Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
Classification and Staging of Morgellons Disease: Lessons from Syphilis
Authors Middelveen MJ , Martinez RM, Fesler MC, Sapi E, Burke J, Shah JS , Nicolaus C , Stricker RB
Received 24 November 2019
Accepted for publication 16 January 2020
Published 7 February 2020 Volume 2020:13 Pages 145—164
DOI https://doi.org/10.2147/CCID.S239840
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Video abstract presented by Melissa C. Fesler.
Views: 79880
Marianne J Middelveen,1 Roberto M Martinez,2 Melissa C Fesler,3 Eva Sapi,4 Jennie Burke,5 Jyotsna S Shah,6 Carsten Nicolaus,7 Raphael B Stricker3
1Atkins Veterinary Services, Calgary, AB, Canada; 2Martinez Veterinary Services, Calgary, AB, Canada; 3Union Square Medical Associates, San Francisco, CA, USA; 4Department of Biology and Environmental Science, University of New Haven, West Haven, CT, USA; 5Australian Biologics, Sydney, NSW, Australia; 6IGeneX Laboratories, Palo Alto, CA, USA; 7BCA-Clinic, Augsburg, Germany
Correspondence: Raphael B Stricker
Union Square Medical Associates, 450 Sutter Street, Suite 1504, San Francisco, CA 94108, USA
Email [email protected]
Introduction: Morgellons disease (MD) is a contested dermopathy that is associated with Borrelia spirochetal infection. A simple classification system was previously established to help validate the disease based on clinical features (classes I-IV).
Methods: Drawing on historical and pathological parallels with syphilis, we formulated a more detailed staging system based on clinical features as well as severity of skin lesions and corresponding histopathological infection patterns, as determined by anti-Borrelia immunohistochemical staining.
Results: Clinical classes I-IV of MD are further categorized as mild, moderate and severe, or stages A, B and C, respectively, based on histopathological findings. Stage A lesions demonstrated little or no immune infiltrates and little or no disorganization of cells; macrophages were not present, and hemorrhage was negligible. Extracellular isolated spirochetes and intracellular staining of keratinocytes in the lower epidermis was occasionally seen. Stage C lesions demonstrated positive staining of keratinocytes in the stratum basale and stratum spinosum and positive intracellular staining of macrophages for Borrelia. Aggregate Borrelia colonies were frequently encountered, hemorrhage was frequent, and intracellularly stained fibroblasts were occasionally seen. Stage B lesions demonstrated a pattern intermediate between Stages A and C.
Conclusion: The enhanced staging system provides objective criteria to assess the severity of dermopathy in MD. Further studies are needed to determine the optimal treatment for MD based on this staging system related to Borrelia infection.
Keywords: Morgellons disease, Lyme disease, Borrelia burgdorferi, relapsing fever Borrelia, tick-borne disease, syphilis, Treponema pallidum
Introduction
Morgellons disease (MD) is a dermatological condition in which lesions that contain unusual filamentous inclusions and/or projections spontaneously arise.1–5 The filaments are distinctive in part due to their varied white, red, blue, green or black coloration, and because visually they resemble microscopic textile fibers.6–9 In addition, the dermopathy may be accompanied by formication (sensation of something crawling on skin). Coupled with the mistaken belief that the fibers are derived from textiles, healthcare professionals frequently and erroneously diagnose the condition as a form of delusional infestation (DI) or the legacy terms delusional parasitosis (DP) and delusions of parasitosis (DOP).6,7,10
In addition to filamentous dermopathy, patients frequently experience Lyme-like symptoms such as musculoskeletal, neurological and cardiovascular manifestations suggestive of spirochetal etiology.1–5,8 Cohort studies indicate that most patients with MD test positively for Borrelia infection and/or have a clinical Lyme disease (LD) diagnosis.4,11–13 Two separate cohort studies have demonstrated that MD afflicts approximately 6% of LD patients.11,14 Thus it has been suggested that MD is a physiological response to spirochetal infection in genetically predisposed patients, and there is an abundance of experimental data to support the hypothesis.8,9
Borrelia infection is caused by members of the Borrelia genus encompassing the LD group, also known as Borrelia burgdorferi (Bb) sensu lato (Bbsl), and the Relapsing Fever Borrelia (RFB) complex, the causative agents of LD and relapsing fever (RF), respectively. Bbsl and RFB have been repeatedly and consistently detected in tissue and fluid specimens taken from MD subjects.8,9,12,13,15 Furthermore, using sensitive molecular methodologies, Bb and RFB have been directly detected and cultured from skin lesions demonstrating MD pathology,8,9,12,13,15–18 thus satisfying many of the criteria outlined in Fredricks and Relman’s molecular guidelines for establishing disease causation.19 Borrelia spirochetes have been detected in MD tissue and body fluid specimens, both directly in dermatological specimens and in cultures from 25 North American MD patients using a combination of microscopic, histopathological and molecular methods. Most of the Borrelia species detected were genetically identified as Bb sensu stricto (Bbss), but B. garinii and B. miyamotoi were also confirmed.12
In a separate study, 90% of a cohort of 30 MD patients tested positively for exposure to and/or infection with Borrelia spirochetes using serological and molecular techniques. Of these, 53% of the cohort tested positive for Bb, RFB or both using PCR amplification and confirmatory sequencing.13 To date, to the best of our knowledge, five independent laboratories have confirmed the presence of Borrelia DNA in MD skin specimens using PCR technology and confirmatory DNA sequencing, and seven independent laboratories have detected Borrelia DNA by direct testing or in cultures of blood, genital secretions and skin specimens taken from MD patients.8,9,13,20 If sensitive and specific detection methods are used, the detection of Borrelia spirochetes directly in MD patient specimens is consistent and reproducible, thus providing evidence suggestive of causality.
In addition to members of the genus Borrelia, other members of the Spirochaetaceae family might be able to induce MD. Various members of the genus Treponema cause a comparable condition, bovine digital dermatitis, in cattle.5 T. denticola has been detected in some MD skin specimens, although not independently of Bbsl.5 Because Treponema is known to cause a comparable condition in an animal model, and because treponemes are spirochetes like Borrelia spp., it is possible that T. denticola or other pathogenic treponemes could be key etiologic factors in the evolution of MD in some patients. In support of that hypothesis, there is historical evidence linking MD-like cases to treponemal infection, as discussed below.
