Back to Journals » Integrated Blood Pressure Control » Volume 7
Aortic stiffness: pathophysiology, clinical implications, and approach to treatment
Authors Sethi S, Rivera O, Oliveros R, Chilton R
Received 20 December 2013
Accepted for publication 11 February 2014
Published 23 May 2014 Volume 2014:7 Pages 29—34
DOI https://doi.org/10.2147/IBPC.S59535
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Salil Sethi, Oscar Rivera, Rene Oliveros, Robert Chilton
University of Texas Health Science Center, San Antonio, TX, USA
Abstract: Aortic stiffness is a hallmark of aging, and classic cardiovascular risk factors play a role in accelerating this process. Current changes in medicine, which focus on preventive care, have led to a growing interest in noninvasive evaluation of aortic stiffness. Aortic stiffness has emerged as a good tool for further risk stratification because it has been linked to increased risk of atherosclerotic heart disease, myocardial infarction, heart failure, and stroke. This has led to the invention and validation of multiple methods to measure aortic stiffness. Pulse wave velocity is emerging as the gold standard for evaluation of aortic stiffness. This review focuses on the pathophysiology involved in aortic stiffness, methods available for evaluation of aortic stiffness, the importance of central pressure as a predictor of future cardiovascular events, and therapies that affect aortic stiffness.
Keywords: aortic stiffness, pulse wave velocity
Introduction
Arterial stiffening is a hallmark of the aging process and atherosclerosis, with a reduction in normal aortic compliance. The aorta serves as a conduit for delivery of an adequate blood supply to peripheral tissues, while dampening sudden oscillations in blood pressure produced by intermittent ventricular ejections.1 During youth, most large arteries are very compliant and elastic, but stiffen as we age. Aortic stiffness is described as elastic resistance to deformation,1 and is affected by complex interactions between vascular smooth muscle cells and the extracellular matrix containing elastin, collagen, and fibrillin fibers.1 Historically, three individuals are credited with describing aortic stiffness, ie, Thomas Young in his Croonian Lecture of 1908,2 and Adriaan Isebree Moens and Diederik Korteweg for the Moens–Korteweg equation.3
Pathophysiology
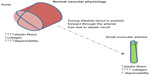
The aorta not only serves as a conduit during systole but also acts as a reservoir for blood. Its elastic properties allow the aorta to store half of the cardiac ejected blood volume per beat. Aortic recoil during diastole pushes the remaining stored volume forward into the peripheral circulation. This phenomenon is known as the Windkessel function, which is the oldest model described for the arterial system (Figure 1).3 In normal subjects, left ventricular ejection causes a pressure pulse with a relatively slow pulse wave velocity at 5–7 m/s.1 This pulse wave during diastole gets reflected at different points located mainly in distal arteries at the branching origin of arterioles. This reflected wave interacts with the incident waveform during early diastole and produces a dicrotic notch. The final shape and waveform of aortic blood pressure is determined by summation of these two pressure waves.4 In young compliant vessels, the reflected wave returns during diastole and causes the pulse pressure to be higher in the peripheral rather than central arteries. This phenomenon is known as pulse pressure amplification.4 However, because of the increase in pulse wave velocity as we age, the reflected wave reaches the central pulse in systole, boosting the systolic blood pressure. This increase in systolic blood pressure when divided by pulse pressure is called the augmentation index.5 This index is a marker of aortic stiffness, which increases as the elastic fibers within the arterial wall (elastin) become disrupted due to mechanical stress (see Figures 2 and 3).
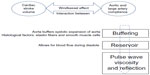  | Figure 1 Physiologic properties of the aorta: Windkessel effect. The proximal aorta absorbs the energy of the left ventricular ejection and dampens the pulsatile flow. |
How to measure aortic stiffness and central blood pressure
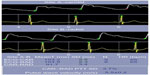
The pulse waveform at any point in the vascular tree is determined by the amplitude and duration of left ventricular ejection and the amplitude and velocity of the reflected wave. The velocity of the antecedent wave increases as stiffness of the vascular bed increases. The principle of applanation tonometry is utilized by several commercially available devices, eg, SphygmoCor (AtCor Medical Inc., Itasca, IL, USA), to generate a peripheral pulse waveform.6 Pulse wave velocity is calculated by measuring the transit time of the pulse waveform at two sites along the vasculature, thus providing a regional assessment of aortic stiffness. As shown in Figure 4, pulse wave velocity is calculated by dividing the distance (measured manually with a tapeline between the two recording sites; the carotid artery and radial artery in this example) by the transit time (measured between the feet of the pressure waveforms at these sites). This is the current gold standard for measurement of aortic stiffness. Pulse wave velocities gradually increase in the peripheral arteries, and are estimated to be 4 m/s in the ascending aorta, 5 m/s in the abdominal aorta, 7 m/s in the brachial arteries, and 8 m/s in the iliac arteries.7
In 1993, Karamanoglu et al8 performed an invasive assessment of 14 patients and confirmed the accuracy of a mathematically formulated generalized transfer function that was devised to determine central blood pressure from easily obtainable peripheral pressure. The accuracy of this mathematical calculation has now been validated in several studies;9,10 most recently, Laugesen et al reported on the accuracy of this estimation in patients with type 2 diabetes mellitus.11
