Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 16
Apolipoprotein E Gene Polymorphism Effects on Lipid Metabolism and Risk of Cerebral Infarction in Northwest Han Chinese Population
Authors Wang Y, Yang S, Zhang S, Lu X, Ma W
Received 27 January 2023
Accepted for publication 31 March 2023
Published 5 April 2023 Volume 2023:16 Pages 303—312
DOI https://doi.org/10.2147/PGPM.S404663
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Yaqi Wang,1,* Shuang Yang,1,* Suya Zhang,1,* Xiaoyu Lu,2 Wenbing Ma1,2
1Department of Pharmacology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, 710061, People’s Republic of China; 2Department of Biological Science and Bioengineering, Key Laboratory of Biomedical Information Engineering of the Ministry of Education, School of Life Science and Technology, Xi’an Jiaotong University, Xi’an, Shaanxi, 710049, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wenbing Ma, Department of Pharmacology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, 710061, People’s Republic of China, Tel/Fax +86-29-85323240, Email [email protected]
Background: The apolipoprotein E (ApoE) genetic variation may contribute to the development of Cerebral Infarction (CI). Serum lipid levels are known risk factors for CI, but the effect of the ApoE gene polymorphism on lipid metabolism remains unclear. This retrospective cohort study was designed to determine the role of ApoE genotypes in CI risk and the relationships between ApoE gene polymorphism and serum lipid levels among the population of northwest China.
Patients and Methods: 517 CI patients and 517 non-CI controls were enrolled in the study. Polymerase chain reaction and hybridization were utilized to determine the ApoE gene polymorphisms.
Results: The ϵ3/ϵ4 genotype and ϵ4 allele frequency were significantly higher in CI patients than in controls. When stratified by age and sex, statistically significant differences in the distribution and frequency of the ϵ3/ϵ4 genotype and ϵ4 allele were found between patients and controls. Compared to ϵ2 carriers, ϵ4 carriers had significantly lower ApoE levels and higher low-density lipoprotein cholesterol (LDL-C), ApoB and ApoB/ApoA-I levels in both two groups. Additionally, control participants with ϵ4 carriers had significantly higher levels of lipoprotein and total cholesterol (TC) levels than ϵ2 carriers, while CI patients with ϵ4 carriers had a significantly lower level of ApoA-I. After adjusting for other established risk factors, drinking, hypertension, lipoprotein, triglycerides (TG) and ϵ4 allele were significant independent risk factors for CI, which was shown to be associated with a nearly two-fold CI risk.
Conclusion: This study demonstrated that ϵ4 allele is independent risk factors for CI among patients in Northwest China. ApoE polymorphism was associated with CI, which was partly mediated through blood lipids and may also be mediated through non-lipid pathways. These data might be of great clinical significance in individualized preventive and therapeutic strategies.
Keywords: apolipoprotein E, cerebral infarction, gene polymorphism, Northwest China
Introduction
Stroke is the second leading cause of disability and mortality worldwide, with CI being the most common subtype.1 Research has shown that the incidence of stroke in Shaanxi, Shandong and Xinjiang grew most dramatically from 2013 to 2019, of which Shaanxi and Xinjiang are both Northwest China.1 CI, which accounts for 70% to 80% of all strokes, is brought on by abnormalities in the cerebral blood flow that result in hypoxia and ischemia and local cerebral ischemic necrosis or brain softening.2 Complex environmental and genetic variables contribute to the development of CI.3 Through association studies, a number of potential genes have been researched in CI, however, the findings are debatable.4
Recently, mounting evidence indicates that ApoE is a candidate gene in the development of CI. ApoE is an arginine-rich alkaline protein, which is present in plasma chylomicron, low-density lipoprotein and very low-density lipoprotein.5 The ApoE gene is situated on chromosome 19q13.2 in humans and has been discovered to be highly expressed in the brain. Genotypes were also determined for the two functional single nucleotide polymorphisms (SNPs) rs429358 (388T > C) and rs7412 (526C > T), resulting in three different alleles (ε2, ε3 and ε4) and six different genotypes (ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4 and ε4/ε4). ApoE plays an important role in cholesterol transport, and plasma lipoprotein metabolism and thus affects the serum lipid profiles in the body.5 Numerous studies indicate that ε4 allele was associated with significantly higher serum TC and LDL-C, thereby contributing to the increasing burden of cardiovascular diseases, including CI.6 Although the mechanism responsible for the association between ApoE polymorphisms and CI, the exact mechanism remains controversial. The distribution of ApoE allele frequencies can vary across different ethnic groups.7 The association between ApoE polymorphism and the risk of developing CI in the Northwest China has also not been examined in any studies. Therefore, the aim of the present study was to investigate the role of ApoE genotypes in the risk of CI in the Northwest Chinese Han population.
