Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
Association Between Daily Dietary Eicosatetraenoic Acid Intake and the Lower Risk of Psoriasis in American Adults
Authors Zhan J , Tang X, Wang F , Han J
Received 9 August 2021
Accepted for publication 30 September 2021
Published 23 October 2021 Volume 2021:14 Pages 1541—1549
DOI https://doi.org/10.2147/CCID.S333288
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Jipang Zhan, Xuhua Tang, Fang Wang, Jiande Han
Department of Dermatology, the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, 510080, People’s Republic of China
Correspondence: Jiande Han
Department of Dermatology, the First Affiliated Hospital of Sun Yat-sen University, No. 58 Zhongshan Second Road, Guangzhou, Guangdong, 510080, People’s Republic of China
Tel +86-138-0274-3924
Fax +86-020-8293-8840
Email [email protected]
Purpose: Unlike eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), the relationship between eicosatetraenoic acid (ETA) and psoriasis remains unclear. Therefore, We performed a cross-sectional study in the general American population to investigate the association between daily dietary ETA, EPA, and DHA intake and the risk of psoriasis.
Participants and Methods: This study applied data from the National Health and Nutrition Examination Survey (NHANES) 2003– 2006 and 2009– 2014. Dietary n3 polyunsaturated fatty acids (PUFA) were calculated based on two 24-hour dietary recall interviews. We defined psoriasis by responding to the question “Have you ever been told by a doctor or other health care professional that you had psoriasis?”. Multivariable logistic regression analysis, trend tests, subgroup analysis, and interaction tests were used to evaluate the associations of ETA, EPA, and DHA intake with the risk of psoriasis, respectively.
Results: A total of 15,733 participants were included in this study. In our optimal multivariate-adjusted model, the odds ratio (OR) with 95% confidence interval (CI) of psoriasis were 0.30 (0.12, 0.88), 1.92 (0.78, 4.74), 1.28 (0.72, 2.27) for daily dietary ETA, EPA, and DHA intake, respectively. Trend tests showed a dose–effect relationship between daily dietary ETA intake and the lower risk of psoriasis. Subgroup analysis and tests for interaction showed that the association was stable in different subgroups.
Conclusion: Our study revealed that there might be a dose–effect association of daily dietary ETA intake with the lower risk of psoriasis in American adults.
Keywords: psoriasis, diet, eicosatetraenoic acid, long-chain n3 polyunsaturated fatty acid, NHANES
Introduction
Psoriasis is a chronic inflammatory skin disease affecting about 2–3% of the world’s population.1 Psoriasis negatively affects patients’ quality of life; it may lead to impair appearance, itching, and other discomforts and could even cause social disability and depression.2 In general, psoriasis is considered a multifactorial disease involving genetic components, diet, lifestyle, and environmental factors.3 Patients with psoriasis often have higher dietary fat intake and less fish or dietary fiber intake, which may be related to the incidence and severity of psoriasis.4 In addition, the severity of psoriasis has a relation to lower levels of circulating n3 polyunsaturated fatty acids (n3 PUFA), and the course of psoriasis was associated positively with a higher ratio of saturated fatty acids to unsaturated fatty acids.5 These findings indicate that the risk and condition of psoriasis are strongly associated with the intake and metabolism of fatty acids. Therefore, a diet involving fatty acids is an essential part of the comprehensive management of psoriasis.
N3 polyunsaturated fatty acids (n3 PUFA) are essential fatty acids that cannot be synthesized de novo by humans and must be obtained from the diet. N3 PUFA is divided into short-chain (≤C18) n3 PUFA including α-linolenic acid (ALA, 18:3n-3) and stearidonic acid (SDA, 18:4n-3), and long-chain (≥C20) n3 PUFA including eicosapentaenoic acid (EPA, 20:5n-3); docosahexaenoic acid (DHA, 22:6n-3); and the less-studied eicosatetraenoic acid (ETA, 20:4n-3). Long-chain n3 PUFA are biosynthesized from ALA (Figure 1), and the metabolic pathway is limited in the human body.6 Therefore, the best way of acquiring these n3 PUFA is from dietary sources.7 The focus here is on long-chain n3 PUFA attributable to their wholesome effects in human disease. Numerous studies have consistently revealed their crucial roles in inhibiting chronic diseases, including inflammatory, carcinogenic, adipogenic, diabetogenic, and atherogenic diseases.8 Moreover, previous studies have also found that supplementation of n3 PUFA (rich in EPA and DHA) improves the condition of psoriasis,3,9–12 but other studies have not found similar results.13,14 More researches are needed to confirm the relationship between n3 PUFA intakes and psoriasis. Previous studies have focused mainly on EPA and DHA, but no ETA. Therefore, this study aimed to explore the relationship between three kinds of long-chain n3 PUFA (ETA, EPA, and DHA) intakes in the daily diet and the risk of psoriasis through a cross-sectional study with a large sample size.
