Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 6
Arbekacin: another novel agent for treating infections due to methicillin-resistant Staphylococcus aureus and multidrug-resistant Gram-negative pathogens
Authors Matsumoto T
Received 22 April 2014
Accepted for publication 17 June 2014
Published 26 September 2014 Volume 2014:6 Pages 139—148
DOI https://doi.org/10.2147/CPAA.S44377
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Tetsuya Matsumoto
Department of Microbiology, Tokyo Medical University, Tokyo, Japan
Abstract: Arbekacin sulfate (ABK), an aminoglycoside antibiotic, was discovered in 1972 and was derived from dibekacin to stabilize many common aminoglycoside modifying enzymes. ABK shows broad antimicrobial activities against not only Gram-positive bacteria including methicillin-resistant Staphylococcus aureus (MRSA) but also Gram-negative bacteria such as Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae. ABK has been approved as an injectable formulation in Japan since 1990, under the trade name Habekacin, for the treatment of patients with pneumonia and sepsis caused by MRSA. The drug has been used in more than 250,000 patients, and its clinical benefit and safety have been proven over two decades. ABK currently shows promise for the application for the treatment of multidrug-resistant Gram-negative bacterial infections such as multidrug-resistant strains of P. aeruginosa and Acinetobacter baumannii because of its synergistic effect in combination with beta-lactams.
Keywords: synergistic effect, Habekacin, MRSA, multidrug-resistant Gram-negative bacteria
Introduction
Arbekacin (ABK) (Meiji Seika Pharma Co, Ltd, Tokyo, Japan) has the hydroxy amino-butyryl group as its chemical structure and is classified as a kanamycin family aminoglycoside (Figure 1).1 ABK causes membrane damage and binds both to the 50S and the 30S ribosomal subunits, resulting in codon misreading and inhibition of translation.2 ABK is not inactivated by aminoglycoside-inactivating enzymes such as (3′) aminoglycoside-phosphotransferase (APH), (4′) aminoglycoside-adenyltransferase (AAD), or AAD (2″) and has a weak affinity for (6′-IV) aminoglycoside-acetyltransferase (AAC). Therefore, ABK exhibits antimicrobial activity against Gram-positive and -negative pathogens including strains resistant to gentamicin (GM), tobramycin (TOB), and amikacin (AMK). In particular, ABK has strong antimicrobial potency against methicillin-resistant Staphylococcus aureus (MRSA) and has been used in Japan since 1990 under the trade name Habekacin (Meiji Seika Pharma Co., Ltd. Tokyo, Japan), to treat sepsis and pneumonia caused by MRSA. In addition, Habekacin has also been used in Korea since 2000.
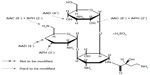  | Figure 1 Structural formula of arbekacin sulfate. |
Principal pharmacology (in vitro antibacterial activities)
ABK showed strong antimicrobial activity against Gram-positive bacteria such as S. aureus3 and Staphylococcus epidermidis.4 Antibacterial activities of ABK, GM, TOB, and AMK against 54 methicillin-susceptible S. aureus clinical isolates were determined and the results are shown in Table 1.3 The minimal inhibitory concentration (MIC) for 90% of the organisms (MIC90) of ABK was 1 μg/mL, whereas MIC90 of GM, TOB, and AMK were 4, 8, and 16 μg/mL, respectively.3 Furthermore, the MIC90 of ABK against S. epidermidis was 0.5 μg/mL and it was stronger than that of AMK (MIC90 4 μg/mL).4 ABK also has superior antibacterial activity against Gram-negative bacteria including Pseudomonas aeruginosa.3,5
The antibacterial activities of ABK against strains producing aminoglycoside-inactivating enzymes were investigated as well as the antibacterial activities of ABK against tested organisms without the influence of aminoglycoside-inactivating enzymes.6 The bactericidal effects of ABK against S. aureus and Escherichia coli were better than those of AMK and GM, and the bactericidal effects against Klebsiella pneumoniae and P. aeruginosa were comparable with AMK and GM.7
Stability to aminoglycoside-inactivating enzymes
ABK was stable to the aminoglycoside-inactivating enzymes produced by MRSA, such as APH, AAD, and AAC.8 Although GM, AMK, TOB, and kanamycin (KM) were completely inactivated by APH (2″), ABK still showed about 50% activity against APH (2″). Furthermore, ABK was not inactivated by AAD (4′) and APH (3′), and also showed stability to these enzymes. These results suggest the excellent antibacterial activities of ABK against MRSA strains.
