Back to Journals » Patient Related Outcome Measures » Volume 10
Are patient-reported outcomes useful in post-treatment follow-up care for women with early breast cancer? A scoping review
Authors Riis CL , Bechmann T , Jensen PT , Coulter A , Steffensen KD
Received 26 November 2018
Accepted for publication 30 January 2019
Published 27 March 2019 Volume 2019:10 Pages 117—127
DOI https://doi.org/10.2147/PROM.S195296
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lynne Nemeth
Cathrine Lundgaard Riis,1–3 Troels Bechmann,1,2 Pernille Tine Jensen,4,5 Angela Coulter,2,3,6 Karina Dahl Steffensen1–3
1Department of Oncology, Vejle Hospital, Vejle, Denmark; 2Institute of Regional Health Research, Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark; 3Center for Shared Decision Making, Vejle, Denmark; 4Department of Gynecology and Obstetrics, Odense University Hospital, Odense, Denmark; 5Department of Clinical Research, University of Southern Denmark, Odense, Denmark; 6Nuffield Department of Population Health, University of Oxford, Oxford, UK
Background: Patient-reported outcomes (PROs) are frequently used to evaluate treatment effects and quality of life in clinical trials. The application of PROs in breast cancer clinics is evolving but their use to generate real-time information for use in follow-up care is uncommon. This proactive use might help to shift healthcare delivery toward a more patient-centered approach by acting as a screening tool for unmet needs or a dialogue tool to discuss issues proposed by the patient.
Aims: This review aims to determine the effects and feasibility of using PROs proactively during follow-up care in early breast cancer.
Materials and methods: A systematic search was conducted in January 2019 in PubMed, Cochrane Library, Embase, and CINAHL. Studies that exclusively concerned women treated for early breast cancer where PROs were used as a proactive tool during follow-up were included.
Results: The search revealed a total of 653 records and four eligible studies were identified; three of which concerned the use of PROs both as a screening tool and as a dialogue tool, and one study in which PROs were used solely as a screening tool. The studies explored the feasibility of collecting and integrating PROs in the clinic and their ability to detect otherwise unrecognized problems. All of the included studies were prone to bias, but they point to potential benefits in respect of better symptom management in follow-up care.
Conclusion: Our search identified a small number of low to moderate quality studies of the proactive use of PROs during follow-up after treatment for early stage breast cancer. The limited evidence available suggests that PROs may be useful for providing a more complete picture of the patient’s symptoms and problems, possibly leading to improvements in symptom management.
Keywords: proactive, patient-reported outcome, PRO, breast cancer, follow-up
Introduction
Early stage breast cancer patients experience multiple symptoms following diagnosis and treatment.1,2 In policy-making and healthcare research, patient involvement has been valued as a way to assess whether the healthcare system delivers what matters most to patients.3,4 Accurate assessment of health status and quality of life is essential for improving well-being and rehabilitation in cancer care.4–6
Patient-reported outcomes (PROs) measure quality of life, physical and social functioning, symptoms, side effects, and emotional well-being. According to the US Food and Drug Administration, PRO data are “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.”7 These measures are distinct from, but complementary to, disease-focused outcomes such as survival, mortality, and other clinical outcomes.8 Self-reported data are collected using questionnaires that can be repeated over time or used as a single evaluation, depending on the purpose.9 Systematically collected PRO data are being used increasingly in clinical trials of cancer treatments,10,11 mainly as a secondary outcome to support a primary clinical outcome.12,13
Breast cancer is the most frequent cancer among women, with an estimated 2.08 million new cases diagnosed worldwide in 2018, constituting 24.2% of all cancers.14 Advances in breast cancer treatments and improved diagnostics continue to increase the population of breast cancer survivors around the world. Within this population, PRO data have the potential to provide valuable information about long-term side effects and quality of life during survivorship.15,16 PROs are mainly used to evaluate treatment effects. Their use in real time, to inform decisions in follow-up care for individual patients, is relatively rare, but may have the potential to shift healthcare delivery toward a more patient-centered approach.5,17
