EPVSs
Table 2 presents the motor performance of the groups. The BG-EPVS group had lower gait speed and cadence, shorter stride length (Figure 3), and longer TUG duration. Tinetti gait test and balance test scores were lower in the BG-EPVS group (P<0.05). Gait speed, standing balance, chair stand, and total scores on the SPPB were lower in BG-EPVS group (P<0.05). Spearman correlation analysis showed that large numbers of BG-EPVSs were negatively related to gait speed, gait cadence, and stride length and positively related to TUG duration (Table 3). Large numbers of BG-EPVSs were negatively related to Tinetti gait test and balance test scores and SPPB gait speed, standing balance, chair stand, and total scores. We further conducted partial correlation analysis to adjust for confounding factors of general clinical characteristics (age, sex, history of hypertension, diabetes, stroke, BUN, creatinine, and homocysteine) and other CSVD markers (WMH, lacunae, and CMBs). The negative correlation between large numbers of BG-EPVSs and gait speed, stride length, balance, and general physical performance did not change after adjustment (Table 3). Large numbers of BG-EPVSs were not independently related to TUG duration or gait cadence after adjusting for confounding factors.
 |
Table 2 Motor performance of BG-EPVS and control groups |
 |
Table 3 Correlations between motor function and BG-EPVSs |
 |
Figure 3 Violin plot of quantitative gait parameters. (A–C) Gait speed, gait cadence, and stride length of BG-EPVS group and control group, respectively. |
To identify the effect of BG-EPVSs on motor function, we took the gait parameters, Tinetti test, and SPPB scores as dependent variables and conducted multiple linear regression analysis. This showed that large numbers of BG-EPVSs were an independent risk factor of lower gait speed, shorter stride length, poor balance, and poor general physical performance after adjusting for age, sex, history of hypertension, diabetes, and stroke, BUN, creatinine, homocysteine, Fazekas score, and number of lacunae and CMBs. Detailed results are presented in Table 4.
 |
Table 4 Multiple linear regression analysis of motor function and BG-EPVSs |
Discussion
In the present study, we explored the association between large numbers of BG-EPVSs and motor function in elderly individuals with CSVDs. Gait, balance, and general physical performance were covered in motor-function assessments. We found that large numbers of BG-EPVSs were independently related to lower gait speed and shorter stride length after adjusting for other CSVD markers (WMH, CMBs, and lacunae). We also found that large numbers of BG-EPVSs were independently related to poor balance and general physical performance.
Previous studies have investigated the relationship between motor function and other CSVD MRI markers (WMH, lacunar infarction, and CMBs).11 The LADIS study adopted SPPB, single-leg stance time, and walking speed to assess motor function, and found that SPPB total score, single- leg stance time, and walking speed decreased with increased WMH and that frontal deep WMH was significantly associated with balance disturbances.25 The RUN DMC study found that WMH and lacunar infarcts were both independently associated with most gait parameters and that stride length was the most sensitive parameter related to WMH.24 WMH in BG and lacunar infarcts in the thalamus were related to lower velocity. The study also found a higher number of CMBs in the BG was independently related to shorter stride length and poorer performance on Tinetti tests.26 However, to the best of our knowledge, clinical studies specifically addressing the association between EPVSs and gait in CSVD patients are sparse.
Shin et al27 explored the relationship between BG-EPVSs, EPVSs in white matter (WM-EPVSs), and motor function in patients with PD. They found that severe BG-EPVSs were associated with worse motor symptoms. Chung et al28 investigated the association between BG-EPVSs and long-term motor outcomes in patients with PD. They found that the PD-EPVS+ group exhibited more severely decreased dopamine transporters, and the risk of freezing gait was higher in the PD-EPVS+ group. The PD-EPVS+ group required higher doses of dopaminergic medications for effective symptom control than the PD-EPVS– group. In the present study, we explored the relationship between BG-EPVSs and motor performance in elderly CSVD patients. We also found that severe BG-EPVSs were independently related to worse gait performance and balance, similar to the results of previous studies. Sun et al29 investigated the correlation between CSVD burden and motor performance in community-dwelling populations. They found that EPVSs were not associated with motor performance, different from our results. They classified patients with more than five BG-EPVSs on the BG slice containing the most EPVSs into the EPVS group. The different results might be attributed to different grouping methods and study populations.