Antibacterial activity against MRSA
ABK showed the most potent antibacterial effect against clinically isolated MRSA strains among the tested aminoglycosides (GM, TOB, and AMK), and the antibacterial effect of ABK was equivalent to that of vancomycin (VCM).3,9–12 Figure 2 shows the cumulative percentage of MIC against MRSA with the antimicrobial susceptibility surveillance conducted in Japan.9–12 The antimicrobial activity of ABK was more potent than the other anti-MRSA drugs except daptomycin.
The susceptibility of MRSA to ABK has not changed since 1990 when ABK was launched. In another surveillance, the MICs of ABK, VCM, teicoplanin (TEIC), and linezolid (LZD) against 228 MRSA clinical isolates in Japan were determined. The results showed that MIC90/MIC50 of VCM and ABK had not significantly changed in the period from 1990 to 2006 even though MIC90 of TEIC and LZD were slightly increased during the period.13
Bactericidal effect of ABK against MRSA
ABK also shows concentration-dependent bactericidal activity.14–18 Viable counts of MRSA were rapidly decreased in a short period after the addition of ABK in comparison with those of VCM, TEIC, and LZD (Figure 3).18
Post antibiotic effect of ABK
Post antibiotic effect is another characteristic of aminoglycoside antibiotics. When MRSA was treated either with ABK or VCM with the same concentration, the bactericidal activity of VCM was weaker than ABK, and the post antibiotic effect was shorter compared with ABK.14
Inhibition of toxic shock syndrome toxin-1 (TSST-1) by ABK
The effect of ABK, VCM, and TEIC on the production of TSST-1 by MRSA strains has been reported.19 In logarithmic phase cultures, ABK, VCM, and TEIC inhibited TSST-1 production by 85, 10, and 25%, respectively, at the concentration of one fourth of each MIC (Figure 4).
Antibacterial activities against multidrug-resistant P. aeruginosa
Multidrug-resistant strains of P. aeruginosa have been an important issue and the strains with the MICs of AMK ≥32 μg/mL, imipenem ≥16 μg/mL, and ciprofloxacin ≥4 μg/mL are defined as multidrug-resistant P. aeruginosa (MDRP) in Japan. It is difficult to treat patients with MDRP infections and colistin (CL) may be a good candidate for treatment. Because CL is not approved for clinical use in Japan, many doctors in Japan are interested in combination therapy such as beta-lactam antibiotics and aminoglycoside antibiotics.
Antibiotic combination therapy study groups studied the effective combination regimen against MDRP and demonstrated that ABK plus aztreonam (AZT) was the most promising combination, the other promising regimens were AZT plus AMK and AZT plus GM (Figure 5).20
Antibiotic combination therapy study groups also reported that a combination of ABK plus AZT showed synergistic effects as well as the combinations of CL plus rifampin, and AMK plus AZT (Figure 6).21 These results suggest that ABK is a useful agent for MDRP infections used in combination therapies.
Antibacterial activities against multidrug-resistant Acinetobacter baumannii-calcoaceticus
Recently, ABK has also attracted attention for its antibacterial effect against A. baumannii-calcoaceticus. Zapor et al22 examined the in vitro antibacterial activity of ABK against A. baumannii-calcoaceticus isolated from clinical specimens at The Walter Reed Army Medical Center during the Global War on Terrorism. Additionally, the in vitro MIC of ABK against 200 Acinetobacter baumannii-calcoacetics isolates recovered from wounded soldiers was determined. The median MIC was 2 μg/mL (range: 0.5 to >64 μg/mL). A total of 97.5% of the isolates had ABK MICs of <8 μg/mL and 86.5% had MICs of <4 μg/mL. There was no association between the ABK MIC and susceptibility to 16 other antibiotics or the specimen source. Moreover, synergy testing suggested an enhanced effect of ABK-carbapenem combinations.22
Efficacy in mouse mixed infection model (in vivo)
Since ABK has shown potent activities against both MRSA and P. aeruginosa, the effect of ABK in a mixed infection model using MRSA and P. aeruginosa was investigated. The median effect dose (ED50) that calculated the life and death on 7 days after administration was 19.5 mg/kg for ABK and >100 mg/kg for VCM. Thus, ABK showed a protective polymicrobial effect on MRSA and P. aeruginosa infections.23
Pharmacokinetics in adults
A multi-center collaborative open clinical study was conducted in patients infected with MRSA to evaluate the efficacy, safety, and the pharmacokinetics-pharmacodynamics (PK-PD) of ABK. The patients were administered 200 mg of ABK once daily, and the patients with severe renal dysfunction (creatinine clearance ≥80 mL/min) showed changes in the pharmacokinetic parameters such as prolongation of half-life, decrease of total clearance, and increase of area under the curve (0–24 hr) (AUC0–24) (Table 2 and Figure 7A).24
On the other hand, the pharmacokinetics in healthy volunteers with normal renal function did not change on 400 and 600 mg single dose or on multiple administrations of ABK over a period of 7 days (Table 2 and Figure 7B).25 These data suggest that renal clearance and total clearance do not decrease at a high dose, and ABK has no tendency toward accumulation if renal function is normal.
