Back to Journals » International Medical Case Reports Journal » Volume 9
Arterial stiffness, as monitored by cardio–ankle vascular index, is affected by obstructive sleep apnea, blood glucose control, and body weight – a case with 8 years follow up
Authors Shimizu K , Yamamoto T, Shirai K
Received 23 May 2016
Accepted for publication 7 July 2016
Published 9 August 2016 Volume 2016:9 Pages 231—235
DOI https://doi.org/10.2147/IMCRJ.S113377
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ronald Prineas
Kazuhiro Shimizu,1 Tomoyuki Yamamoto,2,3 Kohji Shirai2,4
1Department of Internal Medicine, Toho University Sakura Medical Center, Chiba, Japan; 2Department of Vascular Function, Toho University Sakura Medical Center, Chiba, Japan; 3Biological Information Analysis Section, Fukuda Denshi Co., Ltd., Tokyo, Japan; 4Department of Internal Medicine, Mihama Hospital, Chiba, Japan
Abstract: The cardio–ankle vascular index (CAVI) is an indicator of arterial stiffness from the heart to the ankles. The CAVI increases as arteriosclerosis progresses, but it can be decreased by appropriate treatment. There are several risk factors for coronary artery disease, however, the degree of stress caused by each separate risk factor to arteries cannot be assessed. CAVI increases with age and according to the severity of atherosclerosis. We found that CAVI also changes in response to the control of risk factors, which may be associated with the functional stiffness of arteries. CAVI can be a useful indicator of risk control for coronary artery disease. We followed a patient aged 71 years who had diabetes mellitus and obstructive sleep apnea (OSA) by measuring CAVI for 8 years from age 63. He underwent coronary artery bypass grafting due to angina pectoris when he was 63 years old. Before coronary artery bypass grafting, CAVI was 11.8 on the right and 11.5 on the left. Three years later he was found to have OSA and received treatment with continuous positive airway pressure. There was a marked improvement in CAVI after continuous positive airway pressure (age 68; right 10.4, left 10.2). However, following a gradual increase in body weight and worsening of diabetes mellitus, CAVI showed an increasing trend. CAVI decreased with biguanides treatment, but increased again with an increase in body weight. In conclusion, CAVI responded to the patient’s conditions including obesity, diabetes mellitus, and OSA. CAVI is not only a marker of arterial stiffness, but can also be a useful indicator of physiological status; it may be effective in total risk control for coronary artery disease.
Keywords: obstructive sleep apnea, continuous positive airway pressure, cardio-ankle vascular index, biguanides, obesity
Introduction
Cardio–ankle vascular index (CAVI), an indicator of the overall stiffness of the artery from the origin of the aorta to the ankle, is elevated in patients who have coronary risk factors. Both organic and functional factors are thought to be associated with arterial stiffness assessed by CAVI, and the latter can be improved by changes in lifestyle.1,2
The principle of CAVI is based on the stiffness parameter β theory proposed by Hayashi et al.2 Arterial stiffness was originally assessed within a limited region. For the assessment of stiffness of long arteries, Bramwell-Hill’s equation was adapted, which is based on the assumption that change in vascular caliber is associated with pulse wave velocity.1 Therefore, CAVI is calculated using systolic and diastolic blood pressure and pulse wave velocity.1 The vascular screening system, VaSera (Fukuda Denshi Co., Ltd., Tokyo, Japan), has been developed as a method to assess arterial stiffness based on this theory. The reproducibility of CAVI using this device is consistent (coefficient of variance: average 3.8%).1
Several clinical studies have shown that CAVI is increased in patients with atherosclerotic diseases as well as in those with coronary risk factors, however it decreases in response to control of those risk factors.2
We followed a patient who had diabetes mellitus and obstructive sleep apnea (OSA) by measuring CAVI for 8 years. OSA is defined as a condition with the presence of at least five obstructive respiratory events (eg, apnea and hypopneas) per hour during sleep; it is found in 9%–26% of middle-aged people without specific risk factors for the disorder. OSA is associated with an increased prevalence of cardiovascular and cerebrovascular disease, as well as insulin resistance.3,4
Case report
The present case was a 71-year-old male patient who had diabetes mellitus, hypertension, dyslipidemia, and OSA. He had a history of acute myocardial infarction in the mid of the left coronary artery when he was 49 years old. Written informed consent was obtained from the patient to publish patient data.