PROs can potentially be used as a screening tool to customize supportive care for breast cancer survivors. If patients are provided with PROs at several fixed time points during follow-up, the measurements can be used to allocate the patients to the intervention needed. Exposure of new problems may give an early indication of recurrence of breast cancer or of the appearance of unacceptable sequelae to prier treatment.18,19 Reports of fewer problems may be an indicator of effective supportive care, potentially avoiding further interventions and unnecessary clinical appointments, which could be beneficial to both the patient and the healthcare system.20–23 The use of PROs as a dialogue tool in the clinical consultation could help to prompt discussion on the patient’s individual needs, potentially revealing otherwise undetected physical or psychological effects.10,21,24
The potential benefits of using PROs proactively during follow-up care are somewhat different from their use during active treatment. During treatment, symptoms may arise and change rapidly, and patients are usually scheduled for mandatory consultations in order to handle side effects properly and to be prescribed the continued treatment. In this case, PROs are primarily used to illuminate the dialogue about symptom management.6,25,26 In follow-up care, breast cancer patients experience a more varying set of needs, but with fewer opportunities to report problems to the clinician. Here, the potential of a screening tool to detect patients’ individual needs may be helpful to support personalized rehabilitation.1
The present study reviews the literature on the proactive and real-time use of PROs in post-treatment follow-up care for early stage breast cancer patients. We seek to evaluate the feasibility and potential effects of PROs used proactively as screening tools to help clarify the patients’ individual needs and as discussion prompts to enhance the quality, depth, and breadth of dialogue in the clinical consultation.
Materials and methods
Scoping reviews are systematic literature reviews in a broad topic area that provide relevant and quantified results about the knowledge available on a particular topic and aim to rapidly map and synthesize the evidence to emphasize what is known. Scoping reviews are used to identify knowledge gaps, set research agendas, and identify implications for decision-making.27,28
Search strategy
A systematic search was conducted in November 2017 and updated through January 9, 2019 in PubMed, using the key words “breast cancer” and the MeSH term “breast neoplasms” combined with the key words “patient reported outcome*” and the MeSH terms “patient outcome assessment” and “patient reported outcome measures.” No restrictions on language, year, or type of study design were applied and relevant references were examined for additional studies. A similar search strategy was applied to the Cochrane Library, Embase, and CINAHL databases. Please refer to Supplementary materials for the entire search string.
Selection criteria
Studies that met the following criteria were included: 1) the study population exclusively concerned women treated for early stage breast cancer; and 2) PROs were used proactively as a screening or a dialogue tool during follow-up care. In the first selection stage, all relevant citations were screened based on the title and abstract for the use of PROs in a breast cancer population during follow-up. Secondly, full-text articles of potentially eligible studies were obtained and assessed for eligibility. Both procedures were performed by one reviewer (CLR), but any doubt of eligibility was resolved by achieving consensus among three reviewers (CLR, TB, KDS). We excluded studies if the population consisted of mixed cancer types or if some patients had metastatic disease, and we excluded studies if PROs were collected as an outcome measure but not used proactively. Hence, only studies concerned with the proactive use of PROs were included, by which we mean data reported by a patient and used to inform care for the same patient during follow-up.
Information was extracted in a standardized format according to a prespecified Data Extraction Sheet29 to summarize the studies under the following headings: patients, study methods, aims and outcome, assessments, description of the PROs, how they were used, principal findings, and comments. Extracted data are presented in Table 1.
A PRO used as a screening tool was defined as a tool for allocating breast cancer patients to the most optimal supportive care based on symptomatology. A PRO used as a dialogue tool was defined as a tool, which revealed the patient’s physical and psychological symptoms or concerns ahead of a clinical visit and contributed to the discussion of those at the clinical visit. Follow-up care was defined as the post-treatment rehabilitation assignment, which follows and complements the primary surgery and adjuvant treatment with chemotherapy and/or radiotherapy. Defining the initiation of follow-up care may be difficult, since some patients receive only surgery while others have several kinds of adjuvant treatments, including up to 10 years of endocrine therapy. In the current review, the management of acute side effects from chemotherapy and radiotherapy is not considered to be part of follow-up care. Consequently, follow-up initiates at the end of these treatments. Adjuvant endocrine treatment is recommended for 5–10 years and is thereby included as one of the challenges follow-up care needs to provide for.