Pharmacokinetics in children
Recommended initial dosing regimens were 5 mg/kg every 48 hours for preterm infants (postnatal age was within 28 days), 5 mg/kg every 24 hours for preterm infants (postnatal age was 28 days or more), and 4 mg/kg every 24 hours for term infants. These initial dosing regimens could manage the maximum concentration (Cmax) 7–15 μg/mL and trough concentration (Ctrough) 0–2 μg/mL in 72.2%–93.5% of infant patients.26
Administration of ABK once daily in neonates has been investigated; the mean serum peak and Ctrough of ABK were 15.2±4.3 μg/mL and 2.0±1.4 μg/mL, respectively. Overall clinical effectiveness was 78.9% and no adverse effect was observed. During the period of administration, serum creatinine levels of some cases increased slightly, although the highest was 0.27 mg/dL but returned to baseline (pre-dose value) promptly after stopping ABK administration. Therefore, it is supposed that ABK therapy once daily in neonates is a treatment option.27
Distribution of ABK
The PK-PD parameters of ABK in bronchial epithelial lining fluid (ELF) were investigated and the mean Cmax in serum and bronchial ELF were 26.0±12.2, and 10.4±1.9 μg/mL, respectively.28 The ratio of concentrations of the drug in bronchial ELF to Cmax in serum was 0.465±0.188. These data suggest that transitivity of ABK to the respiratory tract was relatively good, because transitivity of aminoglycosides to the lungs is about 30% in general.29
It has been reported that volume of distribution of aminoglycosides generally correlates with the extracellular fluid30,31 and tissue fluids, such as interstitial fluid or synovial fluid, with a sufficient concentration of the drug infiltrating a surgical wound site and subcutaneous tissue.32–38 Distribution of ABK from circulating blood to a wound site was evaluated in patients with wound infection caused by S. aureus who were treated with 200 mg of ABK once daily. In this study, high levels of distribution in the wound exudate, 46.2%–55.3%, were observed.39
Therapeutic drug monitoring of ABK
Therapeutic drug monitoring (TDM) of ABK is required for maximizing efficacy while minimizing toxicities. In the population of patients with normal renal function, the target peak concentration (Cpeak) value of 15–20 μg/mL was not achieved with once daily administration of 150–200 mg as the approved dose, and a higher dosing regimen is required to improve clinical efficacy. A clinical practice guideline for TDM of ABK was developed by the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring.40 Experts recommend 300 mg/day (5.5–6.0 mg/kg) to reach the target concentration.
PK-PD parameters
The PK-PD parameter of ABK which was associated with a therapeutic effect was Cmax/MIC and/or AUC/MIC, with a low correlation of efficacy observed in T>MIC, and the highest correlation coefficient observed in Cmax/MIC.41–43 It was shown that the probability of cure/improvement rose when the Cmax of ABK was increased, with an odds ratio of 6.7 for a change in Cmax from 7.9–12.5 μg/mL.44 In other studies, a key determinant of clinical efficacy of ABK was considered to be Cmax/MIC, and the appropriate Cmax/MIC value which showed a good correlation between bacteriological efficacy was 8 or higher.44–48
Clinical efficacy
There are several reports on clinical efficacy, bacteriological efficacy, and safety against MRSA infection which compared the treatment of VCM and ABK.49,50 Hwang et al50 reported that the bacteriological efficacy responses of ABK and VCM were 71.2% and 79.5%, respectively, and the clinical efficacy responses of those were 65.3% and 76.1%, respectively, and that there was no statistically significant difference between ABK and VCM. The incidence of complications was significantly higher in the VCM group (32.9%) in comparison with the ABK group (15.1%) (P=0.019). ABK was not inferior to VCM, and it could be a good alternative drug for the treatment of MRSA infection.49 However, further prospective randomized trials are needed to confirm this finding.50
Clinical trial for re-assessment of higher dose regimen
There is a report on a clinical study to examine the efficacy and safety of ABK in patients with pneumonia or sepsis caused by MRSA.51 In this study, the target Cpeak was initially set at 15–20 μg/mL and TDM was conducted. The efficacy rate was 87.5% (7/8 patients) for sepsis, 90.5% (19/21 patients) for pneumonia, and 89.7% (26/29 patients) in total (Table 3).