DI is defined as the fixed, false belief of being infested with parasites or other infectious agents. In 1938, the Swedish physician Ekbom published a series of case studies describing patients with formication coupled with the false belief of insect infestation,21 and consequently cases of DI are often referred to as “Ekbom’s syndrome”.6,7 Ekbom reported that patients in his study presented collections of hairs, skin and sand-like particles that are comparable to what we see in MD self-collected specimens.21 MD is frequently associated with formication, and some MD patients mistakenly believe that they have a parasitic infection or infestation. Consequently, MD has been misrepresented as a form of Ekbom’s syndrome.8,9 Interestingly, three out of seven of Ekbom’s subjects had syphilis, and three years before Ekbom’s studies were published, the French physician Vié reported that 6/8 of his DI cohort had documented cases of syphilis.21,22 Although we have not detected T. pallidum in any MD subjects to date, given that there is a historical association with T. pallidum infection in comparable cases, it is reasonable to hypothesize that T. pallidum could be an etiologic factor in a subset of MD patients.
Although spirochetal infection appears to be the key etiologic factor in MD evolution, MD patients have a high incidence of other co-infecting tickborne diseases, with Babesia infection being most frequently reported.4,11 Other pathogens have been detected directly in MD dermatological specimens, although less frequently and less consistently than Borrelia spp. These pathogens include the co-infecting tickborne pathogens Bartonella henselae and Rickettsia spp., and the common human pathogens Helicobacter pylori (Hp) and T. denticola.8,13,15,23–25 Mixed Bb and Hp aggregate structures consistent with biofilms that contain alginate and amyloid protein structures have been identified in MD skin specimens.15 Confocal microscopy of mixed aggregates revealed that Bb occupied the central portion of aggregates while Hp occupied the periphery, suggesting that they could have different functional roles, but also suggesting that Bb infection was established first followed by Hp infection.15 In either case, the results suggest that mixed Borrelia biofilms may play a role in MD development and contribute towards Borrelia persistence and disease chronicity. Although the role of other pathogens and mixed infections in MD skin remains to be determined, it is possible that other microorganisms play a part in MD development and could be co-involved etiological factors in individual patients.
Histochemical studies demonstrate that the cutaneous filaments in MD are not self-implanted synthetic man-made textile fibers, but are hair-like human biofilaments composed of the structural proteins keratin and collagen, the products of keratinocytes and fibroblasts, respectively.5,8,9,17,26 The cellular base of attachment at the stratum basale is nucleated and continuous with surrounding skin cells, consistent with human cellular origin.5,8,9,17,26 Furthermore, the characteristic blue color seen in some fibers results from melanin pigmentation, and blue filaments contain melanocytes and melanin granulation, providing irrefutable evidence that these are not synthetic manufactured fibers.5,8,9,17 Thus, the skin fibers seen in MD patients are biofilaments that appear to be aberrantly produced in response to spirochetal infection.
The classification of diseases provides a foundation for identifying causation of morbidity and mortality, thus allowing actions to be taken to save lives and lessen suffering.27 Classification of diseases is epidemiologically fundamental as it provides a common language, allowing data regarding health-related concerns to be compiled and shared between medical professionals.27 The following clinical classification system for MD, based on duration and location of MD lesions, was previously proposed:8,9,12
- Early localized: lesions/fibers present for less than three (3) months and localized to one area of the body (head, trunk, extremities).
- Early disseminated: lesions/fibers present for less than three (3) months and involving more than one area of the body (head, trunk, extremities).
- Late localized: lesions/fibers present for more than six (6) months and localized to one area of the body (head, trunk, extremities).
- Late disseminated: lesions/fibers present for more than six (6) months and involving more than one area of the body (head, trunk, extremities).
The purpose of the above classification scheme was to provide a clinical framework to validate and standardize the diagnosis of MD. The present study applies the above classification scheme in conjunction with an additional staging system based on the severity of lesions along with corresponding histological evidence of infection. Disease staging is a classification system using objective medical criteria to evaluate disease severity and assess disease progression.28 Because Borrelia spp. are the key pathogens encountered in MD tissue, we focused our staging system on the infection patterns revealed by anti-Borrelia immunohistochemical (IHC) staining. Together, classification and staging provide corroborative data to validate this neglected dermopathy. In addition, staging will provide a framework to help healthcare professionals assess disease evolution and customize treatments based on the stage of infection.
Materials and Methods
Cohort Selection
Patients from across North America were selected for study, providing they met the case definition for MD as determined by a health care practitioner, and that they had lesions suitable for histological sectioning. The case definition used in this study required the presence of spontaneously-developing cutaneous lesions with embedded or projecting red, white, blue, green or black filaments. Prior testing for Borrelia infection was not required. The MD patients were then classified for duration and location of MD lesions, as previously proposed.8,9,12
The study was performed in accordance with the Declaration of Helsinki. Written informed consent for participation was obtained from all subjects, both MD patients and control subjects, in accordance with the specimen collection protocol approved by the Western Institutional Review Board (WIRB), Puyallup, WA. Consent to publish results was obtained from all subjects. The University of New Haven IRB Committee also approved the study as exempt under 45 CFR 46.101(b)(4). The identification, health status and demographic information of study subjects were not provided to research laboratories. All human samples were submitted to participating laboratories and processed in a blinded manner.
Histology
Dermatological specimens were submitted to McClain Laboratories LLC, Smithtown, NY. Skin specimens were taken from lesions showing MD pathology. The MD skin specimens collected for histological study mainly consisted of thickened callus material removed from lesions exhibiting embedded or projecting filaments. Normal commercially available human skin (BioChain Institute, Newark, CA) was included as a negative control. The MD skin specimens along with normal healthy skin were sent for histological processing in a blinded manner.
For further comparison, additional controls were as follows: Liver from a mouse experimentally infected with Bb, used for positive comparison as previously described.12,29 Negative controls included: a culture pellet of mixed Gram-positive bacteria (Staphylococcus spp. and Micrococcus spp.) combined with gelatin; a culture pellet of mixed Gram-negative bacteria (Escherichia coli and Klebsiella spp.) combined with gelatin; a biopsy from a psoriasis case; and a biopsy from skin with fungal infection. All specimens including experimental specimens and controls were formalin-fixed, blocked and sectioned, then IHC-stained to detect Borrelia using an unconjugated rabbit anti-Bb polyclonal antibody,30 incubated with an alkaline phosphatase probe (Biocare Medical #UP536L) followed by a chromogen substrate (Biocare Medical #FR805CHC), and counterstained with hematoxylin. Titration was conducted to define optimal antibody dilutions. Borrelia fluorescent in situ hybridization (FISH) was performed as previously described.12,29 Samples were visualized using a Nikon Eclipse E200 microscope, and images were taken with a Nikon DS-L3 camera.