Clinical experience and other studies have indicated that moderate or severe CSVDs can lead to clinical symptoms.24,30 Mild CSVDs are generally considered clinically silent. For example, gait disturbances are only attributed to moderate or severe WMH and/or the presence of more than three lacunar infarcts.24 Therefore, in the present study we classified subjects with high-degree BG-EPVSs into the BG-EPVS group and the rest into control group. As we all know, subcortical WM is another common region of EPVSs, but the anatomical structure, mechanisms, and risk factors of BG-EPVSs and WM-EPVSs are different.6,9 WM-EPVSs are related to cerebral amyloid angiopathy, whereas BG-EPVSs are related to hypertensive arteriopathy and recognized as an MRI marker of CSVDs. Additionally, BG play an important role in motor-function control. Therefore, in the present study we explored the relationship between BG-EPVSs and motor function. The consequences of large WM-EPVSs should be explored in the future.
Gait velocity is determined by both stride length and gait cadence. In the present study, gait velocity, stride length, and gait cadence were reduced in patients with large numbers of BG-EPVSs. However, BG-EPVSs were significantly related only to gait velocity and stride length and not to cadence after adjusting for WMH, lacunae, and CMBs. Therefore, we think that stride length may be a more sensitive indicator than gait cadence related to BG-EPVSs. Apart from the lower velocity, we found that patients with large numbers of BG-EPVSs were characterized by lower Tinetti balance and SPPB scores, which indicated large numbers of BG-EPVSs were related to poor balance and general physical performance.
The potential pathophysiological mechanisms underlying the association between large numbers of BG-EPVSs and motor function are not completely understood. Motor, balance, and gait abilities are based on the integrity of the complex motor-circuit network that connects the BG and brain-stem structures with the frontal cortex. This network’s function may be challenged by a critical number of lesions in subcortical regions.25 PVSs are thought to serve as a protolymphatic system and play an important role in maintaining neural homeostasis.6 Sulcal cerebrospinal fluid is cleared through arachnoid granulations or enters the parenchyma via the PVSs, where it combines with interstitial fluid prior to exiting the brain. This PV drainage system also allows for the clearance of toxic metabolites within the parenchyma and plays a role in the brain’s immunological response.31 EPVSs are thought to be the result of PV blockage and disrupt the normal function of PVSs. Although a few EPVSs visible on MRI can be normal, the presence of many is not normal, and they have been shown to be associated with some age-related disorders, including cognitive dysfunction, Parkinson's syndrome, WMH, and lacunar infarction.6,7,32 Studies have found that the BG–thalamocortical circuit, brain-network efficiency, and loss of WM-microstructure integrity are involved in gait impairment in subjects with CSVDs.33,34 Recent findings showed that EPVSs lead to WM-microstructure damage by causing lymphatic circulation dysfunction. The glymphatic aberration results in the accumulation of toxic metabolic products that are harmful to the brain microenvironment.35 Therefore, we speculate that large numbers of BG-EPVSs might disrupt the functioning of the BG, brain network, and brain microenvironment, then lead to poor motor performance. This hypothesis should be tested and verified by multimodal MRI (eg, functional MRI and diffusion-tensor imaging) in the future.
Some limitations in the present study must be mentioned. Firstly, it was performed in a single center and the cohort may not represent the general population. Secondly, this was an observational study, and causal relationships between motor dysfunction and BG-EPVSs could not be established. Thirdly, the mechanisms underlying the association between BG-EPVSs and motor function were not explored. A multicenter prospective cohort study should be performed in the future with multimodal MRI being used to explore the mechanisms. Fourthly, we did not include brain-atrophy assessments, which are recognized as another potentially relevant manifestation of CSVDs (although still poorly characterized) and related to motor dysfunction in elderly individuals.36,37 Despite these limitations, this study is the first to find that large numbers of BG-EPVSs were associated with poor gait, balance, and general physical performance in elderly individuals with CSVDs by both quantitative and semiquantitative measures. These novel findings provide some information about the consequences of large numbers of BG-EPVSs and risk of motor disturbances in elderly people.
In addition, we would like to mention that the relationship between large numbers of BG-EPVSs and cognitive impairment should also be explored in elderly silent CSVD patients in the future. Many studies have demonstrated that other CSVD markers (WMH, CMBs, and lacunae) are related to cognitive impairment.10 Grau-Olivares el al found up to 57.8% of first acute lacunar stroke patients had mild neuropsychological disorders.38 The incidence of cognitive impairment in patients with large numbers of BG-EPVSs is not clear, research on the relationship between large numbers of BG-EPVSs and cognitive impairment insufficient, and results have not been consistent.14,16
Conclusion
We found that large numbers of BG-EPVSs were independently related to gait disturbances, poor balance, and general physical performance. Patients with large numbers of BG-EPVSs had lower gait speed and shorter stride length. Causal relationships and mechanisms should be further tested and explored by longitudinal studies in the future.