Methods
Subjects
A total of 517 Chinese CI patients (males: females = 344:173) and 517 controls (males: females = 332:185) were enrolled into the study. The CI patients and the controls had similar proportions of sex and age. All participants were enrolled from May 2018 to May 2019 in the First Affiliated Hospital of Xi’an Jiao Tong University. All participants are ethnically Han Chinese and residents living in Northwest China. The demographic and clinical characteristics of study participants are presented in Table 1. Diagnostic criteria of CI patients were as referred to in the Fourth National Cerebrovascular Disease Conference of China, and the diagnosis was confirmed by computed tomography or Magnetic Resonance Imaging. The exclusion criteria were as follows: patients with malignancy, autoimmune disease, chronic kidney disease, and incomplete heart function and current use of lipid-lowering drugs. The control group were recruited from people without a history of normal neurological examination results, immunological diseases, stroke, or cerebrovascular diseases receiving health examinations in our hospital during the same study period. This study was conducted in accordance with the Declaration of Helsinki, approved by the Ethical Committee of the First Affiliated Hospital of Xi’an Jiao Tong University and we have got the informed consent of all patients.
 |
Table 1 Characteristics of the Study Population |
DNA Extraction and Genotyping
Blood samples from each participant were obtained from the cubital vein and collected in tubes containing ethylene diamine tetra acetic acid (EDTA). This was part of a large number of cases collected looking at different diseases and this particular gene. The details of the experimental procedure were reported in our previous paper.8
Statistical Analyses
Data analysis was performed using SPSS statistical software version 16.0. Continuous variable data with a normal distribution are presented as means ± standard deviation and as numbers and percentages for categorical variables and categorical variables were expressed as a number (%). Student’s t-test and χ2 test were initially used to test the difference between CI and control groups. ApoE genotype and allele frequencies were tested for Hardy-Weinberg equilibrium by chi-square test. The chi-square test and ANOVA were used to analyze the association between specific ApoE genotypes and clinical characteristics. Logistic regression analysis was used to assess the interactions between ApoE genotypes and various factors. A value of p<0.05 was considered statistically significant.
Results
Baseline Clinical Characteristics of the CI and Control Groups
Baseline clinical characteristics of the patients with CI and control groups are summarized in Table 1. A total of 517 Chinese patients with CI, consisting of 344 males and 173 females ages 26–91 years (mean=61.52 years, SD=11.24), and 517 controls participants, comprising 332 males and 185 females ages 23–93 years (mean=61.26 years, SD=14.29), were included in the present study. Most of the study participants originate from northwest China. Age and sex distribution were not significantly different between the two groups (P=0.742 and 0.472, respectively). The proportions of drinking (P=0.002), hypertension (P=0.003) and diabetes (P<0.001) were significantly higher in the CI group than in the control group. TG level was significantly higher in the CI group compared to the control group (P=0.011), while no significant differences were found in TC, high-density lipoprotein (HDL), LDL-c, creatinine (Cr), ApoA-I, ApoB, ApoB/A-I, ApoE, lipoprotein, direct bilirubin (DB) and indirect bilirubin (IB) levels between the two groups (all P > 0.05).