Based on the results, it was recommended that the dosage regimen of ABK should be initially set at 5–6 mg/kg or higher, and adjusted to achieve Cpeak at 10–15 μg/mL or higher and Ctrough lower than 2 μg/mL for treatment of patients with MRSA pneumonia or sepsis. With this strategy, low incidence of adverse drug reactions and higher clinical efficacy would be achieved. As for clinical effects, the efficacy rates for sepsis and pneumonia observed in this study were higher than the 70% efficacy rate which was observed in two other studies.24,52 This high efficacy rate might be attributable to the higher concentration of ABK designed in this study. As the result of TDM intervention, the patients with higher Cpeak at the final TDM than at the first TDM showed a 100% efficacy rate.
A study in elderly patients with pneumonia or sepsis caused by MRSA after once daily administration of ABK at the mean dose of 269.2 mg/day has been reported.53 Cpeak values for all patients, in whom ABK treatment had been effective, were 15 μg/mL or higher. Their results and another report’s results by Kimura et al54 suggest that therapy at high doses of ABK is recommendable even in old people, but that the control of Ctrough is crucial.
Combination therapy against multidrug-resistant Gram-negative bacteria
The combined effect of aminoglycosides and monobactams was studied using the Break-point Checkerboard Plate against MDRP.55 Based on the result, a combination of AZT and ABK was selected as the anti-infective agent for MDRP treatment and the treatment result was reported. Since ABK also shows antibacterial activity against Gram-negative resistant bacteria, ABK as combination therapy can be used as a treatment option.
Adverse effect of ABK
Nephrotoxicity is a major adverse drug reaction to aminoglycoside antibiotics.56–59 The incidence of renal-related adverse drug reactions after administration of ABK was related to Ctrough. When Ctrough was 1, 2 or 5 μg/mL, the estimated rate of adverse drug reactions were 2.5, 5.2, and 13.1% respectively, and the incidence of renal-related adverse drug reactions increased with a higher Ctrough.44 The incidence of ABK-induced nephrotoxicity was observed in all patients when ABK was administrated at a total dose of over 5,000 mg, while it was 4% at a total dose of less than 5,000 mg.45
It is supposed that ototoxicity of aminoglycoside occurs because of the gradual drug accumulation of endolymph and perilymph in the inner ear.60–63 In addition, the results of some meta-analyses reported that there was no difference between single dosing and divided dosing in the incidence of ototoxicity.64,65 Yamasoba et al reported that the cochlea could easily be damaged by aminoglycoside antibiotics because of mitochondrial point mutation at location 1555, and that hearing loss might occur with the administration of small amounts of aminoglycoside antibiotics.66,67 This might suggest that hearing loss might occur in a patient who is not taking aminoglycoside antibiotics, but that the hearing loss is due to a familial or hereditary condition.
Conclusion
ABK has been used for the treatment of MRSA infections for over 20 years in Japan and about 15 years in Korea. Clinical evidence achieved in these two countries revealed the safety and efficacy of this drug. Since ABK shows good antibacterial activity against Gram-negative bacteria in addition to MRSA, some physicians reported the high efficacy of ABK for the treatment of multidrug-resistant Gram-negative bacterial infections such as A. baumannii and P. aeruginosa. Therefore, it is expected that ABK will be a good potential antibiotic as an additional treatment option, such as in combination with beta-lactams (eg, AZT), for serious infections due to its potent antibacterial activities against both MRSA and multidrug-resistant Gram-negative bacteria.
Disclosure
T Matsumoto has served as a speaker for Pfizer Inc., Meiji Seika Pharma Co, Ltd, MSD Co, Ltd, and Dainippon Sumitomo Pharma Co, Ltd.
