Back to Journals » Vascular Health and Risk Management » Volume 19
Ascorbic Acid vs Calcitriol in Influencing Monocyte Chemoattractant Protein-1, Nitric Oxide, Superoxide Dismutase, as Markers of Endothelial Dysfunction: In Vivo Study in Atherosclerosis Rat Model
Authors Heriansyah T , Dimiati H, Hadi TF , Umara DA, Riandi LV, Fajri F, Santosa SF, Wihastuti TA , Kumboyono K
Received 19 December 2022
Accepted for publication 21 February 2023
Published 12 March 2023 Volume 2023:19 Pages 139—144
DOI https://doi.org/10.2147/VHRM.S401521
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Harry Struijker-Boudier
Teuku Heriansyah,1 Herlina Dimiati,1 Tjut Farahiya Hadi,1 Dimas Arya Umara,1 Lian Varis Riandi,2 Fauzan Fajri,3 Sukmawan Fajar Santosa,4 Titin Andri Wihastuti,5 Kumboyono Kumboyono6
1Department of Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Syiah Kuala, Banda Aceh, Aceh, Indonesia; 2Department of Parasitology, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Aceh, Indonesia; 3Department of Animal Model, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Aceh, Indonesia; 4Integrated Research Laboratory, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Aceh, Indonesia; 5Department of Basic Nursing, Faculty of Health Sciences, Universitas Brawijaya, Malang, Jawa Timur, Indonesia; 6Department of Nursing, Faculty of Health Sciences, Universitas Brawijaya, Malang, Indonesia
Correspondence: Tjut Farahiya Hadi, Email [email protected]
Introduction: Ascorbic acid and calcitriol were frequently utilized in conjunction as therapy during the COVID-19 pandemic, and individuals with minor symptoms had notable improvements. There have been a few studies, often with conflicting findings, that examine the use of them for endothelium restoration and numerous clinical trial studies that failed to establish the efficacy. The aim of this study was to find the efficacy of ascorbic acid compared to calcitriol on the inflammatory markers monocyte chemoattractant protein-1 (MCP-1), nitric oxide (NO), and superoxide dismutase (SOD), as protective agents which play an important role in the early stages of atherosclerosis formation. This study was an experimental in vivo study.
Methods: The total of 24 male Rattus norvegicus strain Wistar rats were divided into 4 groups, namely: control/normal group (N), atherosclerosis group (DL) given atherogenic diet, atherosclerosis group given atherogenic diet and ascorbic acid (DLC), and atherosclerosis group given atherogenic diet and calcitriol (DLD) treatment for 30 days.
Results: Ascorbic acid and calcitriol treatment was significantly effective (P< 0.05) in lowering expression of MCP-1 and increasing NO and SOD level. Calcitriol was superior to ascorbic acid in increasing SOD (P< 0.05). There was no significant difference between ascorbic acid and calcitriol in decreasing MCP-1 and increasing NO (P> 0.05).
Discussion: Both treatments could reduce MCP-1, and increase NO and SOD by increasing antioxidants. In this study calcitriol was superior to ascorbic acid in increasing SOD, but not NO and decreasing MCP-1. According to the theory, it was found that calcitriol through nuclear factor erythroid 2-related factor 2 (Nrf2) causes a direct increase in the amount of SOD. Nrf2 is an emerging regulator of cellular resistance to oxidants.
Conclusion: Ascorbic acid and calcitriol treatment was able to reduce MCP-1 and increase NO and SOD in atherosclerosis rat. Calcitriol was significantly superior in increasing SOD levels compared to ascorbic acid.
Keywords: ascorbic acid, calcitriol, monocyte chemoattractant protein-1, nitric oxide, superoxide dismutase, atherosclerosis
Introduction
Despite the fact that COVID-19 has killed countless populations over the past two years, heart disease is still the leading cause of mortality worldwide. Much research conducted during the COVID-19 pandemic concentrated on vascular inflammation, which turned out to be the pathomechanism for COVID-19 and is similar to the pathogenesis of atherosclerosis.1 The potential for the use of various antioxidants as part of the management of atherosclerosis is increased by systemic inflammatory processes, oxidative stress, fat buildup, cell death, and fibrosis in arteries that cause the onset of many systemic disorders, including atherosclerosis.2
Ascorbic acid and calcitriol were frequently utilized in conjunction as therapy during the COVID-19 pandemic, and individuals with minor symptoms had notable improvements.2 In theory, ascorbic acid and calcitriol could aid in the process of restoring endothelial function.3,4 Endothelial damage is the underlying cause of atherosclerosis.5
It has long been understood that ascorbic acid contributes to a number of crucial endothelium processes.5 These functions, according to earlier research, include boosting type IV collagen synthesis and deposition in the basement membrane, promoting endothelial proliferation, preventing apoptosis, scavenging free radicals, and encouraging endothelial cells to produce nitric oxide (NO), which regulates blood flow. Another theory describes how ascorbic acid could stop the oxidation of low-density lipoprotein (LDL).6
Through the bioactivity of endothelial NO synthase (eNOS), calcitriol promotes NO production.6 Although the mechanism is unclear, prior research suggests that vitamin D functions as an anti-inflammatory to impact TNF-cytokines as pro-inflammatory cytokines.7 High dosages of vitamin D were found to dramatically lower serum TNF levels in a study on inflammatory bowel disease. Calcitriol can control NO production by stimulating nitric oxide synthase 3 (NOS3) transcription through the binding of vitamin D receptors (VDR).8 But it was not clear enough.