Allele and Genotype Frequencies of ApoE and CI Risk
The distribution of the ApoE genotype and allele frequencies in CI cases and control subjects are summarized in Table 2. The genotype distribution of this polymorphism in patients with CI and control participants was in accordance with the Hardy-Weinberg equilibrium (P = 0.45 and 0.50, respectively). Genotype ε3/ε3 (63.8%) was the most common type in CI groups, followed by ε3/ε4 (22.8%), ε2/ε3 (9.9%), ε4/ε4 (1.5%), ε2/ε4 (1.2%), and ε2/ε2 (0.8%), whereas control participants were ε3/ε3 (71.0%), followed by ε2/ε3 (13.3%), ε3/ε4 (12.6%), ε2/ε4 (2.1%), ε4/ε4 (0.6%) and ε2/ε2 (0.4%). The allele frequency of ε2, ε3, and ε4 was 6.3%, 80.2% and 13.5% respectively in patients with CI; 8.1%, 83.9% and 7.9% respectively in control participants. The distribution of ApoE genotypes and alleles in the two groups was significantly different (χ2=24.424 and P<0.001, χ2=18.472 and P<0.001, respectively).
 |
Table 2 The Distributions of Genotypes and Alleles of the ApoE Gene in the CI Patients and Controls |
The frequencies of ε3/ε4 genotype (OR =2.057, 95% CI = 1.477–2.864, P<0.001) and ε4 allele (OR =1.818, 95% CI = 1.364–2.424, P<0.001) were significantly higher in CI patients than in control participants. Further, patients with CI had a significantly lower ε3/ε3 (OR =0.721, 95% CI = 0.555–0.937, P=0.017) genotype and ε3 allele (OR =0.773, 95% CI = 0.617–0.969, P=0.029) frequencies than those of control participants (P < 0.05).
To explore the relationship between ApoE genotype and CI, we conducted further analysis stratified by age (dichotomized into ≤60 years and >60 years) and sex. The results showed that ε3/ε4 frequency was significantly higher in patients with CI compared to the control participants (OR =3.067, 95% CI = 1.675–5.614, P<0.001 in age ≤60 years; OR =1.735, 95% CI = 1.156–2.604, P=0.008 in age >60 years and OR =2.206, 95% CI = 1.474–3.301, P<0.001 for males), but not for females (OR =1.746, 95% CI = 0.973–3.134, P=0.078). Additionally, the variance in allele ε4 between patients with CI and controls was also statistically significant (OR =2.072, 95% CI = 1.281–3.353, P=0.003 in age ≤60 years; OR =1.704, 95% CI = 1.189–2.444, P=0.003 in age>60 years; OR =1.709, 95% CI = 1.201–2.432, P=0.001 for males and OR =2.046, 95% CI = 1.246–3.361, P=0.004 for females) (Table 3).
 |
Table 3 Stratified Analyses Between ApoE Polymorphism and Risk of CI |
Relationships Between Serum Lipid Profile and ApoE Alleles
Table 4 describes the association between serum lipid profiles and allelic carrier status (ε2, ε3 and ε4 groups). Participants with ε2/ε4 genotype (n= 17) were excluded because they play opposing roles in lipid metabolism and the incidence of CI. In the patients with CI, ε4 carriers had significantly higher LDL-C, ApoB and ApoB/ApoA-I and lower levels of ApoA-I and ApoE levels than ε2 carriers. LDL-C, ApoB, ApoB/ApoA-I and ApoE levels of the control participants showed similar trends to those in CI groups. Additionally, control participants with ε4 carriers had significantly higher levels of lipoprotein and TC levels than ε2 carriers.
 |
Table 4 Relationships Between Serum Lipid Profile and ApoE Allele in CI Patients and Control Participants |
Logistic Regression Analysis of CI Risk Factors
We performed a multivariate logistic regression analysis to determine which statistically significant variables in the univariate analysis could be used as independent predictors of CI. Univariate logistic analysis showed that drinking (OR =1.701, 95% CI = 1.167–2.478, P=0.006), hypertension (OR =1.885, 95% CI = 1.417–2.508, P<0.001), ApoE levels (OR =0.983, 95% CI = 0.969–0.997, P=0.017), lipoprotein (OR =1.001, 95% CI = 1.000–1.001, P=0.046), TG (OR =1.363, 95% CI = 1.035–1.797, P=0.028) and ε4 allele (OR =1.954, 95% CI = 1.359–2.810; P<0.001) were significant independent risk factors for CI (Table 5). Furthermore, to exclude the confounding effect of age (≤60 years and >60 years) and sex, a multivariate logistic regression analysis stratified according to age and sex was performed. In both age groups, ε4 carriers were associated with increased risk of CI (age ≤60 years: OR = 2.970, 95% CI = 1.553–5.678, P = 0.001; age >60 years: OR = 1.715, 95% CI = 1.082–2.719, P = 0.022). Also, ε4 carriers was significantly associated with a higher risk of CI for males (OR =2.182, 95% CI = 1.398–3.407, P=0.022), but not for females (OR =1.500, 95% CI = 0.779–2.887, P=0.225) (Table 6).