References
Kondo S. | |
Tanaka N, Matsunaga K, Hirata A, Matsuhisa Y, Nishimura T. Mechanism of action of Habekacin, a novel amino acid containing aminoglycoside antibiotic. Antimicrob Agents Chemother. 1983;24(5):797–802. | |
Watanabe A, Yanagihara K, Matsumoto T, et al. Nationwide surveillance of bacterial respiratory pathogens conducted by the Surveillance Committee of Japanese Society of Chemotherapy, Japanese Association for Infectious Diseases, and Japanese Society for Clinical Microbiology in 2009: general view of the pathogens’ antibacterial susceptibility. J Infect Chemother. 2012;18(5):609–620. | |
Yamaguchi K, Ishii Y, Iwata M, et al. Meropenem | |
Nishino T, Sakurai M. In vitro activity of everninomicin (SCH 27899). In: Proceedings of the 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy; October 17–20, 1993; Louisiana, New Orleans. Abstract No 462. | |
Okamoto R, Iyobe S, Mitsuhashi S. HBK | |
Kazuno Y, Tsuneta S, Tamra A, et al. Bactriological evaluation of a new aminoglycoside antibiotic, HBK. Chemother. 1986;34:61–71. | |
Matsuhashi Y, Yamamoto H. | |
Niki Y, Hanaki H, Yagisawa M, et al. Japanese Society of Chemotherapy. The first nationwide surveillance of bacterial respiratory pathogens conducted by the Japanese Society of Chemotherapy. Part 1: a general view of antibacterial susceptibility. J Infect Chemother. 2008;14(4):279–290. | |
Niki Y, Hanaki H, Matsumoto T, et al. Nationwide surveillance of bacterial respiratory pathogens conducted by the Japanese Society of Chemotherapy in 2007: general view of the pathogens’ antibacterial susceptibility. J Infect Chemother. 2009;15(3):156–167. | |
Niki Y, Hanaki H, Matsumoto T, et al. Nationwide surveillance of bacterial respiratory pathogens conducted by the Japanese Society of Chemotherapy in 2008: general view of the pathogens’ antibacterial susceptibility. J Infect Chemother. 2011;17(4):510–523. | |
Takesue Y, Watanabe A, Hanaki H, et al. Nationwide surveillance of antimicrobial susceptibility patterns of pathogens isolated from surgical site infections (SSI) in Japan. J Infect Chemother. 2012;18(6):816–826. | |
Mikuniya T, Kato Y, Muto-Kobayashi Y, et al. Prevalence of drug resistant gene and changes in susceptibility of methicillin-resistant Staphylococcus aureus strains isolated from 1990 to 2006 in Japan to antimicrobial agents. Jpn J Chemother. 2009;61(5):37–40. | |
Watanabe T, Ohashi K, Matsui K, Kubota T. Comparative studies of the bactericidal, morphological and post-antibiotic effects of arbekacin and vancomycin against methicilin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1997;39(4):471–476. | |
Flandrois JP, Fardel G, Carret G. Early stages of in vitro killing curve of LY146032 and vancomycin for Staphylococcus aureus. Antimicrob Agents Chemother. 1988;32(4):454–457. | |
Asseray N, Jacqueline C, Le Mabecque V, et al. Activity of glycopeptides against Staphylococcus aureus infection in a rabbit endocarditis model: MICs do not predict in vivo efficacy. Antimicrob Agents Chemother. 2005;49(2):857–859. | |
Takahashi T, Matsumoto F, Miyazaki S. Comparison of in vitro antibacterial activity of arbekacin, vancomycin and teicoplanin against methicillin-resistant Staphylococcus aureus. Jpn J Chemother. 1999; 47(2):103–107. | |
Kurazono M, Yamada K, Hirai Y, Ida T, Inoue M. MRSA | |
Miyata A, Araake M, Ogawa H, Hanaki H, Hiramatsu K. MRSA | |
Araoka H, Baba M, Tateda K, et al. In vitro combination effects of aztreonam and aminoglycoside against multidrug-resistant Pseudomonas aeruginosa in Japan. Jpn J Infect Dis. 2012;65(1):84–87. | |
Kataoka H, Ida T, Ishii Y, et al. Analysis of the influence of drug resistance factors on the efficacy of combinations of antibiotics for multidrug-resistant Pseudomonas aeruginosa isolated from hospitals located in the suburbs of Kanto area, Japan. Journal of Global Antimicrobial Resistance. 2013;1(2):91–96. | |