Assessment of risk of bias
Risk of bias at the individual study level was assessed using the Risk of Bias in Non-randomized Studies – of Interventions (ROBINS-I) tool.30 The ROBINS-I tool is based on the Cochrane Risk of Bias tool for randomized controlled trials (RCTs) and was developed for intervention studies that did not use randomization, so in a review with heterogeneous study designs this tool was found to provide the best comparison. Risk of bias is assessed within specified bias domains, and review authors are asked to document the information on which judgements are based. Seven domains were assessed: confounding, selection, classification, departures from intended interventions, missing data, outcome measurement, and selective reporting. An assessment of bias was reported for each of these domains and summed to an overall judgement presented in Table 2. Two review authors (CLR, TB) used the ROBINS-I tool and independently assessed the risk of bias across the seven domains for each included study. Any disagreements were discussed and resolved by consensus.
Results
As shown in the PRISMA (Figure 1),29,31 a total of 653 records were identified and screened for eligibility. Ninety-six articles concerned women treated for early stage breast cancer with PROs used as a primary or secondary outcome measure in a follow-up setting. These were further scrutinized and those articles not concerned with the proactive use of PROs in follow-up care were discarded, leaving four studies that met the eligibility criteria.
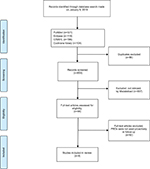  | Figure 1 PRISMA diagram. Abbreviation: PRO, patient-reported outcome. |
The extracted four articles were of recent date, published from 2012 until 2017. A summary of the abstracted information is presented in Table 1. Sample sizes ranged from 102 to 172 patients. Three out of four articles used electronic surveys and multiple assessments over time to achieve a longitudinal perception of the patient’s condition during follow-up.32–34 In the fourth study, printed PRO questionnaires were provided to patients with a prepaid envelope for completion at a single time-point as a cross-sectional survey analysis.35
Characteristics of PRO tools
Three studies used generic, validated PRO tools to capture the patient’s perspective of health, quality of life, anxiety, depression, or other related issues.32,33,35 In one study,33 the content of the questionnaires included both validated surveys such as the 36-Item Short-Form Health Survey36 and the 8-item Patient Health Questionnaire depression scale (PHQ-8),37 as well as a non-validated symptom questionnaire modified from the Memorial Symptom Assessment Scale. The questionnaire also included a free text area for patients to pose questions or report concerns to their provider. The PHQ-8 is validated as a diagnostic and severity measure for depressive disorders in large clinical trials. The Hospital Anxiety and Depression Scale (HADS) is another widely used and validated tool to measure anxiety and depression.38 The HADS was used together with the European Organization for Research and Treatment of Cancer quality of life questionnaire39 in a pilot study.32 This study also used the validated distress thermometer, a numerical scale from 0 (no distress) to 10 (extreme distress), to allocate patients into the study by a cutoff point of >7. This score has been shown to identify breast cancer patients suffering from moderate to severe distress.40 The fourth study used a non-specified web-based health questionnaire with no further documentation about the origin or validation.34
Design, feasibility, and principal findings
The use of PROs both as a screening tool and as a dialogue tool was investigated in three studies.32–34 In a Danish RCT study,32 PROs were used as a screening tool to detect those patients who were in need of a nurse navigator intervention to relieve distress, anxiety, and depression. From a cohort of 116 newly diagnosed breast cancer patients, 50 patients reported scores that indicated a high level of distress. These 50 patients were randomized 1:1 to the intervention group or the control group. The remaining 66 patients were observed and completed PROs at baseline, 6 months, and 12 months. The nurse navigator aimed to improve rehabilitation and supportive care by empathetic listening and actively engaging in dialogue. Using PROs for assessment of the patients’ needs provided topics for psychoeducation, goal-setting, and debriefing. The dialogue was conducted face-to-face or by telephone and could be assisted by referrals to existing rehabilitation offers. At 12 months, the intervention group reported lower levels of distress, anxiety, and depression compared to the control group. No significant effects were seen on health-related quality of life. The intervention was found to be feasible and useful for patients with high distress scores at the time of diagnosis.32