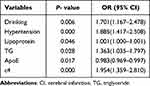 |
Table 5 Logistic Regression Analysis of the Risk of CI in Northwest of China Population |
 |
Table 6 Multiple Logistic Regression Analysis for CI Patients and Control Subjects |
Discussion
CI is the most prevalent subtype of stroke, which is one of the main causes of mortality and long-term impairment globally.9 Because of the aging and expanding population in China, as well as the rising incidence of risk factors, the burden of stroke is anticipated to rise considerably.10 Despite advances in the prevention and treatment of CI, there are still few therapies that have been proven to enhance outcomes following CI.11 Therefore, investigating the pathogenesis of CI actively and preventing cerebral infarction is of great importance. This study analyzed the relationship between genetic polymorphisms of ApoE and CI in the Han Chinese population in Northwest China.
Numerous studies have shown that various environmental variables and genetic variations contribute to the etiology of CI.12 Previously published studies showed that ApoE polymorphisms have been associated with CI, although with varying degrees of success.13,14 In the present case, the ε3/ε3 genotype and ε3 allele were significantly lower in patients than the controls, indicating its potential protective role. In contrast, patients exhibited a greater prevalence of the 3/4 genotype and 4 alleles than did controls, consistent with previous findings.15 Additionally, statistically significant differences in the distribution and frequencies of the ε3/ε4 genotype and ε4 allele in males and ε4 allele in females were observed between patients and controls. In contrast, a Chinese study demonstrated that ε3/ε4 genotype played a protective role against CI and ε2/ε4 genotype may be a potential risk factor in males.16 There were 6190 cases and 6248 controls in 54 studies, and a meta-analysis showed that the ApoE mutant allele 4 was a risk factor for cerebral infarction in Han Chinese people.17 In the current study, multivariate logistic regression analysis indicated that the ε4 allele was associated with a 1.954-fold increased risk of CI after adjusting for conventional risk factors. The impact size of the link between the ε4 allele and the risk of CI was shown to diminish with age when the data were stratified by age. A substantial correlation between the risk of CI and the ε4 allele was seen in patients with CI who were both younger than 60 and older than 60. This result was consistent with a previous meta-analysis.18 In general, the results of our study are consistent with those published by Wang et al19 yet are in contrast with those reported by Sudlow et al.20 These inconsistent results might be the result of variations in participant ethnicities, detection techniques, sample sizes, research designs, and other factors. Interestingly, Clinical studies showed inconsistent data, yet outcomes from several animal experiments were encouraging. ApoE-deficient mice had increased susceptibility to focal cerebral ischemia, ε4 allele could increase infarct size and functional impairment.21,22
There is significant debate and uncertainty around the molecular processes causing the excess of the ε4 allele in CI.23 It has been reported that genetic variations of the ApoE play a major physiological role in lipoprote in metabolism.24 The ε4 allele has been consistently associated with increased TG, LDL-C, ApoB levels and decreased ApoE levels.25,26 The current investigation verified that although LDL-C did not alter considerably, TG levels were noticeably higher in CI patients. The ε2 carriers had significantly lower LDL-C level than ε3 or ε4 carriers in both two groups. Furthermore, logistic regression analysis showed that TG level was a strong independent risk factors for CI. Lipoprotein, a complex lipoprotein, is composed of low-density lipoprotein attached to apolipoprotein by a disulfide bond.27 The LPA gene, which produces ApoA and is unaffected by age, gender, diet, exercise, or the majority of lipid-lowering pharmacological regimens, is the primary regulator of plasma concentrations of lipoprotein.28 Lipoprotein had been highlighted as an important and independent CI risk factor in Caucasian, Japanese, and Chinese populations.29–31 In the present study, the mean level of lipoprotein tended to be higher in patients with CI than in the control participants, although the difference was not statistically significant. Nevertheless, ε2 carriers had significantly lower lipoprotein levels than ε3 or ε4 carriers among the control participants, but no significant differences were observed between ɛ2, ɛ3 or ɛ4 carriers and lipoprotein levels among patients with CI. Multivariate Logistic regression analysis showed plasma lipoprotein level was independently associated with CI after adjustment for traditional risk factors and lipid parameters, which was consistent with previous studies across different ethnic groups31,32 but contradictory with other.33