Zapor MJ, Barber M, Summers A, et al. In vitro activity of the aminoglycoside antibiotic arbekacin against Acinetobacter baumannii calcoaceticus isolated from war-wounded patients at Walter Reed Army Medical Center. Antimicrob Agents Chemother. 2010;54(7):3015–3017. | |
Overview of Habekacin Injectable Solution (additional pediatric indication). In-house document of Meiji Seika Kaisha, Ltd. | |
Aikawa N, Kohno S, Kaku M, WatanabeA, Yamaguchi K, Tanigawara Y. An open clinical study of arbekacin 200 mg qd in patients infected with methicillin-resistant Staphylococcus aureus (MRSA) – A clinical pharmacology study. Jpn J Chemother. 2008;56(3):299–312. | |
Sunakawa K, Hori S. | |
Suzuki K, Tanikawa K, Matsuzaki T. Pharmacokinetics and dosing of arbekacin in preterm and term newborn infants. Pediatr Int. 2003;45(2):175–179. | |
Kinoshita D. | |
Funatsu Y, Hasegawa N, Namkoong H, et al. Penetration of arbekacin sulfate to the lung tissue. Proceedings of the 52nd interscience conference on antimicrobial agents and chemotherapy; September 9–12, 2012; San Francisco. Abstract No 2068. | |
Carcas AJ, Garcia-Satue JL, Zapater P, Frias-Iniesta J. Tobramycin penetration into epithelial lining fluid of patients with pneumonia. Clin Pharmacol Ther. 1999;65(3):245–250. | |
Kimura T, Sunakawa K, Matsuura N, Kubo H, Shimada S, Yago K. Population pharmacokinetics of arbekacin, vancomycin, and panipenem in neonates. Antimicrob. Agents Chemother. 2004;48(4):1159–1167. | |
Koga K, Kusawake Y, Ito Y, Sugioka N, Shibata N, Takada K. Enhancing mechanism of Labrasol on intestinal membrane permeability of the hydrophilic drug gentamicin sulfate. Eur J Pharm Biopharm. 2006;64(1):82–91. | |
Blaser J, Rieder HL, Luthy R. Interface-area-to-volume ratio of interstitial fluid humans determined by pharmacokinetic analysis of netilmicin in small and large skin blisters. Antimicrob Agents Chemother. 1991;35(5):837–839. | |
Dee TH, Kozin F. Gentamicin and tobramycin penetration into synovial fluid. Antimicrob Agents Chemother. 1977;12(4):548–549. | |
Thys JP, Serruvs-Schoutens E, Rocmans P, Herchuelz A, Vanderlinden MP, Yourassowsky E. Amikacin concentration in uninfected post thoracotomy pleural fluid and in serum after intravenous and intrapleural injection. Chest. 1984;85(4):502–505. | |
Kozak AJ, Gerding DN, Peterson LR, Hall WH. Gentamicin intravenous infusion rate: effect on interstitial fluid concentration. Antimicrob Agents Chemother. 1977;12(5):606–608. | |
Chisholm GD, Waterworth PM, Calnan JS, Garrod LP. Concentration of antibacterial agents in interstitial tissue fluid. Br Med J. 1973;1(5853):569–573. | |
Lorentzen H, Kallehave F, Kolmos HJ, Knigge U, Bulow J, Gottrup F. Gentamicin concentrations in human subcutaneous tissue. Antimicrob Agents Chemoter. 1996;40(8):1785–1789. | |
Rosin E, Ebert S, Uphoff TS, Evans MH, Schultz-Darken NJ. Penetration of antibiotics into the surgical wound in a canine model. Antimicrob Agents Chemoter.1989;33(5):700–704. | |
Hayashi M, Ooi K, Yamada, et al. | |
Okada K, Kimura T, Mikamo H, et al. Clinical practice guidelines for therapeutic drug monitoring of arbekacin: A consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Infect Chemother. 2014;20(1):1–5. | |
Shimizu A, Maebashi K, Niida M, et al. S03044. Pharmacokinetic-pharmacodynamic (PK-PD) study of arbekacin using mouse MRSA thigh infection model. In-house document of Meiji Seika Kaisha, Ltd; 2003. | |
Mattie H, Craig WA, Pechere JC. Determination of efficacy and toxicity of aminoglycosides. J Antimicrob Chemother. 1989;24(3):281–293. | |
Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26(1):1–12. | |
Sato R, Tanigawara Y, Kaku M, Aikawa N, Shimizu K. Pharmacokinetic- pharmacodynamic relationship of arbekacin for treatment of patients infected with methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2006;50(11):3763–3769. | |