Serology: IGeneX Laboratory
Western blot (WB) assays were performed as described previously to detect IgM and IgG antibodies reactive to Bb and RFB.13,31,32
PCR: University of New Haven
All skin specimens including skin from MD specimens and normal skin from healthy controls were submitted for PCR testing in a blinded manner. All PCR assays were performed in triplicate. Borrelia DNA was extracted as previously described, and Borrelia gene targets were detected by either real-time PCR using a published TaqMan assay targeted to a 139-bp fragment of the Borrelia 16S rRNA gene, or by nested PCR targeting the following genes: 16S rRNA, flagellin (Fla), OspC, uvrA and pyrG genes.12,20,29,33–35 PCR amplification was confirmed by Sanger sequencing and compared by BLAST analysis using the GenBank database (National Center for Biotechnology Information), as described previously.12,20,29
PCR: Australian Biologics
DNA was extracted from culture pellets using the DNeasy Blood and Tissue® kit (Qiagen) as previously described.12,14,16,20,36 Specimens were tested for the presence of Borrelia DNA and Treponema denticola/Treponema pallidum DNA targets. Blinded samples were run in duplicate with positive and negative controls using real-time PCR targeting the Borrelia 16S rRNA and endpoint PCR targeting the rpoC gene, as previously described.12,14,16,20,36 Sanger sequencing followed by BLAST analysis was used for gene analysis, as described previously.12,14,16,20,36
PCR: IGeneX
Multiplex PCR was performed for the detection of Bb and RFB in clinical samples as described previously.13 Sanger sequencing was performed on all positive Bb and RFB amplicons, confirming Borrelia derivation.
Results
Cohort
Sixteen subjects were selected who met the case description for MD as determined by a healthcare professional and who had suitable dermatological specimens for study. PCR and serological evidence of exposure to or infection with Borrelia and other co-involved pathogens is summarized in Table 1. All patients in our study had evidence of infection and/or exposure to Borrelia. Eight patients had serologic evidence of infection and/or exposure to Bb, and two patients had serologic evidence of infection and/or exposure to RFB. Twelve patients had detectable Bbsl DNA and six patients had detectable RFB DNA in clinical specimens. Nine patients had evidence of infection and/or exposure to co-involved pathogens as follows: Hp (9), Bartonella henselae (5), Babesia spp. (2), T. denticola (2), Ehrlichia chaffeensis (1), Anaplasma phagocytophilum (1), Chlamydia pneumonia (1), Toxoplasma spp. (1), and Mycoplasma spp. (1). Of the subjects in our study, 14/16 were tested by PCR technology for the presence of T. denticola and T. pallidum DNA. All were negative for T. pallidum, and 2/14 were positive for T. denticola DNA, but T. denticola was not found independently of Borrelia DNA.
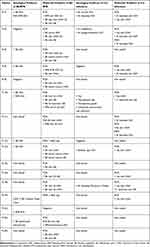 |
Table 1 Summary of Patient Data |
Histology
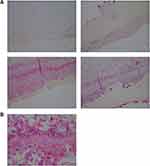
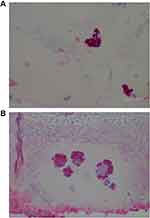
The degree of severity of the clinical presentation corresponded with the degree of severity of the histological patterns. A few sections had visible positively stained individual spirochetes and/or cysts (Figure 1). However, most Borrelia infection was associated with basal keratinocytes or was present in aggregate colonies, and to a lesser extent among keratinocytes in the stratum spinosum. In general, all lesions, regardless of the degree of severity, had visible intracellular and intercellular Bb infection of keratinocytes (Figure 2A and B). As the degree of severity progressed, the degree of cell disarrangement, hemorrhage, presence of macrophages stained with anti-Bb immunostains, presence of fibrin, vacuoles or necrosis, and presence of Borrelia aggregate colonies became increasingly apparent (Figure 2–6). The histological observations and classifications of individual subjects and corresponding data are summarized in Tables 2 and 3. Localized lesions tended to be less severe (Stages A or B), while disseminated lesions tended to be more severe (Stages B or C). Disseminated lesions that were less severe were associated with partial treatment with antibiotics. Staging sorted by classification is summarized in Table 4.
 |
Table 2 Summary of Clinical Classification, Histological Pattern and Severity, and Subsequent Stage Assignment |
 |
Table 3 Summary of Stage and Corresponding Histological Findings of Bb Immunostaining |
 |
Table 4 Clinical Classification and Histological Staging of MD Cohort (n=16 Patients) |
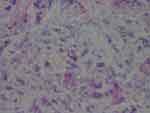 |
Figure 1 Individual spirochete among keratinocytes, stratum basale, detected with anti-Bb immunostain. 1000× magnification. |
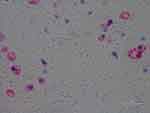 |
Figure 3 Intracellular staining of macrophages detected with anti-Bb immunostain. 1000× magnification. |
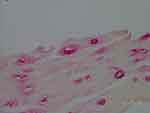 |
Figure 4 Vacuoles in epidermis reactive with anti-Bb immunostain. 1000× magnification. |
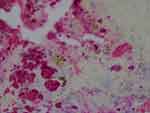 |
Figure 6 Aggregate Bb colonies detected with anti-Bb immunostain interspersed with unusual brown and blue melanin deposits. 1000× magnification. |
The results of histological controls were as follows: anti-Bb immunostaining showed positive reactivity in the experimentally Bb-infected mouse liver positive controls, and failed to show reactivity in all negative controls, including mixed Gram-positive bacteria, mixed Gram-negative bacteria, sections of normal human skin, biopsy sections of human skin with fungal infection, and biopsy sections of human skin with psoriasis.
Discussion
Case Definitions of MD
Various working case definitions have been previously proposed for MD, including the following:
A somatic LD-like illness associated with spontaneously appearing, slowly healing, filamentous, ulcerative skin lesions, with the key diagnostic criterion being colored, white, or black filaments protruding from or embedded in skin.8,9
Case definitions are important in medicine because they provide criteria used to determine whether an individual has a disease, condition or health event of interest.37 In recognition that the development of MD may involve mixed infection, we would like to refine and further develop the working case definition for MD as follows:
Morgellons disease is a dermatological condition characterized by multicolored filaments embedded within or projecting from skin. It is predominantly associated with spirochetal infection caused by members of the genus Borrelia, as well as infection with other tick-borne and non-tick-borne pathogens including Helicobacter pylori and Treponema denticola.