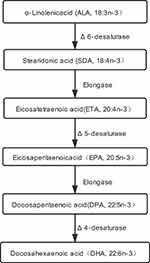 |
Figure 1 Possible biosynthesis and metabolic pathway of long-chain n3 PUFA. |
Materials and Methods
The NHANES study was approved by the institutional review board of the National Centre for Health Statistics, CDC, USA, and informed consent was obtained from participants prior to the research.
Study Population and Purpose
National Health and Nutrition Examination Survey (NHANES) is the annual population survey of the USA using a multistage, stratified, probability-sampling design. We analyzed publicly available data with 50,938 individuals from NHANES 2003–2006, 2009–2014 to determine the relationship between daily dietary n3 PUFA intakes and the risk of psoriasis in this study (there is no question on psoriasis in 2007–2008 survey). Individuals with responses to the psoriasis question and complete dietary data of the first-day and the second-day total nutrients, aged 18–60 years, were selectively selected. Finally, a total of 15,733 participants were included in the study (Figure 2).
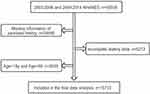 |
Figure 2 Flowchart of the screening process for the eligible participants selection from National Centre for Health Statistics (NHANES). |
Definition of Psoriasis
We identified the diagnosis of psoriasis from the 2003–2006 “dermatology” section and the 2009–2014 ‘medical conditions’ section of the questionnaire data. The presence of psoriasis was determined by the response to the question “Have you ever been told by a doctor or other health care professional that you had psoriasis?”.
Daily Dietary N3 Polyunsaturated Fatty Acid
The average daily dietary n3 PUFA intakes were calculated from two 24-hour dietary recall interviews of total nutrient intakes conducted personally in the mobile examination center and by phone 3 to 10 days later, respectively. In our study, n3 PUFA, including ETA, EPA, and DHA, was obtained from daily foods but not dietary supplements or medications.
Covariates
The following variables were included as potential confounders: Gender, age, marital status, BMI, veteran (Have you ever served on active duty in the US Armed Forces, military Reserves, or National Guard), the ratio of family income to poverty (PIR), smoking (Do you now smoke cigarettes), drinking alcohol (Drinking alcohol at least 12 times per year or not). All these covariates were obtained from demographics data and questionnaire data (Alcohol Use and Smoking - Cigarette/Tobacco Use - Adult).
Statistics
Continuous variables with normal distribution were described as mean ± standard deviation and compared using Student’s t-tests between psoriasis and non-psoriasis groups. Categorical variables were described as percentages and compared by the χ2 test between the two groups. Multivariable logistic regression analyses were adopted to determine the association between daily dietary n3 PUFA (ETA, EPA, and DHA) intakes and the risk of psoriasis while accounting for covariates. No covariates were adjusted in Model 1. Model 2 adjusted for gender, age, veteran, marital status, PIR. Model 3 adjusted for gender, age, veteran, marital status, PIR, BMI, drinking alcohol, and smoking.
Additional trend tests were performed to examine the dose–effect relationship between the daily dietary n3 PUFA intakes and psoriasis. Moreover, subgroup analyses and interaction tests were conducted to test the robustness of associations between the dietary n3 PUFA intake with psoriasis in different subgroups. The results are presented with an odds ratio (OR) with a 95% confidence interval (CI) and p-value. Two-tailed p <0.05 was considered statistically significant. R (http://www.R-project.org) and EmpowerStats (http://www.empowerstats.com) were used for all statistical analyses.