ApoE is a multifunctional protein that is crucial for the metabolism of lipoproteins and can be utilized as a disease diagnostic marker.34 The levels of ApoE showed a decreasing tendency in the CI group compared with the control group, without statistical difference. However, other investigations revealed that the plasma ApoE level in CI patients was considerably elevated and was strongly related to the risk of CI in the Asian population.18,35 We also observed that ɛ4 carriers had a significantly lower ApoE level than ɛ2 carriers in both groups, which was consistent with previous findings.36 According to recent research, high ApoB levels and the ApoB/A-I ratio were better predictors of the risk of CI risk than traditional lipid parameters.37 We found that there was a tendency for a decrease ApoA-I levels in CI patients compared to controls, while ApoB and ApoB/ApoA-I levels tended to increase, but these three indicators were not statistically significant. In comparison to coronary events, data on ApoB and ApoA-I in connection to CI risk are rather few in the literature. Moreover, ε2 carriers had significantly lower ApoB and ApoB/ApoA-I than ε3 and ε4 carriers in both groups, and significantly ApoA-I levels in patients with CI, but no significant difference was observed in the control group. These results are consistent with previous studies.15
The strength of this study is that this is the first study about the relationship between CI and ApoE gene polymorphism in Northwest Han Chinese population. Association of lipid levels with ApoE gene polymorphisms was included in the final analysis and any potential confounding variables or comorbidities were eliminated. There were some inherent limitations presented in our study. (1) Due to a lack of original data and the size of this retrospective investigation, it was difficult to examine potential gene-environment interactions. (2) The study’s limited sample size, might partially contribute to the results’ volatility (3) The study was conducted only in Northwest Chinese populations, and whether these findings will also be true in other populations needs further investigation.
Conclusions
In conclusion, the present study suggested that the ɛ4 allele is associated with CI in the Northwest Han Chinese population. The ε4 allele, drinking, hypertension, lipoprotein and TG levels are independent risk factors for CI among patients in Northwest China.
ApoE polymorphism was associated with CI, which was partly mediated through blood lipids and may also be mediated through non-lipid pathways. Due to the small sample size, more research with a larger sample group is required to refute our findings. Our research provides useful genomic data which may become assist in optimizing individual preventative and therapeutic strategies.
Ethics Approval and Consent to Participate
This study was approved by the Ethical Committee of the First Affiliated Hospital of Xi’an Jiao Tong University and we got the informed consent of each patients.
Acknowledgments
We gratefully acknowledge all sample donors who participated in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Natural Science Basic Research Program of Shaanxi (Program No. 2021-SF-130) and the Foundation of the First Affiliated Hospital of Xi’an Jiaotong University (ProgramNo. 2020ZYTS-08).
Disclosure
The authors declare that they have no competing interests.
References
1. Tu WJHY, Yan F, Yan F, et al. Prevalence of stroke in China, 2013–2019: a population-based study. Lancet Reg Health West Pac. 2022;28:100550. doi:10.1016/j.lanwpc.2022.100550
2. Wang H, Fu X, Ju J, et al. Acupuncture for patients recovering from lacunar infarction A protocol for systematic review and meta-analysis. Medicine. 2021;100(25):e26413.
3. Yang X, Yang S, Xu H, et al. Superoxide dismutase gene polymorphism is associated with ischemic stroke risk in the China Dali Region Han Population. Neurologist. 2021;26:27–31. doi:10.1097/NRL.0000000000000301
4. Terni E, Giannini N, Brondi M, et al. Genetics of ischaemic stroke in young adults. Bba Clin. 2015;3:96–106. doi:10.1016/j.bbacli.2014.12.004
5. Buczynska A, Sidorkiewicz I, Lawicki S, et al. The significance of apolipoprotein E measurement in the screening of fetal down syndrome. J Clin Med. 2020;9(12):3995. doi:10.3390/jcm9123995
6. Lee J-H, Hong S-M, Shin Y-A. Effects of exercise training on stroke risk factors, homocysteine concentration, and cognitive function according the APOE genotype in stroke patients. J Exerc Rehabil. 2018;14:267–274. doi:10.12965/jer.1836108.054
7. Zhu A, Yan L, Shu C, et al. APOE epsilon 4 modifies effect of residential greenness on cognitive function among older adults: a longitudinal analysis in China. Sci Rep. 2020;10:1–8.