Infection Associated with MD
Our objective in this study was to correlate histological observations with lesion severity in order to create a staging system to track the evolution of MD lesions. We relied on IHC and/or PCR to identify Borrelia infection. Using anti-Bb IHC, we observed distinctive patterns of spirochetal infection that correlated with the severity of the lesions (Figure 1–7). Positive staining occurred in association with keratinocytes of the lower epidermis for all levels of severity. Mild lesions demonstrated little or no immune infiltrates and little or no disorganization of cells; macrophages were not present, and hemorrhage was negligible. Anti-Bb IHC revealed occasional extracellular individual isolated spirochete and cystic forms as well as intracellular staining of keratinocytes in the lower epidermis. Severe lesions demonstrated positive staining of keratinocytes in the stratum basale and stratum spinosum and positive intracellular staining of macrophages. Aggregate Borrelia colonies were frequently encountered, hemorrhage was frequent, and intracellularly stained fibroblasts were occasionally encountered. Moderate lesions demonstrated a pattern that was intermediate between mild and severe lesions (Table 1–4). The specific patterns of spirochetal infection that we observed provide strong evidence that MD is not psychogenic in origin but is the result of an infectious process. The proposed histological staging system is outlined in Table 5.
 |
Table 5 Proposed Histological Staging System (Middelveen Criteria) |
Recent studies linking Borrelia infection and MD provide evidence meeting many of the criteria proposed by Fredricks and Relman’s molecular guidelines for establishing disease causation with sequence-based technology.12,13,15,19 There are approximately 52 recorded species of Borrelia, of which 21 are classified into the LD group and 29 are classified into the RFB group (two species remain unclassified). Both Bb and RFB have been consistently detected in MD specimens.12,13,15,17,18 Although other pathogens have been detected in MD dermatological specimens, Borrelia spirochetes are the pathogens most consistently detected in MD lesions. Therefore, our proposed staging system focuses on the infectious patterns of Borrelia spp. as seen at the cellular level in histological sections from MD lesions.
Comparison Between MD and Syphilis
Classification and staging of disease processes are human concepts that enable us to categorize diseases, but convenient grouping is not found in nature. Comparison between historical accounts of syphilis and syphilis cases that are seen today in immunocompetent subjects demonstrates that spontaneous diminution of virulence has occurred. One hypothesis explaining the decrease in virulence is that many patients with secondary syphilis may have had co-infections with other microbial agents, and that co-infection may have compounded presentation, morbidity, and mortality of syphilis.38 Irrespective of the presence of co-involved infections, staging and classification of syphilis remained centered on infection with T. pallidum. As many MD patients have infections with other tick-borne pathogens and other human pathogens, it is reasonable to assume that like syphilis, co-infections may play a role in augmenting severity of the condition. As spirochetal Borrelia infection appears to be the key denomination factor, our staging system for MD will focus on Borrelia.
Immunohistology of MD
Our cohort had serological and/or DNA evidence of Borrelia spirochetal infection, although prior testing was not required for selection as study subjects. We observed a significant spirochetal burden in all the dermatological specimens from MD lesions that were submitted for study, regardless of location, lesion characteristic, or length of illness. The anti-Bb IHC staining was highly specific for bacteria with morphology consistent with spirochetal infection, and staining was associated with specific cell types, namely keratinocytes, fibroblasts and macrophages. The rabbit anti-Bb polyclonal antibody used in this study is reported to cross-react with T. pallidum, B. hermsii and B. parkeri.30 A Borrelia stain with a broad range of specificity for spirochetal infection that detected both Borrelia spp. and Treponema spp. was intentionally selected for this study in order to capture the full spectrum of spirochetes that could potentially be involved in MD evolution. For comparison purposes, sections of normal human skin were stained to determine possible cross-reactivity with commonly encountered dermatological microorganisms.12 Normal human skin bacterial flora showed no staining.
Biopsy sections of human skin with fungal infection and with psoriasis were examined to determine possible cross-reactivity with other inflammatory dermatological conditions and to rule out non-specific staining with inflammatory proteins and cells. Staining of psoriasis and fungal infection biopsy sections was negative, indicating that the staining was not cross-reactive or non-specific for cells or proteins associated with inflammation. Two subjects did have DNA evidence of T. denticola infection in association with Borrelia infection, but given that every patient had evidence of Borrelia infection it appears that this spirochete species is the key etiologic factor. T. denticola is a common human oral spirochete, and we hypothesize it may have been introduced by mouth-to-hand-to-lesion transmission or self-inoculation.
Developing a Staging System for MD
As Borrelia is a member of the Spirochaetaceae family like T. pallidum, the causative agent of syphilis, it was logical when devising a staging system to make a comparison between MD lesions and syphilitic lesions. Syphilis is classified into three sequential clinical symptomatic stages of infection (primary, secondary and tertiary), along with corresponding characteristic skin lesions of each stage.38,39 Primary syphilitic lesions are localized, occurring 2–3 weeks after initial exposure. If left untreated, the primary lesion heals in 4–5 weeks, but 4–8 weeks after that the infection becomes systemic and secondary syphilis develops. Some individuals have relapsing episodes of secondary syphilis with periods of latency in between and some may go on to develop tertiary syphilis, the late manifestation of the disease.39
We do not see systematic progression of MD in quite the same manner, because MD lesions can develop long after other systemic symptoms are evident. If an erythema migrans (EM) rash was present after a tick bite, that stage of Borrelia infection would be comparable to the primary stage. However, many LD patients have no history of EM rash,40 and by the time filamentous lesions develop in most MD patients, the initial Borrelia infection has passed unnoticed, Lyme-like symptoms are chronic, and the infectious process is well established. Presumably, the progression of LD is affected by the virulence of the Borrelia species, the individual host response to infection and numerous other secondary etiological factors. As stated previously, only a subset of patients with Borrelia infection develop MD lesions,11 but when MD symptoms are present, for most patients the stage of disease progression is comparable to that of secondary syphilis.
Historical Background of Syphilis Staging
Syphilis is a systemic infection associated with multisystem symptoms, and cutaneous secondary syphilitic lesions are localized reactions in tissue induced by metastatic accumulation of treponemes.38 Various classification schemes for syphilis were proposed in the early 20th century. Fournier differentiated lesions based on location on the skin proper versus mucous membranes, while Fox proposed that secondary syphilitic lesions be grouped into early and late syphilids (welts), a classification scheme that gained acceptance by the American Dermatological Association in 1924.38 The secondary syphilitic lesions classified as early lesions by this scheme are described as follows:
- Macular – the earliest secondary lesions, but frequently overlooked, small (4–8 mm), flat, pale pink to violaceous spots, that occur on any part except never on the face;
- Small papular – small localized skin elevations that are easily palpated, frequently erupt after macular rashes, are variable in color, and may occur on any part of the body, but favor the trunk;
- Follicular or pseudovesicular – small (pinpoint to pinhead), round or pointed papules around orifices of follicles or sweat glands, that tend to group, and may occur on any part of the body, but are frequently found on genital and anal regions, the back, upper trunk, arms, thighs and face;
- Lichenoid – flattened, angulated lesions that resemble lichen planus, appearing on any part of the body, but most often on the back, upper trunk, and arms;
- Vesicular – pointed small vesicles that rupture with difficulty, and can appear on any part of the body;
- Psoriasiform – resemble psoriasis lesions, but do not bleed if scale is removed, occurring mostly on the palms of hands and soles of feet, but may also occur on the face, elbows, and knees;
- Corymbiform – nipple-like, usually large lesions surrounded by smaller lesions, occurring on any part of the body.