Data Sharing
Data that support the findings of this study are available from the corresponding author on reasonable request.
Ethical Standards
This study was approved by the Ethics Committee of Beijing Chaoyang Hospital affiliated with Capital Medical University and was conducted in accordance with the Declaration of Helsinki (ethics approval 2020-216). All participants provided informed written consent.
Acknowledgment
We would like to thank all participants included in the study.
Funding
This work is supported by the National Natural Science Foundation of China (grant 82001244). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors declare that they have no competing interests for this work, the materials or methods used, or the findings specified in this paper.
References
1. Verghese J, LeValley A, Hall CB, Katz MJ, Ambrose AF, Lipton RB. Epidemiology of gait disorders in community-residing older adults. J Am Geriatr Soc. 2006;54(2):255–261. doi:10.1111/j.1532-5415.2005.00580.x
2. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–889. doi:10.1007/s12603-009-0246-z
3. Kim YJ, Kwon HK, Lee JM, et al. Gray and white matter changes linking cerebral small vessel disease to gait disturbances. Neurology. 2016;86(13):1199–1207. doi:10.1212/WNL.0000000000002516
4. de Laat KF, Reid AT, Grim DC, et al. Cortical thickness is associated with gait disturbances in cerebral small vessel disease. Neuroimage. 2012;59(2):1478–1484. doi:10.1016/j.neuroimage.2011.08.005
5. MacKinnon CD. Sensorimotor anatomy of gait, balance, and falls. Handb Clin Neurol. 2018;159:3–26.
6. Gouveia-Freitas K, Bastos-Leite AJ. Perivascular spaces and brain waste clearance systems: relevance for neurodegenerative and cerebrovascular pathology. Neuroradiology. 2021;63(10):1581–1597. doi:10.1007/s00234-021-02718-7
7. Rudie JD, Rauschecker AM, Nabavizadeh SA, Mohan S. Neuroimaging of dilated perivascular spaces: from benign and pathologic causes to mimics. J Neuroimaging. 2018;28(2):139–149. doi:10.1111/jon.12493
8. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. doi:10.1016/S1474-4422(13)70124-8
9. Wang ML, Yu MM, Wei XE, Li WB, Li YH. Association of enlarged perivascular spaces with Aβ and tau deposition in cognitively normal older population. Neurobiol Aging. 2021;100:32–38. doi:10.1016/j.neurobiolaging.2020.12.014
10. Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: a clinical review. Neurology. 2019;92(24):1146–1156. doi:10.1212/WNL.0000000000007654
11. Litak J, Mazurek M, Kulesza B, et al. Cerebral small vessel disease. Int J Mol Sci. 2020;21(24):24. doi:10.3390/ijms21249729
12. Moretti R, Caruso P. Small vessel disease-related dementia: an invalid neurovascular coupling? Int J Mol Sci. 2020;21(3):1095. doi:10.3390/ijms21031095
13. Caruso P, Signori R, Moretti R. Small vessel disease to subcortical dementia: a dynamic model, which interfaces aging, cholinergic dysregulation and the neurovascular unit. Vasc Health Risk Manag. 2019;15:259–281. doi:10.2147/VHRM.S190470
14. Jie W, Lin G, Liu Z, et al. The relationship between enlarged perivascular spaces and cognitive function: a meta-analysis of observational studies. Front Pharmacol. 2020;11:715. doi:10.3389/fphar.2020.00715
15. Zhu YC, Dufouil C, Soumaré A, Mazoyer B, Chabriat H, Tzourio C. High degree of dilated Virchow-Robin spaces on MRI is associated with increased risk of dementia. J Alzheimers Dis. 2010;22(2):663–672. doi:10.3233/JAD-2010-100378
16. Gertje EC, van Westen D, Panizo C, Mattsson-Carlgren N, Hansson O. Association of enlarged perivascular spaces and measures of small vessel and Alzheimer disease. Neurology. 2021;96(2):e193–e202. doi:10.1212/WNL.0000000000011046
17. Verwer JH, Biessels GJ, Heinen R, Exalto LG, Emmelot-Vonk MH, Koek HL. Occurrence of impaired physical performance in memory clinic patients with cerebral small vessel disease. Alzheimer Dis Assoc Disord. 2018;32(3):214–219. doi:10.1097/WAD.0000000000000233
18. van der Holst HM, Tuladhar AM, Zerbi V, et al. White matter changes and gait decline in cerebral small vessel disease. Neuroimage Clin. 2018;17:731–738. doi:10.1016/j.nicl.2017.12.007