There have been a few studies, often with conflicting findings, that examine the use of ascorbic acid and calcitriol for endothelium restoration and numerous clinical trial studies that failed to establish the efficacy. In light of the aforementioned and lack of data, the researcher is eager to compare the effects of ascorbic acid and calcitriol on rat endothelium dysfunction in an atherosclerosis model.
Method
The study was an experimental study. The study used 24 male white rats (Rattus norvegicus) aged 4 weeks, with body weights ranging from 50 to 100 g, divided into four treatment groups. Each treatment unit contained six white male rats. The treatment groups were: N (rats given standard diet or control), DL (atherogenic diet), DLC (atherogenic and ascorbic acid), and DLD (atherogenic and calcitriol). The treatment was carried out for 90 days and 30 days of intervention. After an acclimatization period for 2 weeks, 6 rats were given normal diet while 18 rats were chosen randomly and given atherogenic diet (containing 0.2% cholic acid, 2% egg yolk, 5% goat fat, and 92.8% corn rice) ad libitum for 8 weeks.9
The treatment phase was started in the ninth week.10 In this phase, the rats were fed according to the research plans as described above. Particularly in the treatment groups, the rats were given calcitriol dosage for rats (0.009 µg/day) equivalent to 0.25 µg calcitriol dosage recommended for humans, and ascorbic acid (9 mg/day) equivalent to 500 mg ascorbic acid dosage recommended for humans, given for 30 days. At the end of treatment phase, euthanasia was performed by researchers and laboratory staff using intraperitoneal ketamine (Ilium ketamil; Troy Laboratories, Australia) and xylazine (Xyla; Interchemie, Netherlands). Blood was taken from the heart, then placed in venoject. Blood plasma samples were centrifuged using microcentrifuge (MC-12; Benchmark, Sayreville, New Jersey, United States) at 3000 rpm for 10 minutes. It was stored immediately at −80℃. Plasma would be used for examination of NO, MCP-1, and SOD concentration.
NO, MCP-1, and SOD Concentration Measurement
Measurement of NO, MCP-1, and SOD concentration in blood plasma samples of rat was conducted by enzyme-linked immunosorbent assay (ELISA) method. NO concentration was measured with Rat NO ELISA kit (Cat. No. E-BC-K035-M; Elabscience, Houston, Texas). MCP-1 concentration was measured with Rat MCP-1 ELISA kit (Cat. No. E-EL-R0633; Elabscience, Houston, Texas), and SOD concentration was measured with Rat T-SOD ELISA kit (Cat. No. E-BC-K019-M; Elabscience, Houston, Texas).
ELISA for MCP-1 was begun with standard and sample of 100 μL inserted into the well. The sample (except blank well) was incubated at 37℃ for 1 hour. Then 90 μL substrate reagent was added and incubated for 15 minutes at 37℃ (away from light). The reaction was stopped by adding 50 μL stop solution. After 5 minutes, the sample was read using ELISA reader (xMarkTM Microplate Absorbance Spectrophotometer; Bio-Rad, Hercules, California, USA) at 450 nm.
ELISA for NO was begun with standard and sample of 100–300 μL. Add 200 μL of reagent 1, and mix fully with a vortex mixer. Add 100 μL of reagent 2, and mix fully with a vortex mixer. Stand for 15 min at room temperature, centrifuge at 3100g for 10 min. Take 160 μL of supernatant to the corresponding wells of microplate for chromogenic reaction. Add 80 μL of chromogenic reagent to each well, oscillate for 2 min, and stand at room temperature for 15 min. The sample was read using ELISA reader (xMarkTM Microplate Absorbance Spectrophotometer; Bio-Rad, Hercules, California, USA) at 550 nm.
ELISA for SOD was begun with sample of 90 μL inserted into the well of non-enzyme working solution, control enzyme solution, and sample enzyme solution. Shake for 10 s with microplate reader, and cover the plate with sealer. Incubate for 50 min at 37℃. Add 180 μL of chromogenic agent. Shake for 10 s, and stand for 10 min in room temperature. The sample was read using ELISA reader (xMarkTM Microplate Absorbance Spectrophotometer; Bio-Rad, Hercules, California, USA) at 550 nm.