The secondary syphilitic lesions classified as late lesions by this scheme are described as follows:
- Large papular – like small papular, but flattened, may coalesce, occur on any part of the body;
- Annular – papular lesions with a circinate configuration, that spread peripherally forming rings or gyrate patches, occur on any part of the body, but favor mucocutaneous areas;
- Condylomata lata – begin as papules that flatten becoming macerated, with a thick mucoid exudate appear in two forms, one flat moist and excoriated in the center, the other is verrucous, both occur most frequently on the rectum, scrotum, vulva and groin;
- Pustular – originates as a vesicle, resembles a papule with scales, then ruptures becoming flaccid, containing little if any pus, can form scabs over concave ulcerations or may appear as a concave ulceration with no scab formation, occurring on any part of the body, but favoring the face, nose flanks, thighs, palms of hands and soles of feet;
- Rupial – large heavily encrusted pustules that can appear on any part of the body;
- Frambesiform – a papular raspberry-like growth, moist, violaceous, verrucous, that occur on any part of the body, favoring the face, scalp, mouth, nose, and less frequently the axilla, anal and genital regions;
- Pigmentary – vary in size and are not raised, can be hypopigmented or hyperpigmented, and can appear on any part of the body.
Regardless of the location and presentation of lesions, secondary syphilis lesions are local reactions to accumulated treponemes in susceptible tissue.38 Alopecia can occur in untreated cases of secondary syphilis, arising from follicular involvement.38 Condylomata lata are large greyish raised lesions that arise from the breakdown of secondary lesions in moist areas where tissue trauma has occurred such as the groin or axilla.38 The treponemal burden in lesions provides evidence that lesions are progressive stages of the same infectious process.38
Clinical Diagnosis of MD
Excluding the presence of cutaneous filaments seen in MD lesions, we have observed comparable characteristic variation in the manifestation of MD lesions. Like secondary syphilis, MD results from systemic spirochetal infection. Our findings indicate that all MD lesions are associated with a spirochetal burden and, regardless of location and variation in characteristic appearance, represent the same infectious process, comparable to syphilis. Based on this observation, we propose the following criteria and guidelines for diagnosing MD:
Diagnostic criteria (Proposed guidelines for diagnosing MD):
- Primary features (Must include the following):
- Multicolored filaments embedded within or protruding from the skin
- Secondary features (May include one or more of the following):
- Development of calluses
- Ulcerative lesions
- Papules
- Burning, itching, stinging, biting
- Hair loss
- Atypical hair/nail production
- Dry appearance with or without flaking skin
- Edema
- Hyper- or hypo-pigmentation from scarring
- Hypertrophic scarring
- Excoriations
- Slowly healing lesions
- Aging skin
We propose the following MD subtypes (How the above features present, morphological presentation and combination of above features):
- Facial
- Follicular
- Oropharyngeal/Nasopharyngeal
- Ophthalmological
- Central (trunk)
- Appendicular (extremities)
- Genitourinary/Vulvovaginal
- Gastrointestinal/Anorectal
- Vesicular (some start as liquid-filled blisters, especially on the hands)
MD lesions are comparable to secondary syphilitic lesions. However, rather than relying on the systematic progress from initial infection through sequential clinical stages of infection, as has been done with syphilis, our staging focuses on the severity of lesions and corresponding histological findings. Our staging methodology demonstrates that the level of severity is associated with unique histological patterns. Mild (Stage A), moderate (Stage B) and severe (Stage C) MD lesions all demonstrated anti-Bb IHC staining associated with keratinocytes within the stratum basale and/or the stratum spinosum. The intracellular anti-Bb IHC staining of keratinocytes and fibroblasts that we observed is corroborated by findings in other published studies.
Borrelia spirochetes can invade fibroblasts and keratinocytes, and can replicate intracellularly in these cells.41–43 In vitro studies demonstrate that intracellular sequestration enables Borrelia resistance to antibiotic treatment, and may provide a mechanism for persistent infection.41,42 Likewise, T. pallidum can localize intracellularly within keratinocytes, fibroblasts, spermatocytes, interstitial cells and Leydig cells,44–47 and T. denticola can invade human gingival epithelial cells.48 Macrophages and keratinocytes are the cells in which intracellular Borrelia is most frequently observed. Bb gains entrance into macrophages by phagocytosis,49 and interestingly keratinocytes can also act in a phagocytic manner.50,51 Thus, Borrelia may gain entrance into cells thorough a phagocytic process in patients with MD.
Histopathology of MD Lesions
Severe MD disease is associated with inflammatory infiltrate (mainly macrophages), hemorrhage and Borrelia aggregate colonies. Most importantly, severity of lesions corresponded with the level of intracellular anti-Bb IHC staining of macrophages, primarily in the dermis, a finding that suggests chronicity. A similar pattern of intracellular T. pallidum infection within macrophages and aggregate structures was previously recognized in specimens from secondary and tertiary syphilitic patients.52 Furthermore, in murine models, Bb rapidly gains entrance into macrophages through phagocytosis, normally resulting in degradation. However, Bb may survive phagocytosis and persist intracellularly, and infection within macrophages may provide a pathogenic mechanism.49 In MD cases, we do not know if Bb survives phagocytosis by persisting and replicating within macrophages, but we speculate that given the frequency of this finding, it occurs in severe MD cases. As a result, survival within macrophages may contribute to spirochete persistence and recalcitrance, like survival within keratinocytes and fibroblasts. Thus, significant Borrelia accumulation occurs within keratinocytes, fibroblasts and macrophages and, hypothetically, Bb intracellular survival and replication could result in cell rupture and formation of aggregate colonies, as shown recently.15