Results
A total of 15,733 participants were enrolled in this cross-sectional study, including 414 individuals with psoriasis and 15,319 without psoriasis as control. Compared with control, those with psoriasis tend to be elderly, married/widowed/divorced/separated, have larger BMI, and are more likely to be veterans and smokers. There were no significant differences between the two groups in gender, ratio of family income to poverty (PIR) and drinking alcohol. In addition, among the three kinds of n3 PUFA, those with psoriasis had less daily dietary ETA intake (Table 1).
 |
Table 1 Characteristics of Participants by Psoriasis |
In multivariable logistic regression analysis, only the association of ETA with the lower risk of psoriasis was observed, in which the odds ratio (OR) with 95% confidence interval (CI) of psoriasis based on daily dietary ETA intake was 0.36 (0.14, 0.92) in model 1 (Table 2). In model 2, after adjusting for gender, age, veteran, marital status, PIR, daily dietary ETA intake was still associated with the lower risk of psoriasis, in which the OR (95% CI) was 0.35 (0.13, 0.94). In model 3 adjusting for more covariates, the association was still identified with the OR (95% CI) of 0.30 (0.12, 0.88). However, no associations were not found between EPA/DHA and psoriasis from model 1 to model 3 (Table 2). Furthermore, the smooth curve fitting showed the risk of psoriasis decreased linearly with the increase in daily dietary ETA intake (Figure 3A).
 |
Table 2 Univariate and Multivariate Logistic Regression Model of Daily Dietary N3 Polyunsaturated Fatty Acids Intakes and Psoriasis |
The OR (95% CI) of psoriasis based on quintiles of daily dietary ETA intakes presented decreasing trend from the lowest to the highest. Compared with the lowest quintile, the OR (95%) of psoriasis for the highest quintile was 0.71 (0.52, 0.97) (Table 3). Besides, the p-value for trend was 0.031, and the results were stable in the further adjusted model 2 and model 3. The result further revealed the dose–effect association between daily dietary ETA intakes and psoriasis.
 |
Table 3 Trend Test of Association of Daily Dietary ETA Intakes with Psoriasis |
Moreover, subgroup analyses and interaction tests were conducted to test the robustness of associations of the dietary n3 PUFA intake with psoriasis in different subgroups. Tests of interaction between covariates and daily dietary ETA intake in all models were not significant (All p for interaction >0.05), concluding that the association between ETA and psoriasis was stable in different subgroups (Table 4). Additionally, we intuitively observed that the risk of psoriasis decreased linearly with the increase in daily dietary ETA intake within all subgroups (Figure 3B–I).
 |
Table 4 Interaction Test of Association of Daily Dietary ETA Intakes with Psoriasis |
Discussion
This study showed an association between daily dietary ETA intake and the lower risk of psoriasis, and the association was stable in different subgroups. However, there was no significant correlation between daily dietary EPA/DHA intakes and the risk of psoriasis in our study.
Arachidonic acid (AA, 20:4 n-6) metabolites have been considered to be related to psoriasis pathogenesis.10,15,16 Leukotriene B4 (LTB4) and 12-hydroxy eicosatetraenoic acid (12-HETE), the products of arachidonic acid (AA) catalyzed by lipoxygenase, are vital pro-inflammatory chemotactic mediators and higher in psoriatic plaques than in normal skin.17–19 Long-chain n3 PUFA, such as EPA and DHA, are also substrates of lipoxygenase and precursors of anti-inflammatory compounds such as resolvins, protectins, and maresins, which can reduce the production of LTB4.20–23 This lends biological plausibility to the use of long-chain n3 PUFA (EPA and DHA) alone or in combination with other drugs in the treatment of psoriasis. ETA is an isomer of n6 PUFA Arachidonic acid. ETA can serve as a dual inhibitor of cyclooxygenase (COX1 and COX2) and lipoxygenase to block the production of LTB4.24,25 ETA has also been shown to compete with arachidonic acid in the arachnoidal-CoA synthetase step, thereby preventing arachidonic acid uptake.26,27 In addition, Δ 17-8-HETE and Δ 17–8, 15-diHETE, the two metabolites of ETA via the 5-lipoxygenase pathway, have been proved to be natural LTB4 receptor antagonists with anti-inflammatory activity.28 Moreover, ETA can stimulate anti-inflammatory cytokines IL-10 secretion from macrophages in vitro.29 Therefore, ETA might reduce the inflammation of psoriasis or lower the risk of the disease.