Ethic Statement
The research was conducted at the Animal Laboratory, Faculty of Veterinary Medicine, Universitas Syiah Kuala, following adapted guidelines for the care and use of laboratory animals by Institut Teknologi Bandung and National Research Council of The National Academies. The implementation of this research has been approved by Veterinary Ethics Committee, Faculty of Veterinary Medicine, Universitas Syiah Kuala, reference 160/KEPH/VIII/2022.
Statistical Analysis
The normality of data distribution and equal variances in all groups was tested using the Shapiro–Wilk test (P value >0.05), and one-way ANOVA test was used to determine the effect of ascorbic acid and calcitriol administration on the concentration of MCP-1, NO, and SOD. Furthermore, analysis was performed using post hoc test to understand the differences of each parameter in each group. Independent T-test was used to compare ascorbic acid and calcitriol. This statistical test was conducted using SPSS 20 (IBM Cooperation, New York, NY).
Results
All of the rats survived till euthanasia. The Shapiro–Wilk test showed normally distributed data (P value >0.05).
Effect of Ascorbic Acid in MCP-1, NO, and SOD Levels
Table 1 shows that ascorbic acid could reduce MCP-1 level (P=0.006), increase NO level (P=0.01), and increase SOD level (P=0.000). As shown in Table 2, the average decrease in MCP-1 levels in the DLC group was 128 ng/mL (P=0.005). The average increase in NO levels in the DLC group was 33.2 μmol/L (P=0.01). The average increase in SOD levels in the DLC group was 72 U/mL (P=0.000).
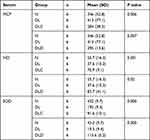 |
Table 1 Effect on MCP-1, NO, and SOD Levels in All Group |
 |
Table 2 Post Hoc Analysis of MCP-1, NO, and SOD Levels in DLC and DLD |
Effect of Calcitriol in MCP-1, NO, and SOD Levels
As shown in Table 1, calcitriol could reduce MCP-1 level (P=0.007), increase NO level (P=0.02), and increase SOD level (P=0.000). Table 2 shows that the average decrease in MCP-1 levels in the DLD group was 117 ng/mL (P=0.006). The average increase in NO levels in the DLD group was 46.1 μmol/L (P=0.02). The average increase in SOD levels in the DLD group was 96 U/mL (P=0.000).
Comparison of Ascorbic Acid Vs Calcitriol in Decreasing MCP-1, and Increasing NO and SOD
Table 3 shows that there is no significant difference between ascorbic acid and calcitriol in decreasing MCP-1. There was no significant difference between ascorbic acid and calcitriol in increasing NO. It showed calcitriol was superior to ascorbic acid in increasing SOD.
 |
Table 3 Comparison of Ascorbic Acid vs Calcitriol in Decreasing MCP-1, and Increasing NO and SOD |
Discussion
In DLC group there was decreasing of MCP-1. This result is the same as research in 2022 that found that there is a relationship with ascorbic acid in the decrease in MCP-1. Ascorbic acid not only contains benefits as antioxidant but as an anti-inflammatory suppresses MCP-1 through inflammatory mechanism.11
The DLC group had an increase in SOD. This is in accordance with previous studies that state that ascorbic acid works as an antioxidant that could increase SOD.12 The DLC group had an increase in NO. This is in accordance with previous studies which stated that ascorbic acid has a direct effect on increasing NO and prevents NO from oxidizing, which will eventually produce free radicals.13
The DLD group had a decrease in MCP-1. This is in accordance with previous studies conducted that found a decrease in MCP-1 through vitamin D receptor (VDR) so that the inflammatory process could be inhibited.14 The DLD group had an increase in SOD. This is in accordance with previous studies that proved that there is a significant increase in SOD in mice with renal impairment.14
The DLD group had an increase in NO. Calcitriol could increase the production of eNOS, which is an enzyme that increases the formation of NO. This is in line with a study conducted before in rats with arterial stiffness.15 In this study, they found a significant increase in NO. The other research observed that nitric oxide (NO) production in ovariectomized rat aortic endothelial cells was restored following a 12-h treatment with calcitriol.16 Another research showed that calcitriol induced a very rapid increase in endothelial NO production via interaction with VDR.3
In this study calcitriol was superior to ascorbic acid in increasing SOD, but not NO and decreasing MCP-1. There has been no prior research comparing the two. According to the theory, it was found that calcitriol through nuclear factor erythroid 2-related factor 2 (Nrf2) causes a direct increase in the amount of SOD. Nrf2 is an emerging regulator of cellular resistance to oxidants.17 Nrf2 regulates the basal and induced expression of an array of antioxidant response element dependent genes to regulate the physiological and pathophysiological outcomes of oxidant exposure.18
Conclusion
This is a novel finding in atherosclerosis rat. The administration of ascorbic acid and calcitriol was able to reduce MCP-1 and increase NO and SOD in atherosclerosis rat. Calcitriol was superior in increasing SOD levels compared to ascorbic acid.