MD filaments originated in subcutaneous locations, emphasizing that these fibers could not have been externally implanted (Figure 7A). MD filaments originating in the basal cell layer reacted intensely with anti-Bb IHC staining (Figure 7B and C). The base of attachment and lower shaft of MD filaments demonstrated a composition of intracellularly anti-Bb IHC-stained keratinocytes that was continuous with the basal cell layer (Figure 7C). This specific staining pattern shows a direct association between MD filament formation and Borrelia infection: the fact that the organism presumed to be responsible for MD dermopathy is visible in areas demonstrating the key diagnostic criterion for MD is suggestive of a causal relationship. The presence of Bbsl, and possibly co-involvement of other pathogens, in MD skin may result in altered gene regulation and expression of keratin, collagen and melanin, that induce the production of aberrant filaments.8,9 In support of this hypothesis, co-culture of human fibroblasts with Bbsl enhances collagen mRNA expression in vitro and stimulates the growth factors responsible for increased collagen production.53
Diagnostic Approach to Syphilis
Secondary syphilis and MD associated with Borrelia infection are both systemic diseases associated with a variety of musculoskeletal, neurological and cardiac symptoms.4,11,39,49 Correctly diagnosing these spirochetal infections is therefore of paramount importance, and recognizing the associated dermopathy can aid in diagnosis. In diagnosing MD, we can take a lesson from the example of syphilis. The diagnosis of syphilis involves a range of different acceptable direct and indirect testing methods. Serological tests are the primary means of diagnosis and include specific treponemal tests and non-specific non-treponemal tests.39 Most diagnoses are based on clinical appearance with supporting serological evidence: reactivity to cardiolipin (VDRL and RPR tests), and/or reactivity to treponemal outer membrane proteins (MHA-TP).49 More recently, automated EIA tests and simple dipstick tests have become available.39
Serological detection is sufficiently sensitive and specific for most cases of syphilis, but serology may lack sensitivity and/or specificity in the early phases and for certain manifestations of the disease; therefore, other methods have been used for detection.54 Although not frequently performed, direct detection of spirochetes in primary and secondary syphilitic lesions by darkfield microscopy is an acceptable means of diagnosis. In addition, diagnosis may be based on histological examination of biopsy material using a silver staining method or IHC staining, and a new variation of IHC staining, focus floating microscopy (FFM), is now available.39,49,54 DNA amplification of T. pallidum gene targets by PCR technology is also an accepted diagnostic method and reportedly has a specificity and sensitivity comparable to serological methods.39
Diagnostic Approach to LD and MD
In contrast to the broad range of acceptable testing methods for syphilis, testing for Borrelia infection in North America, as recommended by the Infectious Diseases Society of America (IDSA) and the US Centers for Disease Control and Prevention (CDC), is focused on detection of LD, and relies on a single, defective, insensitive serological test protocol.31,32,55–57 The recommended testing is a two-tiered test protocol consisting of a screening assay for serum antibodies – either an Enzyme Immunoassay (EIA) or an Immunofluorescence Assay (IFA). If positive, a confirmatory Western Blot is performed. The IgM Western Blot is performed only if the patient has been symptomatic for less than or equal to 30 days; and the IgG Western Blot is performed if the patient has had symptoms for greater than 30 days.58 Patients with late or longstanding LD frequently demonstrate prolonged IgM seroreactivity that should be addressed by testing. Other deficiencies in LD testing arise from the fact that CDC endorsed commercial LD test kits are based on antigens from a single Bbss strain, B-31, and are not capable of detecting antigenic reactivity to the many Bbsl and RFB species and strains that lack cross-reactivity.9,13,32
There are nine species of Bbsl that are said to have human pathogenic potential: B. afzelii, B. bavariensis, B. bissettii, B. burgdorferi sensu stricto, B. garinii, B. kurtenbachii, B. lusitaniae, B. spielmanii, and B. valaisiana.59 Our understanding of Bbsl infection is in its infancy, however, and some species classified as non-pathogenic may yet be shown to cause human disease.60 Our knowledge of RFB is even more limited in scope than our knowledge of Bbsl. In the USA, there is no case definition for relapsing fever (RF) and it is not nationally reportable.61 Most human cases are reported in the Western states and include: B. miyamotoi, B. hermsii, B. lonestari, B. parkeri, B. turicatae, and B. mazzottii.62–68 Bb and RFB are antigenically distinct with unique Western blot band patterns; therefore tests based on Bb antigens do not adequately detect RFB.13,32 Ideally, serological testing for Borrelia spirochetes in MD patients should encompass the full spectrum of spirochetes associated with the dermopathy.
Although it is insensitive, serological testing is helpful for diagnosing LD because most patients with LD do not have spirochetal infection that is detectable by direct detection methods. However, patients with MD have a visible manifestation of infection that is easily identifiable by the MD case definition, and spirochetal infection is detectable in dermatological tissue by direct detection methods, providing that the correct methodologies are used.8,9,12,13,15,17,18 Methodologies that should be accepted as corroborative diagnostic evidence of spirochetal infection in MD patients should include those that can directly detect Borrelia spirochetes in skin specimens from tissue showing dermopathy. This can be accomplished by histological sectioning and staining with silver stains, IHC, FFM and or FISH; and by DNA detection using PCR technology.8,9,12,13,15,17,18 Culturing from skin specimens and from other tissues and bodily fluids can also be an effective means of detecting spirochetal infection in MD patients.8,9,12 It is imperative that reliance on a single faulty serological method for diagnosing suspected LD should be upgraded and that other more sensitive techniques should be included in the repertoire of accepted diagnostic tools for MD.
Conclusions
This is the first proposed staging and classification system for MD that further illuminates this contested illness. The evidence presented here supports MD evolution as being directly associated with spirochetal infection, bearing similar historical and pathological parallels to syphilis. Establishing an MD staging system validates the disease as a true somatic illness and should serve as a template for diagnosis. Further research is needed to understand the mechanisms behind this complex disease process.
Acknowledgments
The authors thank Carlo Maria Mortellaro, DVM and Loris De Vecchi, DVM for their helpful discussion of bovine digital dermatitis. Supported by a grant from the Lindorf Family Foundation, Newark, OH.
Disclosure
Dr Jyotsna Shah is the President and stock owner of IGeneX Inc. Dr Raphael Stricker is the owner of Union Square Medical Associates. The authors report no other conflicts of interest in this work.