19. Stijntjes M, de Craen AJ, van der Grond J, Meskers CG, Slagboom PE, Maier AB. Cerebral microbleeds and lacunar infarcts are associated with walking speed independent of cognitive performance in middle-aged to older adults. Gerontology. 2016;62(5):500–507. doi:10.1159/000444583
20. Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010;41(3):450–454. doi:10.1161/STROKEAHA.109.564914
21. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–356. doi:10.2214/ajr.149.2.351
22. de la Cámara M, Higueras-Fresnillo S, Martinez-Gomez D, Veiga ÓL. Interday reliability of the IDEEA activity monitor for measuring movement and nonmovement behaviors in older adults. J Aging Phys Act. 2019;27(2):141–154. doi:10.1123/japa.2017-0365
23. Gorelick ML, Bizzini M, Maffiuletti NA, Munzinger JP, Munzinger U. Test-retest reliability of the IDEEA system in the quantification of step parameters during walking and stair climbing. Clin Physiol Funct Imaging. 2009;29(4):271–276. doi:10.1111/j.1475-097X.2009.00864.x
24. de Laat KF, van Norden AG, Gons RA, et al. Gait in elderly with cerebral small vessel disease. Stroke. 2010;41(8):1652–1658. doi:10.1161/STROKEAHA.110.583229
25. Poggesi A, Pantoni L, Inzitari D, et al. 2001–2011: a decade of the LADIS (Leukoaraiosis And DISability) Study: what have we learned about white matter changes and small-vessel disease? Cerebrovasc Dis. 2011;32(6):577–588. doi:10.1159/000334498
26. de Laat KF, van den Berg HA, van Norden AG, Gons RA, Olde Rikkert MG, de Leeuw FE. Microbleeds are independently related to gait disturbances in elderly individuals with cerebral small vessel disease. Stroke. 2011;42(2):494–497. doi:10.1161/STROKEAHA.110.596122
27. Shin NY, Park YW, Yoo SW, et al. Adverse effects of hypertension, supine hypertension, and perivascular space on cognition and motor function in PD. NPJ Parkinson's Dis. 2021;7(1):69. doi:10.1038/s41531-021-00214-6
28. Chung SJ, Yoo HS, Shin NY, et al. Perivascular spaces in the basal ganglia and long-term motor prognosis in newly diagnosed Parkinson disease. Neurology. 2021;96(16):e2121–e2131. doi:10.1212/WNL.0000000000011797
29. Su N, Zhai FF, Zhou LX, et al. Cerebral small vessel disease burden is associated with motor performance of lower and upper extremities in community-dwelling populations. Front Aging Neurosci. 2017;9:313. doi:10.3389/fnagi.2017.00313
30. Baezner H, Blahak C, Poggesi A, et al. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008;70(12):935–942. doi:10.1212/01.wnl.0000305959.46197.e6
31. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. doi:10.1126/scitranslmed.3003748
32. Rundek T, Shpiner DS, Margolesky J. Perivascular spaces in basal ganglia - An innocent bystander in Parkinson’s disease? Mov Disord. 2019;34(11):1585–1587. doi:10.1002/mds.27824
33. Cai M, Jacob MA, Norris DG, Duering M, de Leeuw FE, Tuladhar AM. Cognition mediates the relation between structural network efficiency and gait in small vessel disease. Neuroimage Clin. 2021;30:102667. doi:10.1016/j.nicl.2021.102667
34. de Laat KF, Tuladhar AM, van Norden AG, Norris DG, Zwiers MP, de Leeuw FE. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011;134(Pt 1):73–83. doi:10.1093/brain/awq343
35. Che Mohd Nassir CMN, Damodaran T, Yusof SR, et al. Aberrant neurogliovascular unit dynamics in cerebral small vessel disease: a rheological clue to vascular parkinsonism. Pharmaceutics. 2021;13(8):1207. doi:10.3390/pharmaceutics13081207
36. Smith EE, Arboix A. Focal cortical thinning is caused by remote subcortical infarcts: spooky action at a distance. Neurology. 2012;79(20):2016–2017. doi:10.1212/WNL.0b013e3182749f6e
37. Grau-Olivares M, Arboix A, Junqué C, Arenaza-Urquijo EM, Rovira M, Bartrés-Faz D. Progressive gray matter atrophy in lacunar patients with vascular mild cognitive impairment. Cerebrovasc Dis. 2010;30(2):157–166. doi:10.1159/000316059
38. Grau-Olivares M, Arboix A, Bartrés-Faz D, Junqué C. Neuropsychological abnormalities associated with lacunar infarction. J Neurol Sci. 2007;257(1–2):160–165. doi:10.1016/j.jns.2007.01.022
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.