Funding
This research was funded by the Institute of Research and Community Services, Universitas Syiah Kuala, under the Professor Research Grant 141/UN11/SPKPNBP/2022.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Raisi Z, Cooper J, Salih A, et al. Cardiovascular disease and mortality sequelae of COVID-19 in the UK Biobank. Heart. 2023;109(2):119–126. doi:10.1136/heartjnl-2022-321492
2. Infusino F, Marazzato M, Mancone M, et al. Diet supplementation, probiotics, and nutraceuticals in SARS-CoV-2 infection: a scoping review. Nutrients. 2020;12(6):1718. doi:10.3390/nu12061718
3. Molinari C, Rizzi M, Squarzanti DF, Pittarella P, Vacca G, Renò F. 1,25-dihydroxycholecalciferol (Vitamin D3) induces NO-dependent endothelial cell proliferation and migration in a three-dimensional matrix. Cell Physiol Biochem. 2013;31(6):815–822. doi:10.1159/000350099
4. Grano A, De Tullio MC. Ascorbic acid as a sensor of oxidative stress and a regulator of gene expression: the Yin and Yang of vitamin C. Med Hypotheses. 2007;69(4):953–954. doi:10.1016/j.mehy.2007.02.008
5. Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER. l-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem. 2001;276(1):40–47. doi:10.1074/jbc.M004392200
6. Tabrizi R, Akbari M, Lankarani KB, Heydari ST, Kolahdooz F, Asemi Z. The effects of vitamin D supplementation on endothelial activation among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab. 2018;15(1):85. doi:10.1186/s12986-018-0320-9
7. Sokol SI, Srinivas V, Crandall JP, et al. The effects of vitamin D repletion on endothelial function and inflammation in patients with coronary artery disease. Vasc Med. 2012;17(6):394–404. doi:10.1177/1358863X12466709
8. Kim DH, Meza CA, Clarke H, Kim JS, Hickner RC. Vitamin D and Endothelial Function. Nutrients. 2020;12(2):575. doi:10.3390/nu12020575
9. Getz GS, Reardon CA. Animal models of atherosclerosis. ATVB. 2012;32(5):1104–1115. doi:10.1161/ATVBAHA.111.237693
10. Murwani S, Ali M, Muliartha K. Diet aterogenik pada tikus putih (Rattus norvegicus strain Wistar) sebagai model hewan aterosklerosis. JKB. 2006;22(1):6–9. doi:10.21776/ub.jkb.2006.022.01.2
11. Quincey A, Mohan S, Edderkaoui B. Monocyte chemotactic proteins mediate the effects of hyperglycemia in chondrocytes: in vitro studies. Life. 2022;12(6):836. doi:10.3390/life12060836
12. Shakouri Mahmoudabadi MM, Rahbar AR. Effect of EPA and Vitamin C on superoxide dismutase, glutathione peroxidase, total antioxidant capacity and malondialdehyde in type 2 diabetic patients. Oman Med J. 2014;29(1):39–45. doi:10.5001/omj.2014.09
13. Ladurner A, Schmitt CA, Schachner D, et al. Ascorbate stimulates endothelial nitric oxide synthase enzyme activity by rapid modulation of its phosphorylation status. Free Radic Biol Med. 2012;52(10):2082–2090. doi:10.1016/j.freeradbiomed.2012.03.022
14. Fernandez-Robredo P, González-Zamora J, Recalde S, et al. Vitamin D protects against oxidative stress and inflammation in human retinal cells. Antioxidants. 2020;9(9):838. doi:10.3390/antiox9090838
15. Wee CL, Mokhtar SS, Singh KKB, Yahaya S, Leung SWS, Rasool AHG. Calcitriol supplementation ameliorates microvascular endothelial dysfunction in Vitamin D-deficient diabetic rats by upregulating the vascular eNOS PROTEIN EXPRESSION AND REDUCING OXIDATIVE STress. Oxid Med Cell Longev. 2021;2021:1–11. doi:10.1155/2021/3109294
16. Dong J, Ling Wong S, Wai Lau C, et al. Calcitriol restores renovascular function in estrogen-deficient rats through downregulation of cyclooxygenase-2 and the thromboxane-prostanoid receptor. Kidney Int. 2013;84(1):54–63. doi:10.1038/ki.2013.12
17. Nakai K, Fujii H, Kono K, et al. Vitamin D activates the Nrf2-keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am J Hypertens. 2014;27(4):586–595. doi:10.1093/ajh/hpt160
18. Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53(1):401–426. doi:10.1146/annurev-pharmtox-011112-140320
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