References
1. Savely G, Leitao MM. Skin lesions and crawling sensation: disease or delusion? Adv Nurse Pract. 2005;13:16–17.
2. Savely VR, Leitao MM, Stricker RB. The mystery of Morgellons disease: infection or delusion? Am J Clin Dermatol. 2006;7:1–5. doi:10.2165/00128071-200607010-00001
3. Savely VR, Stricker RB. Morgellons disease: the mystery unfolds. Exp Rev Dermatol. 2007;2(5):585–591. doi:10.1586/17469872.2.5.585
4. Savely VR, Stricker RB. Morgellons disease: analysis of a population with clinically confirmed microscopic subcutaneous fibers of unknown etiology. Clin Cosmet Investig Dermatol. 2010;3:67–78. doi:10.2147/ccid.s9520
5. Middelveen MJ, Stricker RB. Filament formation associated with spirochetal infection: a comparative approach to Morgellons disease. Clin Cosmet Investig Dermatol. 2011;4:167–177. doi:10.2147/CCID.S26183
6. Pearson ML, Selby JV, Katz KA; Unexplained Dermopathy Study Team, et al. Clinical, epidemiologic, histopathologic and molecular features of an unexplained dermopathy. PLoS One. 2012;7:e29908. doi:10.1371/journal.pone.0029908
7. Shah R, Taylor RE, Bewley A. Exploring the psychological profile of patients with delusional infestation. Acta Derm Venereol. 2017;97:98–101. doi:10.2340/00015555-2423
8. Middelveen MJ, Stricker RB. Morgellons disease: a filamentous borrelial dermatitis. Int J Gen Med. 2016;9:349–354. doi:10.2147/IJGM.S116608
9. Middelveen MJ, Fesler MC, Stricker RB. History of Morgellons disease: from delusion to definition. Clin Cosmet Investig Dermatol. 2018;11:71–90. doi:10.2147/CCID.S152343
10. Freudenmann RW, Lepping P. Delusional infestation. Clin Microbiol Rev. 2009;22(4):690–732. doi:10.1128/CMR.00018-09
11. Fesler MC, Middelveen MJ, Stricker RB. Clinical evaluation of Morgellons disease in a cohort of North American patients. Dermatol Rep. 2018;10:7660. doi:10.4081/dr.2018.7660
12. Middelveen MJ, Bandoski C, Burke J, et al. Exploring the association between Morgellons disease and Lyme disease: identification of Borrelia burgdorferi in Morgellons disease patients. BMC Dermatol. 2015;15:1. doi:10.1186/s12895-015-0023-0
13. Middelveen MJ, Cruz ID, Fesler MC, Stricker RB, Shah JS. Detection of tick-borne infection in Morgellons disease patients by serological and molecular techniques. Clin Cosmet Investig Dermatol. 2018;11:561–569. doi:10.2147/CCID.S184521
14. Mayne PJ. Clinical determinants of Lyme borreliosis, babesiosis, bartonellosis, anaplasmosis, and ehrlichiosis in an Australian cohort. Int J Gen Med. 2014;8:15–26. doi:10.2147/IJGM.S75825
15. Middelveen MJ, Filush KR, Bandoski C, et al. Mixed Borrelia burgdorferi and Helicobacter pylori biofilms in Morgellons disease dermatological specimens. Healthcare (Basel). 2019;7(2):E70. doi:10.3390/healthcare7020070
16. Mayne P, English JS, Kilbane EJ, et al. Morgellons: a novel dermatological perspective as the multisystem infective disease borreliosis. F1000Research. 2013;2:118. doi:10.12688/f1000research
17. Middelveen MJ, Mayne PJ, Kahn DG, Stricker RB. Characterization and evolution of dermal filaments from patients with Morgellons disease. Clin Cosmet Investig Dermatol. 2013;6:1–21. doi:10.2147/CCID.S39017
18. Middelveen MJ, Burugu D, Poruri A, et al. Association of spirochetal infection with Morgellons disease. F1000Res. 2013;2:25. doi:10.12688/f1000research.2-25.v1
19. Fredricks DN, Relman DA. Sequence-based identification of microbial pathogens: a reconsideration of Koch’s postulates. Clin Microbiol Rev. 1996;9(1):18–33. doi:10.1128/CMR.9.1.18
20. Middelveen MJ, Sapi E, Burke J, et al. Persistent Borrelia infection in patients with ongoing symptoms of Lyme disease. Healthcare (Basel). 2018;6(2):E33. doi:10.3390/healthcare6020033
21. Ekbom KA. Praeseniler dermat-zooenwahn. Acta Psychiatr Scand. 1938;13:227–259. doi:10.1111/j.1600-0447.1938.tb06569.x
22. Vié J L. ’idée délirante d’anthropathie interne.
23. Allen L, Saylor-Hefley C. Morgellons under investigation: identification of associated microorganisms by molecular analysis of epithelial samples;
24. Bandoski C. Evidence for the presence of human pathogens Borrelia and Helicobacter in Morgellons patients’ skin samples;
25. Lewis J, Lloyd VK, Robichaud GA. Evidence of bacterial co-infections in Morgellons and Lyme patients;
26. Middelveen MJ, Rasmussen EH, Kahn DG, Stricker RB. Morgellons disease: a chemical and light microscopic study. J Clin Exp Dermatol Res. 2012;3:2. doi:10.4172/2155-9554.1000140
27. Lindmeier C. WHO releases new international classification of diseases (ICD 11), June 18, 2018. Available from: https://www.who.int/news-room/detail/18-06-2018-who-releases-new-international-classification-of-diseases-(icd-11).
28. Conklin JE, Lieberman JV, Barnes CA, Louis DZ. Disease staging: implications for hospital reimbursement and management. Health Care Financ Rev. 1984;Suppl:13–22.
29. Middelveen MJ, McClain SA, Bandoski C, et al. Granulomatous hepatitis associated with chronic Borrelia burgdorferi infection: a case report. Res Open Access. 2014;1:875.
30. Abcam Datasheet. Anti-borrelia burgdorferi antibody (ab20950), 2015. Available from: https://www.abcam.com/borrelia-burgdorferi-antibody-ab20950.html.
31. Shah JS, Cruz DI, Narciso W, Lo W, Harris NS. Improved sensitivity of Lyme disease Western blots prepared with a mixture of Borrelia burgdorferi strains 297 and B31. Chronic Dis Int. 2014;1(2):7.
32. Liu S, Cruz ID, Ramos CC, Taleon P, Ramasamy R, Shah J. Pilot study of immunoblots with recombinant Borrelia burgdorferi antigens for laboratory diagnosis of Lyme disease. Healthcare (Basel). 2018;6(3):E99. doi:10.3390/healthcare6030099
33. Margos G, Hojgaard A, Lane RS, et al. Multilocus sequence analysis of Borrelia bissettii strains from North America reveals a new borrelia species, Borrelia kurtenbachii. Ticks Tick Borne Dis. 2010;1:151–158. doi:10.1016/j.ttbdis.2010.09.002
34. Clark KL, Leydet B, Hartman S. Lyme borreliosis in human patients in Florida and Georgia, USA. Int J Med Sci. 2013;10:915–931. doi:10.7150/ijms.6273
35. O’Rourke M, Traweger A, Lusa L, et al. Quantitative detection of Borrelia burgdorferi sensu lato in erythema migrans skin lesions using internally controlled duplex real time PCR. PLoS One. 2013;8:e63968. doi:10.1371/journal.pone.0063968
36. Mayne PJ. Investigation of Borrelia burgdorferi genotypes in Australia obtained from erythema migrans tissue. Clin Cosmet Investig Dermatol. 2012;5:69–78. doi:10.2147/CCID.S31913
37. CDC. Introduction to epidemiology, 2012. Available from: https://www.cdc.gov/csels/dsepd/ss1978/lesson1/section5.html.