In an RCT from the USA, a web-based system for symptom management after treatment of breast cancer was evaluated against standard care.33 The online questionnaire included a free text area for patients to pose questions or report concerns to their provider. The information was used in the triage of patients for additional follow-up appointments and aimed at facilitating patient–clinician dialogue, when present. The authors hypothesized that PROs would reduce time to symptom management and decrease the number of breast cancer-related hospital appointments, but no reduction in the use of healthcare resources was demonstrated.33 Participants in the intervention group reported a significant higher mean of 7.36 new or changed symptoms during the 18-month study period compared to the standard care arm with a mean of 3.2 new or changed symptoms. The authors concluded that the online questionnaires facilitated better reporting and more timely assessment of symptoms, particularly for those symptoms not deemed by the patient to be urgent and in need of immediate attention. There was a comparatively low questionnaire completion rate of 50%, which the authors suggested could be caused by the electronic interface, which might have inhibited its use by some patients.33
In a retrospective analysis of 106 breast cancer patients, the impact of using a web-based health questionnaire on symptom reporting, physician documentation of symptoms, and symptom management was investigated for its potential of screening for otherwise undetected symptoms and as a dialogue tool in the management of those.34 The study sample was randomly selected from a population of patients who had filled in a follow-up questionnaire before their appointment and had given consent for data to be used in clinical research. The sample was argued to be representative for the whole breast cancer population. A summary of the information reported by the patients was available for clinician review before the patient’s visit. The questionnaire completion rate was nearly 80% for first appointments and about 40% for follow-up visits in the population from which the sample was extracted. The primary finding was a significantly higher incidence of symptoms reported by the patient than documented by the clinician.34
In the fourth study, a prospective study of 172 participants, PRO measures were used solely as a screening tool to identify levels of distress in breast cancer survivors approaching discharge from hospital-based follow-up care to community-based care at least 2 years past diagnosis.35 This study met the inclusion criterion of proactive use by the fact that patients who reported scores that indicated significant distress were contacted and referred to supportive services. Only a minority of patients reported high anxiety and depression scores on the generic PRO instruments used in this study, but the authors concluded that screening with PROs for psychological/emotional distress should be a vital part of follow-up care. The response rate was 75%.35
Biases in the studies
The assessment of risk of bias by the ROBINS-I tool is presented in Table 1. Only the pilot study32 achieved moderate bias assessment as a consequence of the RCT design and high response rates. The remaining three studies were assessed as having a high risk of selection bias, confounding, and missing data. The scores according to the ROBINS-I tool across seven domains are reported in Table 2.
Discussion
The majority of the publications on the use of PROs within breast cancer patients deal with the patient’s evaluation of a treatment.10,41 Using PRO data in clinical trials examining side effects of adjuvant endocrine therapy has revealed a higher frequency of both physical and psychosocial symptom burden than based on clinicians’ assessment.42–46 Thus, one of the potential benefits with the application of systemically obtained PRO data, used proactively in survivorship, may be to provide a more comprehensive evaluation that includes the patient’s perception of problems experienced during follow-up care.11,42–46
PROs collected ahead of a clinical visit and actively used as part of informed care for individual patients seem far more challenging than including PROs as an outcome measure in a clinical trial to assess treatment effects.47,48 Implementation of the proactive use of PROs in everyday care involves changing the clinical culture and workflow, requiring extra training and support for those unfamiliar with these tools. Introducing changes in clinical procedures in the face of busy work schedules can lead to a disturbed work pattern, delays, and resistance from clinical staff. Clarification of the appropriate use and interpretation of PROs and their impact on the healthcare system still needs further investigation.49–52
The present review identified four studies exploring the potential of using PROs as a dialogue tool and a screening tool during follow-up in breast cancer patients. The studies differ concerning design, the selected PRO tools, and how they evaluated the potential of applying PROs proactively during follow-up care. The range of study designs, from a randomized clinical trial to an observational study, complicates comparison of the principal findings but still gives a perspective on the potential for using PRO data proactively.