8. Ma W, Zhang L, Luo L, et al. Effect of apolipoprotein E ε4 allele on the progression of carotid atherosclerosis through apolipoprotein levels. Pharmgenomics Pers Med. 2022;15:653–661. doi:10.2147/PGPM.S367471
9. Varotto L, Bregolin G, Paccanaro M, et al. Network meta-analysis on patent foramen ovale: is a stroke or atrial fibrillation worse? Neurol Sci. 2021;42:101–109. doi:10.1007/s10072-020-04922-4
10. Li S, Chen L, Xu C, et al. Expression profile and bioinformatics analysis of circular RNAs in acute ischemic stroke in a South Chinese Han population. Sci Rep. 2020;10:1–4.
11. Owen B, Akbik O, Torbey M, et al. Incidence and outcomes of intracerebral haemorrhage with mechanical compression hydrocephalus. Stroke Vasc Neurol. 2021;6(3):328–336. doi:10.1136/svn-2020-000401
12. Zhang L-J, Yuan B, Li -H-H, et al. Associations of genetic polymorphisms of SAA1 with cerebral infarction. Lipids Health Dis. 2013;12(1). doi:10.1186/1476-511X-12-130
13. Banerjee I, Gupta V, Ganesh S. Association of gene polymorphism with genetic susceptibility to stroke in Asian populations: a meta-analysis. J Hum Genet. 2007;52(3):205–219. doi:10.1007/s10038-006-0098-x
14. Um JY, Moon KS, Lee KM, et al. Polymorphism of angiotensin-converting enzyme, angiotensinogen, and apolipoprotein E genes in Korean patients with cerebral infarction. J Mol Neurosci. 2003;21:23–28. doi:10.1385/JMN:21:1:23
15. Wu H, Huang Q, Yu Z, et al. The SNPs rs429358 and rs7412 of APOE gene are association with cerebral infarction but not SNPs rs2306283 and rs4149056 of SLCO1B1 gene in southern Chinese Hakka population. Lipids Health Dis. 2020;19(1):202. doi:10.1186/s12944-020-01379-4
16. Zhong Z, Wu H, Ye M, et al. Association of APOE gene polymorphisms with cerebral infarction in the Chinese population. Med Sci Monit. 2018;24:1171–1177. doi:10.12659/MSM.905979
17. Chen C, Hu Z. ApoE polymorphisms and the risk of different subtypes of stroke in the Chinese population: a comprehensive meta-analysis. Cerebrovasc Dis. 2016;41(3–4):119–138. doi:10.1159/000442678
18. Kumar A, Kumar P, Prasad M, et al. Association between apolipoprotein epsilon 4 gene polymorphism and risk of ischemic stroke: a meta-analysis. Ann Neurosci. 2016;23:113–121. doi:10.1159/000443568
19. Wang Q-Y, Wang W-J, Wu L, et al. Meta-analysis of APOE epsilon 2/epsilon 3/epsilon 4 polymorphism and cerebral infarction. J Neural Transm. 2013;120:1479–1489. doi:10.1007/s00702-013-1019-8
20. Sudlow C, Gonzalez NAM, Kim J, et al. Does apolipoprotein E genotype influence the risk of ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage? Stroke. 2006;37:364–370. doi:10.1161/01.STR.0000199065.12908.62
21. Lawrence DW, Comper P, Hutchison MG, et al. The role of apolipoprotein E episilon (ɛ)-4 allele on outcome following traumatic brain injury: a systematic review. Brain Inj. 2015;29:1018–1031. doi:10.3109/02699052.2015.1005131
22. Laskowitz DT, Sheng HX, Bart RD, et al. Apolipoprotein E-deficient mice have increased susceptibility to focal cerebral ischemia. J Cereb Blood Flow Metab. 1997;17:753–758. doi:10.1097/00004647-199707000-00005
23. Zhuo YY, Wu JM, Kuang L, et al. The discriminative efficacy of retinal characteristics on two traditional Chinese syndromes in association with ischemic stroke. Evid Based Complement Altern Med. 2020;2020:1–8. doi:10.1155/2020/6051831