We found a dose–effect relationship between daily dietary ETA intake and the lower risk of psoriasis, and this association was stable in different subgroups. To our acknowledgment, this has not been found in previous studies. Therefore, ETA supplements or foods rich in ETA may benefit people with a family history of psoriasis and psoriasis patients. ETA is a rare long-chain n-3 PUFA in nature, comprising only 1% of fish oils. ETA is also found in New Zealand green-lipped mussel (Perna canaliculus) and triglycerides of transgenic seeds from Camelina.30 If foods rich in ETA were not available, ETA supplements may be a better choice. Besides, it is reported that consuming plant-derived oils rich in stearidonic acid (SDA, 18:4 n-3) leads to increased tissue concentrations of ETA.28 Therefore, plant-derived oils rich in SDA may be potential alternative sources if both ETA supplements and foods rich in ETA were unavailable.
In this study, we did not find the relationship between daily dietary EPA/DHA intakes and the risk of psoriasis, which is consistent with some studies.2 In previous studies, the doses of EPA and DHA consumed by patients far exceeded that in their daily diet.10,31–33 Moreover, it is reported that there is no benefit of edible fish oil rich in EPA and DHA.14 However, intravenous infusion of large doses of n3 PUFA showed better effects than oral n3 PUFA or fish oil.34 Intravenous n3 PUFA has higher bioavailability and faster hydrolysis of esterified EPA and DHA than oral administration.34,35 Absorption of intravenous 4.2g n3 PUFA is likely to be twice or triple the same dose of the oral way.36 The doses of daily dietary EPA and DHA for 95% percentile participants in this study were 0.18g and 0.30g per day, respectively, far below that in previous studies. Therefore, the daily dietary EPA and DHA dose in our study may be too low to affect the risk of psoriasis, which might account for the absence of correlation between EPA/DHA and psoriasis in our study.
Therefore, supplement with ETA other than EPA and DHA may be more beneficial in the long-term dietary management of patients with psoriasis or those with high risks of developing psoriasis. However, we do not deny the potential effects of EPA and DHA on psoriasis but suggest that EPA and DHA may need higher doses or intravenous injections to reduce the risk and inflammation of psoriasis.
Our research has some limitations. First of all, it is a cross-sectional study, and no causality can be inferred from these results. Second, dietary data were obtained through two 24-hour dietary recall interviews, and this dietary survey method is not that accurate because of misrecording and omission,37 which may lead to inevitable recall bias. Besides, intakes of n3 PUFA from dietary supplements are not available in this study. Finally, the self-reported diagnosis of psoriasis may be subject to the same misclassification errors as other population-based epidemiological studies of psoriasis.1,38 All these limitations may affect the reliability of the conclusion. However, we have tried our best to increase the credibility and reliability of the conclusions in the study. We applied multiple adjustment models to analyze the relationship between n3 PUFA intake and the risk of psoriasis, trend tests to further detect the dose-effect, subgroup analyses, and interaction tests to examine the stability of the association in different subgroups, smooth curve fitting diagram to show the relationship between ETA and psoriasis visually.
Conclusion
This study showed a potential association between daily dietary ETA intake and the lower risk of psoriasis in American adults. However, there is no significant correlation between daily dietary EPA and DHA intakes and the risk of psoriasis. Based on these findings, we assumed that a supplement of ETA might be beneficial in patients with psoriasis and those with a high risk of developing psoriasis. Further prospective randomized controlled trials are needed to examine the efficacy of ETA on psoriasis.
Acknowledgments
We acknowledge the staff at the National Center for Health Statistics at the CDC, who design, collect, administer the NHANES data and release the data available for public use. We are thankful to all study participants for their cooperation.
Disclosure
All authors declare no conflicts of interest in this work.