Three out of four studies used electronic surveys. Apps for phones and tablets focusing on easily accessible electronic surveys are of great importance for the rapid progress of PRO data research.53,54 It is crucial for the collection of real-time data that patients are able to assess their PROs where ever they are.22,55 Electronic collections of PROs also provide the possibility of immediate feedback to the patient and could be combined with some kind of web-based self-management application to support patient education, activation, and empowerment.56 However, electronic questionnaires may not be feasible for all patients due to language or skill barriers, particularly older people who lack computer experience or those with multiple morbidities.57 The technical feasibility of integrating PRO data into the electronic medical record has been provided in some settings and makes the collection of PRO measurements more cost-effective.49,58 Success with the task elsewhere is evolving due to technological improvements and the awareness of the potential benefits of using PROs proactively.58–60
In the study by Thompson et al,35 patients completed paper questionnaires. The researchers obtained a high compliance in completed items for the returned PROs, which may be attributable to the simple design of the study with a single evaluation. Of the 227 patients who voluntarily agreed to participate and who were provided with questionnaires, from a cohort of 323 eligible candidates, only 172 actually completed and returned them. The sample was thus highly selected, with only 53.2% of the cohort represented, calling into question its representativeness, and there was no discussion in the paper to inform judgment about the extent of bias this might have introduced. In research it is acceptable to allocate resources to the management of paper questionnaires, but in routine care it is necessary to minimize workloads and resource use. Infrastructure for data collection and safe storage, data analysis, and data presentation that is readily understood by patients and clinicians are further challenging factors for the implementation of PROs.16,52–54,57,59 In the RCT by Wheelock et al,33 low response rates were suggested to be caused by lack of skills toward the management of an electronic survey. The development of user-friendly, easy-access electronic PRO instruments, and timing – what to measure, when, and why – is crucial for standardized implementation, integration, and feasibility.61–66
Collection of PRO data has been recommended in the evaluation of lifestyle interventions in breast cancer survivorship and to provide complementary information to more traditional clinical indicators.28 Multiple studies have demonstrated that PROs more accurately capture patients’ experience of symptoms and other problems than physicians’ assessments.46,67–69 The studies we reviewed on use of PROs during follow-up care are suggestive of more complete symptom reporting, but they were too biased to be conclusive.33,34
Conclusion
The potential use of PROs in early stage breast cancer as a symptom screening and dialogue tool during follow-up is promising. However, our review reveals limited knowledge of its potential. Of the four studies we found, three were prone to bias and the fourth has limitations general for pilot studies, with low reproducibility and generalizability, since there was only one nurse navigator and a small sample size. We believe PROs could be useful to provide more complete and accurate information in patient–clinician communication, enhancing the quality of dialogue and revealing otherwise undetected symptoms or needs, but this assumption requires testing in more robust studies. If proven, this could lead to better follow-up care and improvements in health-related quality of life. The challenges, which must be overcome to provide more reliable evidence on this matter, include resistance from clinicians and the need to develop user-friendly, easily accessible technology, available for all involved parties. Further investigations in larger-scale studies are needed to understand every aspect of using PROs proactively in follow-up after treatment for breast cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
Hansen DG, Larsen PV, Holm LV, et al. Association between unmet needs and quality of life of cancer patients: a population-based study. Acta Oncol. 2013;52(2):391–399. | ||