24. Hardy TM, de Mendoza VB, Sun YV, et al. Genomics of reproductive traits and cardiometabolic disease risk in African American Women. Nurs Res. 2019;68(2):135–144. doi:10.1097/NNR.0000000000000337
25. Martinez-Magana JJ, Genis-Mendoza AD, Tovilla-Zarate CA, et al. Association between APOE polymorphisms and lipid profile in Mexican Amerindian population. Mol Genet Genomic Med. 2019;7. doi:10.1002/mgg3.958
26. Karahan Z, Ugurlu M, Ucaman B, et al. Relation between apolipoprotein E gene polymorphism and severity of coronary artery disease in acute myocardial infarction. Cardiol Res Pract. 2015;2015:1–4. doi:10.1155/2015/363458
27. Di Maio S, Grueneis R, Streiter G, et al. Investigation of a nonsense mutation located in the complex KIV-2 copy number variation region of apolipoprotein(a) in 10,910 individuals. Genome Med. 2020;12. doi:10.1186/s13073-020-00771-0
28. Enas EA, Varkey B, Dharmarajan TS, et al. Lipoprotein(a): an independent, genetic, and causal factor for cardiovascular disease and acute myocardial infarction. Indian Heart J. 2019;71(2):99–112. doi:10.1016/j.ihj.2019.03.004
29. Koschinsky ML, Marcovina SM. Structure-function relationships in apolipoprotein(a): insights into lipoprotein(a) assembly and pathogenicity. Curr Opin Lipidol. 2004;15:167–174. doi:10.1097/00041433-200404000-00009
30. Gaw A, Murray HM, Brown EA, et al. Plasma lipoprotein(a) Lp(a) concentrations and cardiovascular events in the elderly: evidence from the prospective study of pravastatin in the elderly at risk (PROSPER). Atherosclerosis. 2005;180:381–388. doi:10.1016/j.atherosclerosis.2004.12.015
31. Sun L, Li ZH, Zhang HY, et al. Pentanucleotide TTTTA repeat polymorphism of apolipoprotein(a) gene and plasma lipoprotein(a) are associated with ischemic and hemorrhagic stroke in Chinese - A multicenter case-control study in China. Stroke. 2003;34:1617–1622. doi:10.1161/01.STR.0000078370.12085.02
32. Jurgens G, Taddeipeters WC, Koltringer P, et al. Lipoprotein(a) serum concentration and apolipoprotein(a) phenotype correlate with severity and presence of ischemic cerebrovascular-disease. Stroke. 1995;26:1841–1848. doi:10.1161/01.STR.26.10.1841
33. Lv P, Jin HQ, Liu YY, et al. Comparison of risk factor between lacunar stroke and large artery atherosclerosis stroke: a cross-sectional study in China. PLoS One. 2016;11(3):e0149605. doi:10.1371/journal.pone.0149605
34. Woltjer RL, Reese LC, Richardson BE, et al. Pallidal neuronal apolipoprotein E in pantothenate kinase-associated neurodegeneration recapitulates ischemic injury to the globus pallidus. Mol Genet Metab. 2015;116:289–297. doi:10.1016/j.ymgme.2015.10.012
35. Wang Z, Xia Y, Zhao Y, et al. Intestinal gutsfeature and role of ApoE and glucose metabolism in cerebral infarction patients. Int J Clin Exp Pathol. 2017;10:561–565.
36. Tascilar N, Dursun A, Ankarali H, et al. Relationship of apoE polymorphism with lipoprotein(a), apoA, apoB and lipid levels in atherosclerotic infarct. J Neurol Sci. 2009;277:17–21. doi:10.1016/j.jns.2008.09.034
37. Chou Y-C, Chan P-C, Yang T, et al. Apolipoprotein B level and the apolipoprotein B/apolipoprotein A-I ratio as a harbinger of ischemic stroke: a prospective observation in Taiwan. Cerebrovasc Dis. 2020;49(5):487–494. doi:10.1159/000509452
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