References
1. Johnson JA, Ma C, Kanada KN, Armstrong AW. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28(3):327–332. doi:10.1111/jdv.12105
2. Sarkar R, Chugh S, Bansal S. General measures and quality of life issues in psoriasis. Indian Dermatol Online J. 2016;7(6):481–488. doi:10.4103/2229-5178.193908
3. Clark CCT, Taghizadeh M, Nahavandi M, Jafarnejad S. Efficacy of ω-3 supplementation in patients with psoriasis: a meta-analysis of randomized controlled trials. Clin Rheumatol. 2019;38(4):977–988. doi:10.1007/s10067-019-04456-x
4. Ricketts JR, Rothe MJ, Grant-Kels JM. Nutrition and psoriasis. Clin Dermatol. 2010;28(6):615–626. doi:10.1016/j.clindermatol.2010.03.027
5. Myśliwiec H, Baran A, Harasim-Symbor E, et al. Serum fatty acid profile in psoriasis and its comorbidity. Arch Dermatol Res. 2017;309(5):371–380. doi:10.1007/s00403-017-1748-x
6. Nguyen QV, Malau-Aduli BS, Cavalieri J, Nichols PD, Malau-Aduli AEO. Enhancing omega-3 long-chain polyunsaturated fatty acid content of dairy-derived foods for human consumption. Nutrients. 2019;11(4):743. doi:10.3390/nu11040743
7. Simopoulos AP. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8(3):128. doi:10.3390/nu8030128
8. Calder PC. Very long-chain n-3 fatty acids and human health: fact, fiction and the future. Proc Nutr Soc. 2018;77(1):52–72. doi:10.1017/s0029665117003950
9. Maurice PD, Allen BR, Barkley AS, Cockbill SR, Stammers J, Bather PC. The effects of dietary supplementation with fish oil in patients with psoriasis. Br J Dermatol. 1987;117(5):599–606. doi:10.1111/j.1365-2133.1987.tb07492.x
10. Grimminger F, Mayser P, Papavassilis C, et al. A double-blind, randomized, placebo-controlled trial of n-3 fatty acid based lipid infusion in acute, extended guttate psoriasis. Clin Investig. 1993;71(8):634–643. doi:10.1007/bf00184491
11. Tveit KS, Brokstad KA, Berge RK, et al. A Randomized, Double-blind, Placebo-controlled Clinical Study to Investigate the efficacy of Herring Roe Oil for treatment of Psoriasis. Acta Derm Venereol. 2020;100(10):adv00154. doi:10.2340/00015555-3507
12. Wolters M. Diet and psoriasis: experimental data and clinical evidence. Br J Dermatol. 2005;153(4):706–714. doi:10.1111/j.1365-2133.2005.06781.x
13. Upala S, Yong WC, Theparee T, Sanguankeo A. Effect of omega-3 fatty acids on disease severity in patients with psoriasis: a systematic review. Int J Rheum Dis. 2017;20(4):442–450. doi:10.1111/1756-185x.13051
14. Mayser P, Grimm H, Grimminger F. n-3 fatty acids in psoriasis. Br J Nutr. 2002;87(Suppl 1):S77–82. doi:10.1079/bjn2001459
15. Brain S, Camp R, Dowd P, Black AK, Greaves M. The release of leukotriene B4-like material in biologically active amounts from the lesional skin of patients with psoriasis. J Invest Dermatol. 1984;83(1):70–73. doi:10.1111/1523-1747.ep12261712
16. Kragballe K, Herlin T. Benoxaprofen improves psoriasis. A double-blind study. Arch Dermatol. 1983;119(7):548–552. doi:10.1001/archderm.1983.01650310010002
17. Gupta AK, Ellis CN, Tellner DC, Anderson TF, Voorhees JJ. Double-blind, placebo-controlled study to evaluate the efficacy of fish oil and low-dose UVB in the treatment of psoriasis. Br J Dermatol. 1989;120(6):801–807. doi:10.1111/j.1365-2133.1989.tb01378.x
18. Wan M, Tang X, Stsiapanava A, Haeggström JZ. Biosynthesis of leukotriene B4. Semin Immunol. 2017;33:3–15. doi:10.1016/j.smim.2017.07.012
19. Veale DJ, Torley HI, Richards IM, et al. A double-blind placebo controlled trial of Efamol Marine on skin and joint symptoms of psoriatic arthritis. Br J Rheumatol. 1994;33(10):954–958. doi:10.1093/rheumatology/33.10.954
20. Fogh K, Herlin T, Kragballe K. Eicosanoids in acute and chronic psoriatic lesions: leukotriene B4, but not 12-hydroxy-eicosatetraenoic acid, is present in biologically active amounts in acute guttate lesions. J Invest Dermatol. 1989;92(6):837–841. doi:10.1111/1523-1747.ep12696858
21. Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem. 2012;58(10):1476–1484. doi:10.1373/clinchem.2012.190199
22. Dalli J, Colas RA, Serhan CN. Novel n-3 immunoresolvents: structures and actions. Sci Rep. 2013;3:1940. doi:10.1038/srep01940
23. Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111(10):5922–5943. doi:10.1021/cr100396c
24. Grienke U, Silke J, Tasdemir D. Bioactive compounds from marine mussels and their effects on human health. Food Chem. 2014;142:48–60. doi:10.1016/j.foodchem.2013.07.027
25. McPhee S, Hodges LD, Wright PFA, et al. Anti-cyclooxygenase effects of lipid extracts from the New Zealand green-lipped mussel, Perna canaliculus. Comp Biochem Physiol B Biochem Mol Biol. 2007;146(3):346–356. doi:10.1016/j.cbpb.2006.11.001
26. Treschow AP, Hodges LD, Wright PFA, Wynne PM, Kalafatis N, Macrides TA. Novel anti-inflammatory omega-3 PUFAs from the New Zealand green-lipped mussel, Perna canaliculus. Comp Biochem Physiol B Biochem Mol Biol. 2007;147(4):645–656. doi:10.1016/j.cbpb.2007.04.004
27. Wilson DB, Prescott SM, Majerus PW. Discovery of an arachidonoyl coenzyme A synthetase in human platelets. J Biol Chem. 1982;257(7):3510–3515. doi:10.1016/S0021-9258(18)34808-7
28. Gagnon KJ, Lefort N, Poirier SJ, Barnett DA, Surette ME. 5-lipoxygenase-dependent biosynthesis of novel 20:4 n-3 metabolites with anti-inflammatory activity. Prostaglandins Leukot Essent Fatty Acids. 2018;138:38–44. doi:10.1016/j.plefa.2018.10.005
29. Lefort N, LeBlanc R, Surette ME. Dietary buglossoides arvensis oil increases circulating n-3 polyunsaturated fatty acids in a dose-dependent manner and enhances lipopolysaccharide-stimulated whole blood interleukin-10-a randomized placebo-controlled trial. Nutrients. 2017;9(3):E261. doi:10.3390/nu9030261
30. Poole LB, Parsonage D, Sergeant S, et al. Acyl-lipid desaturases and Vipp1 cooperate in cyanobacteria to produce novel omega-3 PUFA-containing glycolipids. Biotechnol Biofuels. 2020;13:83. doi:10.1186/s13068-020-01719-7
31. Guida B, Napoleone A, Trio R, et al. Energy-restricted, n-3 polyunsaturated fatty acids-rich diet improves the clinical response to immuno-modulating drugs in obese patients with plaque-type psoriasis: a randomized control clinical trial. Clin Nutr. 2014;33(3):399–405. doi:10.1016/j.clnu.2013.09.010
32. Balbás GM, Regaña MS, Millet PU. Study on the use of omega-3 fatty acids as a therapeutic supplement in treatment of psoriasis. Clin Cosmet Investig Dermatol. 2011;4:73–77. doi:10.2147/ccid.s17220
33. Bittiner SB, Cartwright I, Tucker WFG, Bleehen SS. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;331(8582):378–380. doi:10.1016/s0140-6736(88)91181-6
34. Mayser P, Mrowietz U, Arenberger P, et al. Omega-3 fatty acid-based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38(4):539–547. doi:10.1016/s0190-9622(98)70114-8
35. Yamazaki K, Hamazaki T, Yano S, Funada T, Ibuki F. Changes in fatty acid composition in rat blood and organs after infusion of docosahexaenoic acid ethyl ester. Am J Clin Nutr. 1991;53(3):620–627. doi:10.1093/ajcn/53.3.620
36. McCusker MM, Grant-Kels JM. Healing fats of the skin: the structural and immunologic roles of the omega-6 and omega-3 fatty acids. Clin Dermatol. 2010;28(4):440–451. doi:10.1016/j.clindermatol.2010.03.020
37. Chen J, Sun B, Zhang D. Association of dietary n3 and n6 fatty acids intake with hypertension: NHANES 2007–2014. Nutrients. 2019;11(6):1232. doi:10.3390/nu11061232
38. Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9(2):136–139. doi:10.1046/j.1087-0024.2003.09102.x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