Ellegaard M-BB, Grau C, Zachariae R, Bonde Jensen A. Fear of cancer recurrence and unmet needs among breast cancer survivors in the first five years. A cross-sectional study. Acta Oncologica. 2017;56(2):314–320. | ||
Organisation for Economic Co-operation and Development. The next generation of health reforms. Ministerial Statement. Paris: OECD; 2017. Available from: https://www.oecd.org/health/ministerial-statement-2017.pdf. Accessed March 21, 2019. | ||
Kool M, van der Sijp JRM, Kroep JR, et al. Importance of patient reported outcome measures versus clinical outcomes for breast cancer patients evaluation on quality of care. The Breast. 2016;27:62–68. | ||
Basch E, Abernethy AP. Supporting clinical practice decisions with real-time patient-reported outcomes. J Clin Oncol. 2011;29(8):954–956. | ||
Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565. | ||
Center for Drug Evaluation and Research. Guidance for Industry, Patient-reported outcome measures: use in medical product development to support labeling claims. Silver Spring, MD: CDER; 2009. Available from: https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf. Accessed March 21, 2019. | ||
Howell D, Fitch M, Bakker D, et al. Core domains for a person-focused outcome measurement system in cancer (PROMS-Cancer core) for routine care: a scoping review and Canadian Delphi consensus. Value Health. 2013;16(1):76–87. | ||
Coulter A, Potter CM, Peters M, Fitzpatrick R. Cancer PROMs: a scoping study. Macmillan Cancer Support; 2015. | ||
Ohsumi S, Shimozuma K. Current status and future perspectives of patient-reported outcome research in clinical trials for patients with breast cancer in Japan. Breast Cancer. 2013;20(4):296–301. | ||
Tevaarwerk AJ, Wang M, Zhao F, et al. Phase III comparison of tamoxifen versus tamoxifen plus ovarian function suppression in premenopausal women with node-negative, hormone receptor-positive breast cancer (E-3193, INT-0142): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2014;32(35):3948–3958. | ||
Ribi K, Luo W, Bernhard J, et al. Adjuvant tamoxifen plus ovarian function suppression versus tamoxifen alone in premenopausal women with early breast cancer: patient-reported outcomes in the suppression of ovarian function trial. J Clin Oncol. 2016;34(14):1601–1610. | ||
Bernhard J, Luo W, Ribi K, et al. Patient-reported outcomes with adjuvant exemestane versus tamoxifen in premenopausal women with early breast cancer undergoing ovarian suppression (text and soft): a combined analysis of two phase 3 randomised trials. Lancet Oncol. 2015;16(7):848–858. | ||
Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2018;39:1–13. | ||
Fallowfield L, Jenkins V. Psychosocial/survivorship issues in breast cancer: are we doing better? J Natl Cancer Inst. 2015;107(1):335. | ||
Palmer SC, Stricker CT, Demichele AM, et al. The use of a patient-reported outcome questionnaire to assess cancer survivorship concerns and psychosocial outcomes among recent survivors. Support Care Cancer. 2017;25(8):2405–2412. | ||
Gilbert A, Sebag-Montefiore D, Davidson S, Velikova G. Use of patient-reported outcomes to measure symptoms and health related quality of life in the clinic. Gynecol Oncol. 2015;136(3):429–439. | ||
Fu MR, Axelrod D, Guth A, et al. A Web- and Mobile-Based intervention for women treated for breast cancer to manage chronic pain and symptoms related to lymphedema: randomized clinical trial rationale and protocol. JMIR Res Protoc. 2016;5(1):e7p. | ||
Rush CL, Darling M, Elliott MG, et al. Engaging Latina cancer survivors, their caregivers, and community partners in a randomized controlled trial: Nueva Vida intervention. Qual Life Res. 2015;24(5):1107–1118. | ||
Lin NU, Thomssen C, Cardoso F, et al. International guidelines for management of metastatic breast cancer (MBC) from the European School of Oncology (ESO)–MBC Task Force: surveillance, staging, and evaluation of patients with early-stage and metastatic breast cancer. Breast. 2013;22(3):203–210. | ||
Todd BL, Feuerstein M, Gehrke A, Hydeman J, Beaupin L. Identifying the unmet needs of breast cancer patients post-primary treatment: the Cancer Survivor Profile (CSPro). J Cancer Surviv. 2015;9(2):137–160. | ||
Patel RA, Klasnja P, Hartzler A, Unruh KT, Pratt W. Probing the benefits of real-time tracking during cancer care. AMIA Annu Symp Proc. 2012;2012:1340–1349. | ||