He developed effort angina at age 63 years and underwent coronary angiography, as three affected branches were found, coronary artery bypass grafting (CABG) was planned. Before CABG, CAVI was 11.8 on the right and 11.5 on the left. His height was 178 cm, body weight was 76 kg, body mass index was 23.9, and systolic/diastolic blood pressure was 135/81 mmHg. He received oral medication with carvedilol 10 mg/day, losartan 50 mg/day, rosuvastatin 7.5 mg/day, aspirin 100 mg/day, and biguanides 500 mg/day; he administered self-injections of insulin – Humalog Mix® 50 (16-18-20 units). CABG was successfully performed. Three years later, at age 66 years, he was suspected of having OSA because of daytime sleepiness and he underwent polysomnography (PSG), which revealed severe OSA with apnea–hypopnea index (AHI) 56.9 events per hour. Continuous positive airway pressure (CPAP) was started at age 66.
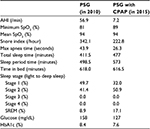
During CPAP, the patient had good compliance in wearing a mask from the early stage; during the medical examination, the usage rate was consistently ≥90% (average 7 hours), and residual AHI was maintained at ≤2 events. Five years after the initial CPAP, at age 71, CPAP-PSG was done. Table 1 shows changes in sleeping parameters between the first and second PSG. After the start of CPAP, apnea disappeared. In addition, the ratio of sleep stage 1 was decreased, while stage 2 and rapid eye movement sleep stage were increased.
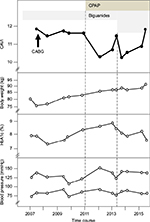
An epidemiological study has shown that CAVI increases with age. In the present case, when the patient was 68 years old, CAVI was 10.4 on the right and 10.2 on the left, showing a marked improvement from baseline (age 63; right 11.8, left 11.5) (Figure 1). CAVI then gradually increased to the baseline level during the subsequent year and a half. Hemoglobin A1c (HbA1c) worsened to 8.9% following an increase in body weight. Owing to an increased dose of biguanides, HbA1c improved, and in response, CAVI also improved. Nevertheless, even after the positive response to treatment with CPAP and biguanides, vascular elasticity increased, as reflected by an increased CAVI, following an increase in body weight due to worsened lifestyle and uncontrolled diabetes. CAVI is little affected by blood pressure level, as shown in Figure 1.
Discussion
OSA is associated with increased cardiovascular morbidity and mortality. A long-term epidemiological study conducted in Spain in 2005 showed that the high incidence of cardiovascular disease in patients with severe OSA was significantly decreased after patients started receiving CPAP.5 Cross-sectional studies showed that OSA patients had a significantly higher CAVI.6 Another study conducted in Japan reported that CAVI improved after treatment with CPAP in patients with OSA.7 The results of a prospective study in Lithuania in 2015 that investigated 2,106 patients with metabolic syndrome showed that, during the mean 3.8-year follow up, the survival rate was significantly lower in patients with CAVI ≥7.95 due to cardiovascular disease. This suggests that in patients with metabolic syndrome CAVI may be considered as a surrogate risk marker of cardiovascular disease.8
CAVI is closely associated with age according to epidemiological studies, although clinical test values should be monitored on the basis of individual cases. CAVI in the present case, which should have shown an increasing trend during the 8-year observation period, showed a decrease after the patient started CPAP. It is unlikely that arteriosclerosis improved in such a short-term period. We consider that CAVI reflects functional changes including the contractural state of vascular smooth muscle cells, which are associated with sympathetic nerve and parasympathetic nerve activities, nitric oxide generating system, and vascular endothelial function. It is known that CPAP therapy reduces chronic activation of the sympathetic nerves caused by OSA.9-11 As a result, we thought that CAVI was decreased by the improvement of vascular endothelial function. We performed PSG with CPAP 5 years after the initial CPAP, and observed a decrease in AHI from 56.9 events/hour to 7.2 events/hour, an improvement of the hypoxic condition, and achievement of a deeper sleep stage (Table 1). Nevertheless, CAVI again increased in this patient, thus, continuous management of lifestyle including body weight is essential in patients with metabolic syndrome. The potential mechanisms underlying the OSA–obesity–metabolic syndrome interaction involve sympathetic activation, oxidative stress, inflammation, and neurohumoral changes.12-14
Diabetes mellitus is also associated with an increase in CAVI, and antidiabetic agents decrease CAVI.15-17 This is considered to occur because the hyperglycemic condition worsens vascular endothelial function, causing contracture of vascular smooth muscle. In the present case, improvement of this functional stiffness was thought to lead to a decrease in CAVI. Biguanides were shown to improve insulin resistance.18 In the present case, after the start of CPAP, the patient had a gradual increase in HbA1c due to an increase in body weight. CAVI improved after biguanides’ dose was increased from 500 mg to 1,000 mg (Figure 1). However, during further observation, HbA1c and body weight increased again, and CAVI also increased again. Increased CAVI suggests existence of cardiovascular risks like underlying vascular stiffening. CAVI represents both functional and organic arterial stiffness, and reflects both the state of smooth muscle contraction and mechanical properties of the arterial wall.2 With an abundance of evidence, continuous monitoring of CAVI to address a sudden change in the values would be necessary to optimize treatment for individual patients. The present case showed that CPAP for OSA and biguanides for diabetes are useful treatments that improve vascular endothelial function, however, the positive effects on CAVI last only for a short term. It may be necessary to improve upstream conditions such as obesity.