Negita K, Yamashita M, Kubota T, et al. Study on therapeutic drug monitoring of arbekacin in patients infected with methicillin-resistant Staphylococcus aureus and its efficacy. Jpn J Pharm Health Care Sci. 2001;27(2):123–131. | |
Nambara M, Ikeue H, Kawasaki E, Tomioka S, Shimokawa F, Tanabe K. Effectiveness and adverse reactions between once daily and every other day administration of arbekacin sulfate. Jpn J Ther Drug Monit. 2003;20(3):241–248. | |
Kobayashi M, Saikyo A, Soma K, Yago K, Sunakawa K. Pharmacokinetic and pharmacodynamics (PK-PD) analysis to determine the optimal method of arbekacin administration. Jpn J Chemother. 2006;54(1):18–24. | |
Tanikaze N, Komatsu M, Shimakawa K, Yamamoto I. | |
Hwang JH, Lee JH, Moon MK, Kim JS, Won KS, Lee CS. The usefulness of arbekacin compared to vancomycin. Eur J Clin Microbiol Infect Dis. 2012;31(7):1663–1666. | |
Hwang JH, Lee JH, Moon MK, Kim JS, Won KS, Lee CS. The efficacy and safety of arbekacin and vancomycin for the treatment in skin and soft tissue mrsa infection: preliminary study. Infect Chemother. 2013;45(1):62–68. | |
Matsumoto T, Hanaki H, Kimura T, et al. Clinical efficacy and safety of arbekacin sulfate in patients with MRSA sepsis or pneumonia: a multi-institutional study. J Infect Chemother. 2013;19(1):128–137. | |
Kawano H, Tanigawara Y. Postmarketing surveillance review of arbekacin sulfate in patients with therapeutic drug monitoring. Jpn J Ther Drug Monit. 2010;27(2):55–71. | |
Yamamoto Y, Izumikawa K, Hashiguchi K, et al. The efficacy and safety of high-dose arbekacin sulfate therapy (once-daily treatment) in patients with MRSA infection. J Infect Chemother. 2012;18(2):241–246. | |
Kimura T, Sunakawa K, Totsuka K, et al. Dose finding study on arbekacin sulfate for appropriate peak levels. Jpn J Chemother. 2011;59(6):597–604. | |
Araoka H, Baba M, Takagi S, et al. Monobactam and aminoglycoside combination therapy against metallo-beta-lactamase-producing multidrug-resistant Pseudomonas aeruginosa screened using a ‘break-point checkerboard plate’. Scand J Infect Dis. 2010;42(3):231–233. | |
Mingeot-Leclercq MP, Tulkens PM. Aminoglycosides: nephrotoxicity. Antimicrob Agents Chemother. 1999;43(5):1003–1012. | |
Swan SK. Aminoglycoside nephrotoxicity. Semin Nephrol. 1997;17(1):27–33. | |
Giuliano RA, Verpooten GA, Verbist L, Wedeen RP, De Broe ME. In vivo uptake kinetics of aminoglycosides in the kidney cortex of rats. J Pharmacol Exp Ther. 1986;236(2):470–475. | |
Rougier F, Claude D, Maurin M, et al. Aminoglycoside nephrotoxicity: modeling, simulation, and control. Antimicrob Agents Chemother. 2003;47(3):1010–1016. | |
Chanbers HE. Chemotherapy of microbial diseases antimicrobial agents: the aminoglycosides. In: Hardman JG, Limbird LE, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 10th edition. New York: McGraw-Hill; 2001:1219–1238. | |
Huy PTB, Mannel C, Menlemans A. Kinetics of aminoglycoside antibiotics in perilymph in animals. In: Lerner SA, Martz GJ, Hawkins JE, editors. Aminoglycoside Ototoxicity. Little, Brown and Company; 1981; 81–97. | |
Tulkens PM, Clerckx-Braun F, Donnez J, et al. Safety and efficacy of aminoglycosides once-a-day: experimental data and randomized, controlled evaluation in patients suffering from pelvic inflammatory disease. J Drug Dev. 1988;1(Suppl 3):71–82. | |
Totsuka K, Shimizu K, Mitomi N, Niizato T, Araake M. Arbekacin | |
Bates DE. Aminoglycoside ototoxicity. Drugs Today (Barc). 2003; 39(4):277–285. | |
Barclay ML, Kirkpatrick CM, Begg EJ. Once daily aminoglycoside therapy – Is it less toxic than multiple daily doses and how should it be monitored? Clin Pharmacokinet. 1999;36(2):89–98. | |
Yamasoba T. Inner ear disorders and mitochondrial DNA mutation. Practica Oto-Rhino-Laryngologica. 2011;104(8):533–540. | |
Hamasaki K, Rando RR. Specific binding of aminoglycosides to a human rRNA construct based on a DNA polymorphism which causes aminoglycoside-induced deafness. Biochemistry. 1997;36(40):12323–12328. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.