Porter I, Gonçalves-Bradley D, Ricci-Cabello I, et al. Framework and guidance for implementing patient-reported outcomes in clinical practice: evidence, challenges and opportunities. J Comp Eff Res. 2016;5(5):507–519. | ||
Stover AM, Basch EM. Using patient-reported outcome measures as quality indicators in routine cancer care. Cancer. 2016;122(3):355–357. | ||
Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197. | ||
Basch E. The rationale for collecting patient-reported symptoms during routine chemotherapy. American Society of Clinical Oncology Educational Book. 2014;34:161–165. | ||
Tricco AC, Lillie E, Zarin W, et al. A scoping review on the conduct and reporting of scoping reviews. BMC Medical Research Methodology. 2016;16(1):1–10. | ||
Howell D, Molloy S, Wilkinson K, et al. Patient-reported outcomes in routine cancer clinical practice: a scoping review of use, impact on health outcomes, and implementation factors. Ann Oncol. 2015;26(9):1846–1858. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine. 2009;151(4):264. | ||
Sterne JAC, Hernán Ma, Reeves BC, Savović J. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:4–10. | ||
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine. 2009;6(7):e1000100. | ||
Mertz BG, Dunn-Henriksen AK, Kroman N, et al. The effects of individually tailored nurse navigation for patients with newly diagnosed breast cancer: a randomized pilot study. Acta Oncologica. 2017;56(12):1682–1689. | ||
Wheelock AE, Bock MA, Martin EL, et al. SIS.NET: a randomized controlled trial evaluating a web-based system for symptom management after treatment of breast cancer. Cancer. 2015;121(6):893–899. | ||
Bock M, Moore D, Hwang J, et al. The impact of an electronic health questionnaire on symptom management and behavior reporting for breast cancer survivors. Breast Cancer Research and Treatment. 2012;134(3):1327–1335. | ||
Thompson J, Coleman R, Colwell B, et al. Levels of distress in breast cancer survivors approaching discharge from routine hospital follow-up. Psycho-Oncology. 2013;22(8):1866–1871. | ||
Ware JE, Sherbourn CD. The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual Framework and Item Selection Author (s): John E. Ware, Jr. and Cathy Donald Sherbourne Published by : Lippincott Williams & Wilkins Stable URL : http://www.jstor.org/stable/3765916 Ac. Medical Care. 1992;30(6):473–483. | ||
Reyes-Gibby CC, Anderson KO, Morrow PK, Shete S, Hassan S. Depressive symptoms and health-related quality of life in breast cancer survivors. J Womens Health. 2012;21(3):311–318. | ||
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. | ||
Groenvold M, Klee MC, Sprangers MA, Aaronson NK, Aaronson NK, Sprangers, Mirjam A.G. and Aaronson. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J Clin Epidemiol. 1997;50(4):441–450. | ||
Ploos van Amstel FK, Tol J, Sessink KH, et al. A specific distress cutoff score shortly after breast cancer diagnosis. Cancer Nurs. 2017;40(3):E35–E40. | ||
Pe M, Dorme L, Coens C, et al. Statistical analysis of patient-reported outcome data in randomised controlled trials of locally advanced and metastatic breast cancer: a systematic review. Lancet Oncol. 2018;19(9):e459–e469. | ||
Snyder CF, Blackford AL, Aaronson NK, et al. Can patient-reported outcome measures identify cancer patients’ most bothersome issues? J Clin Oncol. 2011;29(9):1216–1220. | ||
Oberguggenberger A, Goebel G, Beer B, et al. Getting the whole picture: adding patient-reported outcomes to adjuvant endocrine treatment evaluation in premenopausal breast cancer patients. Breast J. 2014;20(5):555–557. | ||
Snyder CF, Blackford AL, Okuyama T, et al. Using the EORTC-QLQ-C30 in clinical practice for patient management: identifying scores requiring a clinician’s attention. Qual Life Res. 2013;22(10):2685–2691. | ||
Ganz PA, Petersen L, Bower JE, Crespi CM. Impact of adjuvant endocrine therapy on quality of life and symptoms: observational data over 12 months from the mind-body study. J Clin Oncol. 2016;34(8):816–824. | ||
Ellegaard M-BB, Grau C, Zachariae R, Jensen AB. Women with breast cancer report substantially more disease- and treatment-related side or late effects than registered by clinical oncologists: a cross-sectional study of a standard follow-up program in an oncological department. Breast Cancer Res Treat. 2017;164(3):727–736. | ||
Nordan L, Blanchfield L, Niazi S, et al. Implementing electronic patient-reported outcomes measurements: challenges and success factors. BMJ Qual Saf. 2018;27(10):852–856. | ||