Increased CAVI suggests the existence of cardiovascular risks like underlying vascular stiffening, and decreased CAVI suggests their improvement. As for the long term, management of upstream conditions such as obesity may be more necessary. Vascular function parameters including CAVI may be useful not only in epidemiological studies, but also in the assessment of risk factors on an individual patient basis.
Disclosure
Kazuhiro Shimizu declares no conflict of interest related to this work. Tomoyuki Yamamoto is employed by Fukuda Denshi Co., Ltd., was involved in the development of Vasera measuring CAVI, and reports no other conflicts of interest in this work. Kohji Shirai is a visiting professor of the Department of Vascular Function in Toho University, is supported by Fukuda Denshi Co., Ltd., but has no patent and no financial profit, and reports no other conflicts of interest in this work.
References
Shirai K, Utino J, Otsuka K, Takata M. A noble blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb. 2006;13(2):101–107. | ||
Hayashi K, Yamamoto T, Takahara A, Shirai K. Clinical assessment of arterial stiffness with cardio-ankle vascular index: theory and applications. J Hypertens. 2015;33(9):1742–1757. | ||
Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521–530. | ||
Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. | ||
Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. | ||
Kumagai T, Kasai T, Kato M, et al. Establishment of the cardio-ankle vascular index in patients with obstructive sleep apnea. Chest. 2009;136(3):779–786. | ||
Kato M, Kumagai T, Naito R, et al. Change in cardio-ankle vascular index by long-term continuous positive airway pressure therapy for obstructive sleep apnea. J Cardiol. 2011;58(1):74–82. | ||
Laucevičius A, Ryliškyte L, Balsyte J, et al. Association of cardio-ankle vascular index with cardiovascular risk factors and cardiovascular events in metabolic patients. Medicina. 2015;51(3):152–158. | ||
Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003;177(3):385–390. | ||
Narkiewicz K,van de Borne PJ, Cooley RL, Dyken ME, SomersVK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98(8):772–776. | ||
Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol (1985). 1997;83(1):95–101. | ||
Nagayama D, Endo K, Ohira M, et al. Effects of body weight reduction on cardio-ankle vascular index (CAVI). Obes Res Clin Pract. 2013;7(2):e139–e145. | ||
Iguchi A, Yamakage H, Tochiya M, et al. Effects of weight reduction therapy on obstructive sleep apnea syndrome and arterial stiffness in patients with obesity and metabolic syndrome. J Atheroscler Thromb. 2013;20(11):807–820. | ||
Satoh-Asahara N, Kotani K, Yamakage H, et al. Cardio-ankle vascular index predicts for the incidence of cardiovascular events in obese patients: a multicenter prospective cohort study (Japan Obesity and Metabolic Syndrome Study: JOMS). Atherosclerosis. 2015;242(2):461– 468. | ||
Ibata J, Sasaki H, Kakimoto T, et al. Cardio-ankle vascular index measures arterial wall stiffness independent of blood pressure. Diabetes Res Clin Pract. 2008;80(2):265–270. | ||
Nagayama D, Saiki A, Endo K, et al. Improvement of cardio-vascular vascular index by glimepiride in type 2 diabetic patients. Int J Clin Pract. 2010;64(13):1796–1801. | ||
Ohira M, Endo K, Oyama T, et al. Improvement of postprandial hyperglycemia and arterial stiffness upon switching from premixed human insulin 30/70 to biphasic insulin aspart 30/70. Metabolism. 2011;60(1):78– 85. | ||
Scarpello JH, Howlett HC. Metformin therapy and clinical uses. Diab Vasc Dis Res. 2008;5(3):157–167. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.