38. Baughn RE, Musher DM. Secondary syphilitic lesions. Clin Microbiol Rev. 2005;18(1):205–216. doi:10.1128/CMR.18.1.205-216.2005
39. French P. Syphilis. BMJ. 2007;334(7585):143–147. doi:10.1136/bmj.39085.518148.BE
40. Kannangara DW, Patel P. Report of non-Lyme, erythema migrans rashes from New Jersey with a review of possible role of tick salivary toxins. Vector Borne Zoonotic Dis. 2018;18(12):641–652. doi:10.1089/vbz.2018.2278
41. Georgilis K, Peacock M, Klempner MS. Fibroblasts protect the Lyme disease spirochete, Borrelia burgdorferi, from ceftriaxone in vitro. J Infect Dis. 1992;166(2):440–444. doi:10.1093/infdis/166.2.440
42. Klempner MS, Noring R, Rogers RA. Invasion of human skin fibroblasts by the Lyme disease spirochete. Borrelia Burgdorferi. J Infect Dis. 1993;167(5):1074–1081. doi:10.1093/infdis/167.5.1074
43. Chmielewski T, Tylewska-Wierzbanowska S. Interactions between Borrelia burgdorferi and mouse fibroblasts. Pol J Microbiol. 2010;59(3):157–160. doi:10.33073/pjm-
44. Juanpere-Rodero N, Martin-Ezquerra G, Fernandez-Casado A, et al. Cell and tissue interactions of Treponema pallidum in primary and secondary syphilitic skin lesions: an ultrastructural study of serial sections. Ultrastruct Pathol. 2013;37(1):36–42. doi:10.3109/01913123.2011.584498
45. Sykes JA, Miller JN. Intracellular location of Treponema pallidum (Nichols strain) in the rabbit testis. Infect Immun. 1971;4(3):307–314. doi:10.1128/IAI.4.3.307-314.1971
46. Sykes JA, Miller JN, Kalan AJ. Treponema pallidum within cells of a primary chancre from a human female. Br J Vener Dis. 1974;50(1):40–44. doi:10.1136/sti.50.1.40
47. Sykes JA, Kalan J. Intracellular Treponema pallidum in cells of a syphilitic lesion of the uterine cervix. Am J Obstet Gynecol. 1975;122(3):361–367. doi:10.1016/0002-9378(75)90185-4
48. Inagaki S, Kimizuka R, Kokubu E, Saito A, Ishihara K. Treponema denticola invasion into human gingival epithelial cells. Microb Pathog. 2016;94:104–111. doi:10.1016/j.micpath.2016.01.010
49. Montgomery RR, Nathanson MH, Malawista SE. The fate of borrelia burgdorferi, the agent for Lyme disease, in mouse macrophages. Destruction, Survival, Recovery. J Immunol. 1993;150(3):909–915.
50. Schröder JM. The role of keratinocytes in defense against infection. Curr Opin Infect Dis. 2010;23(2):106–110. doi:10.1097/QCO.0b013e328335b004
51. Bigelmayr S, Koenigs A, Kraiczy P. Inter- and intraspecies-specific adhesion of Lyme borreliae to human keratinocytes. Ticks Tick Borne Dis. 2019;10(1):207–212. doi:10.1016/j.ttbdis.2018.10.006
52. Ross EH. An intracellular parasite developing into spirochaetes, found by the jelly method of in vitro staining in syphilitic lesions and in the circulating blood during the secondary stages of the disease. Br Med J. 1912;2(2711):1651–1654. doi:10.1136/bmj.2.2711.1651
53. Aberer E, Surtov-Pudar M, Wilfinger D, Deutsch A, Leitinger G, Schaider H. Co-culture of human fibroblasts and Borrelia burgdorferi enhances collagen and growth factor mRNA. Arch Dermatol Res. 2018;310(2):117–126. doi:10.1007/s00403-017-1797-1
54. Müller H, Eisendle K, Bräuninger W, Kutzner H, Cerroni L, Zelger B. Comparative analysis of immunohistochemistry, polymerase chain reaction and focus-floating microscopy for the detection of Treponema pallidum in mucocutaneous lesions of primary, secondary and tertiary syphilis. Br J Dermatol. 2011;165(1):50–60. doi:10.1111/j.1365-2133.2011.10314.x
55. Stricker RB, Johnson L. Lyme disease diagnosis and treatment: lessons from the AIDS epidemic. Minerva Med. 2010;101(6):419–425.
56. Stricker RB, Johnson L. Lyme disease: the next decade. Infect Drug Resist. 2011;4:1–9. doi:10.2147/IDR.S15653
57. Cook MJ, Puri BK. Commercial test kits for detection of Lyme borreliosis: a meta-analysis of test accuracy. Int J Gen Med. 2016;18(9):427–440. doi:10.2147/IJGM.S122313
58. CDC. Lyme disease. Two tiered testing decision tree, 2011. Available from: https://www.cdc.gov/lyme/healthcare/clinician_twotier.html.
59. Rudenko N, Golovchenko M, Grubhoffer L, Oliver JH
60. Fesler MC, Shah JS, Middelveen MJ, Burrascano JJ, Stricker RB. Lyme disease: diversity of Borrelia species in California and Mexico based on a novel immunoblot assay. J Invest Med. 2020;68:175-176.
61. Forrester JD, Kjemtrup AM, Fritz CL, et al. Tickborne relapsing fever — United States, 1990–2011. MMWR. 2015;64:58–60.
62. Burkot TR, Mullen GR, Anderson R, Schneider BS, Happ CM, Zeidner NS. Borrelia lonestari DNA in adult Amblyomma americanum ticks, alabama. Emerg Infect Dis. 2001;7(3):471–473. doi:10.3201/eid0703.017323
63. Bunikis J, Tsao J, Garpmo U, Berglund J, Fish D, Barbour AG. Typing of Borrelia relapsing fever group strains. Emerg Infect Dis. 2004;10(9):1661–1664. doi:10.3201/eid1009.040236
64. Schwan TG, Raffel SJ, Schrumpf ME, et al. Tick-borne relapsing fever and Borrelia hermsii, Los Angeles County, California, USA. Emerg Infect Dis. 2009;15(7):1026–1031. doi:10.3201/eid1507.090223
65. Vuyyuru R, Liu H, Manser T, Alugupalli KR. Characteristics of Borrelia hermsii infection in human hematopoietic stem cell-engrafted mice mirror those of human relapsing fever. Proc Natl Acad Sci U S A. 2011;108(51):20707–20712. doi:10.1073/pnas.1108776109
66. Cutler SJ. Relapsing fever borreliae: a global review. Clin Lab Med. 2015;35(4):847–865. doi:10.1016/j.cll.2015.07.001
67. Wagemakers A, Staarink PJ, Sprong H, Hovius JW. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol. 2015;31(6):260–269. doi:10.1016/j.pt.2015.03.008
68. Cutler SJ, Ruzic-Sabljic E, Potkonjak A. Emerging borreliae – expanding beyond Lyme borreliosis. Mol Cell Probes. 2017;31:22–27. doi:10.1016/j.mcp.2016.08.003
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.