Greenhalgh J, Meadows K. The effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: a literature review. J Eval Clin Pract. 1999;5(4):401–416. | ||
Schougaard LMV, Larsen LP, Jessen A, et al. AmbuFlex: tele-patient-reported outcomes (telePRO) as the basis for follow-up in chronic and malignant diseases. Qual Life Res. 2016;25(3):525–534. | ||
Calvert M, Kyte D, Duffy H, et al. Patient-reported outcome (PRO) assessment in clinical trials: a systematic review of guidance for trial protocol writers. PLoS ONE. 2014;9(10):e110216. | ||
Snyder CF, Herman JM, White SM, et al. When using patient-reported outcomes in clinical practice, the measure matters: a randomized controlled trial. J Oncol Practice. 2014;10(5):e299–e306. | ||
Smith TG, Castro KM, Troeschel AN, et al. The rationale for patient-reported outcomes surveillance in cancer and a reproducible method for achieving it. Cancer. 2016;122(3):344–351. | ||
Kuijpers W, Groen WG, Oldenburg HS, et al. eHealth for breast cancer survivors: use, feasibility and impact of an interactive portal. JMIR Cancer. 2016;2(1):e3. | ||
Min YH, Lee JW, Shin Y-W, et al. Daily collection of self-reporting sleep disturbance data via a smartphone APP in breast cancer patients receiving chemotherapy: a feasibility study. J Med Internet Res. 2014;16(5):e135. | ||
Abernethy AP, Abernethy APLeBlanc. Patient-reported outcomes in cancer care - hearing the patient voice at greater volume. Nat Rev Clin Oncol. 2017;14(12):763–772. | ||
Melissant HC, Verdonck-de Leeuw IM, Lissenberg-Witte BI, Konings IR, Cuijpers P, van Uden-Kraan CF. “Oncokompas”, a web-based self-management application to support patient activation and optimal supportive care: a feasibility study among breast cancer survivors. Acta Oncol. 2018;57(7):924–934. | ||
Graf J, Simoes E, Wißlicen K, et al. Willingness of patients with breast cancer in the adjuvant and metastatic setting to use electronic surveys (ePRO) depends on sociodemographic factors, health-related quality of life, disease status and computer skills. Geburtshilfe und Frauenheilkunde. 2016;76(05):535–541. | ||
Bennett AV, Jensen RE, Basch E. Electronic patient-reported outcome systems in oncology clinical practice. CA: A Cancer Journal for Clinicians. 2012;62(5):336–347. | ||
Snyder CF, Blackford AL, Wolff AC, et al. Feasibility and value of PatientViewpoint: a web system for patient-reported outcomes assessment in clinical practice. Psychooncology. 2013;22(4):895–901. | ||
Snyder CF, Aaronson NK, Choucair AK, et al. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Quality of Life Research. 2012;21(8):1305–1314. | ||
Wu AW, White SM, Blackford AL, et al. Improving an electronic system for measuring PROs in routine oncology practice. J Cancer Survivorship. 2016;10(3):573–582. | ||
Leblanc M, Stineman M, Demichele A, Stricker C, Mao JJ. Validation of QuickDASH outcome measure in breast cancer survivors for upper extremity disability. Arch Phys Med Rehabil. 2014;95(3):493–498. | ||
Fayanju OM, Mayo TL, Spinks TE, et al. Value-based breast cancer care: a multidisciplinary approach for defining patient-centered outcomes. Annals of Surgical Oncology. 2016;23(8):2385–2390. | ||
Jensen RE, Snyder CF, Abernethy AP, et al. Review of electronic patient-reported outcomes systems used in cancer clinical care. J Oncol Pract. 2014;10(4):e215–e222. | ||
Zhang J, Yao Y-Feng, Zha X-Ming. Development and evaluation of a patient-reported outcome (PRO) scale for breast cancer. 2015;16(18):8573–8578. | ||
Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346(January):f167. | ||
Gordon B-BE, Chen RC. Patient-reported outcomes in cancer survivorship. Acta Oncol. 2017;56(2):166–173. | ||
Berry DL, Blumenstein BA, Halpenny B, et al. Enhancing patient-provider communication with the electronic self-report assessment for cancer: a randomized trial. J Clin Oncol. 2011;29(8):1029–1035. | ||
di Maio M, Basch E, Bryce J, Perrone F. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat Rev Clin Oncol. 2016;13(5):319–325. |
Supplementary material
Supplementary S1 search string: ((((“Patient Reported Outcome Measures”[Mesh]) OR “Patient Outcome Assessment”[Mesh]) OR “patient reported outcome*”[Title/Abstract])) AND ((“breast cancer”[Title/Abstract]) OR “Breast Neoplasms”[Mesh]).
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