 . [Development of arbekacin and synthesis of new derivatives stable to enzymatic modifications by methicillin-resistant Staphylococcus aureus]. Jpn J Antibiot. 1994;47(6):561–574. Japanese.
. [Development of arbekacin and synthesis of new derivatives stable to enzymatic modifications by methicillin-resistant Staphylococcus aureus]. Jpn J Antibiot. 1994;47(6):561–574. Japanese. . [Nationwide surveillance of parenteral antibiotics containing meropenem activities against clinically isolated strains in 2006]. Jpn J Antibiot. 2007;60(6):344–377. Japanese.
. [Nationwide surveillance of parenteral antibiotics containing meropenem activities against clinically isolated strains in 2006]. Jpn J Antibiot. 2007;60(6):344–377. Japanese. [Antibacterial activity of HBK]. Chemother. 1986;34:1–10. Japanese.
[Antibacterial activity of HBK]. Chemother. 1986;34:1–10. Japanese. [The enzymatic mechanisms of resistant to aminoglycoside antibiotics in methicillin-cephem-resistant Staphylococcus aureus]. Jpn J Antibiot. 1988;41(5):523–529. Japanese.
[The enzymatic mechanisms of resistant to aminoglycoside antibiotics in methicillin-cephem-resistant Staphylococcus aureus]. Jpn J Antibiot. 1988;41(5):523–529. Japanese. . [Epidemiological survey of drug resistance of methicillin–resistant Staphylococcus aureus isolated in Japan in 2000]. Jpn J Chemother. 2002;50(8):494–499. Japanese.
. [Epidemiological survey of drug resistance of methicillin–resistant Staphylococcus aureus isolated in Japan in 2000]. Jpn J Chemother. 2002;50(8):494–499. Japanese. Toxic shock syndrome toxin-1(TSST-1)
Toxic shock syndrome toxin-1(TSST-1) . [Effect of Arbekacin of the production of toxic shock syndrome toxin 1 by methicillin-resistant Staphylococcus aureus]. Jpn J Antibiotics. 2001;54(7):372–381. Japanese.
. [Effect of Arbekacin of the production of toxic shock syndrome toxin 1 by methicillin-resistant Staphylococcus aureus]. Jpn J Antibiotics. 2001;54(7):372–381. Japanese. . [Safety and pharmacokinetics of 400 and 600 mg arbekacin sulfate to healthy male volunteers]. Jpn J Antibiotics. 2013;66(2):97–109. Japanese.
. [Safety and pharmacokinetics of 400 and 600 mg arbekacin sulfate to healthy male volunteers]. Jpn J Antibiotics. 2013;66(2):97–109. Japanese. . [Evaluation of once a day of arbekacin administration to neonates as a new object of peak concentration]. Kansenshogaku Zasshi. 2010;84(6):727–733. Japanese.
. [Evaluation of once a day of arbekacin administration to neonates as a new object of peak concentration]. Kansenshogaku Zasshi. 2010;84(6):727–733. Japanese. . [Arbekacin sulfate concentration in peripheral lymph and in serum after intravenous injection: report of four cases]. Jpn J Antibiot. 2012;65(3):207–215. Japanese.
. [Arbekacin sulfate concentration in peripheral lymph and in serum after intravenous injection: report of four cases]. Jpn J Antibiot. 2012;65(3):207–215. Japanese. . [Study of clinical significance of PK/PD (pharmacokinetics/pharmacodynamics) parameters after administering arbekacin to patients with pulmonary methicillin-resistant Staphylococcus aureus infection]. Jpn J Chemother. 2004;52(9):469–473. Japanese.
. [Study of clinical significance of PK/PD (pharmacokinetics/pharmacodynamics) parameters after administering arbekacin to patients with pulmonary methicillin-resistant Staphylococcus aureus infection]. Jpn J Chemother. 2004;52(9):469–473. Japanese. . [Evaluation of once-daily administration of arbekacin]. Jpn J Antibiotics. 1994;47(6):676–692. Japanese.
. [Evaluation of once-daily administration of arbekacin]. Jpn J Antibiotics. 1994;47(6):676–692. Japanese.