Pulse wave Doppler ultrasound can also be used to measure pulse wave velocity. This technique uses the same principle of transit time as measured by the time delay in the onset of the pulse wave at two points in the vascular tree from the QRS complex. This method has been validated and is closely correlated with the results obtained by applanation tonometry.12
Clinical importance of aortic stiffness
With the current changes in medical practice, which focus on preventive care, there has been a growing interest in noninvasive methods for evaluation of aortic stiffness to help identify individuals at higher risk of future cardiovascular events. Aortic stiffness is an independent predictor of vascular morbidity and mortality (Figure 5),13 as evidenced by studies performed in patients with hypertension,14 diabetes mellitus,15 end-stage renal disease,16 and age >70 years.17 More recently, central blood pressure has been used to predict long-term cardiovascular outcomes, and values >130/90 mmHg were diagnostic for hypertension and predicted a worse prognosis.18 The traditional risk factors associated with atherosclerosis have also been associated with early arterial stiffening; among them are smoking, hypertension, diabetes mellitus, and hypercholesterolemia.19 Fukuda et al assessed aortic stiffness, as measured by brachial ankle pulse wave velocity, in 192 patients who underwent coronary angiography. Aortic stiffness was not only correlated with a higher incidence of coronary artery disease but also had a negative correlation with coronary flow reserve.20
  | Figure 5 Meta-analysis of 17 longitudinal studies that evaluated aortic pulse wave velocity and followed up 15,877 subjects for a mean of 7.7 years. |
It has been recognized since the 1980s that normal brachial cuff arterial pressure does not correlate with left ventricular hypertrophy as determined by echocardiography.21 Further, the CAFE (Conduit Artery Function Evaluation) trial, a substudy of ASCOT (Anglo-Scandinavian Cardiac Outcome Trial), showed that even when target brachial pressure was achieved using different therapeutic regimens, there were differences in central blood pressure between the regimens that translated into different clinical outcomes in the treatment groups.22 The Strong Heart Study investigators noticed that pulse pressure was more closely related to vascular hypertrophy and extent of atherosclerosis than was systolic pressure. Central pulse pressure had a stronger relationship with subclinical manifestations of cardiovascular disease (arteriosclerotic heart disease, myocardial infarction, stroke, heart failure, and sudden cardiac death) than brachial pulse pressure.23 In this study, men and women with a central pulse pressure ≥50 mmHg had a two-fold increased risk of future cardiovascular events even when corrected for other cardiovascular risk factors. The predictive value of central pulse pressure remained true even in individuals over the age of 60 years.23
In addition, a stronger association between aortic stiffness and future cardiovascular events was found in individuals with hypertension in a substudy of ASCOT. This substudy identified that wave reflection index, even when adjusted for age, sex, heart rate, brain natriuretic protein level, fasting glucose, antihypertensive regimen, and carotid intima-media thickness, remained a predictor of future cardiovascular events (P=0.003).24 A study by Pereira et al showed that, independent of hypertension and other risk factors for arteriosclerotic heart disease, individuals with aortic stiffness had a 1.39 (95% confidence interval 1.08–1.72; P=0.02) increase in relative risk of fatal stroke.25 Other studies have shown an association between increased aortic stiffness and microvascular cerebral disease, including lacunar infarcts, in individuals with hypertension.26 Although there has been a correlation between aortic stiffness and microvascular cerebral ischemic changes, studies performed to define etiologic role of aortic stiffness in pathogenesis of cognitive decline and dementia have yielded both positive and negative results.27,28
It has been hypothesized that nonenzymatic glycation of the arterial wall, among other factors, results in increased aortic stiffness in patients with type 2 diabetes.29 Hyperglycemia can cause nonenzymatic glycosylation of several proteins, including collagen and elastin. Insulin resistance and high insulin concentrations, elevated triglycerides, and low high-density lipoprotein cholesterol play an important role in diabetes-related vascular stiffness. Even after adjustment for potential cardiovascular risk factors, including microvascular and macrovascular complications, ambulatory blood pressure, and metabolic control, carotid–femoral pulse wave velocity was predictive of cardiovascular events, including myocardial infarction, cardiac death, stroke, peripheral vascular disease, and heart failure.30 Mansour et al showed that one standard deviation in pulse wave velocity was associated with a 47% increase in cardiovascular events in type 2 diabetics. Pulse wave velocity was a stronger predictor of cardiovascular events than age, systolic blood pressure, sedentary lifestyle, and waist circumference.31
Since the 1980s, there have been multiple models created to measure aortic stiffness by noninvasive methods. Because of the differences in techniques used, the evidence has become somewhat confusing and contradictory, but in general, pulse wave velocity has been shown to be an independent risk factor for future cardiovascular events, mortality, and stroke. Past studies have shown that pulse wave velocity could be additive to the Framingham risk score for assessment of cardiovascular risk in individuals with diabetes and hypertension.14,31 A large prospective trial should be performed to determine if this tool can be used to risk stratify individuals better and if therapy aiming to change the parameters of pulse wave velocity can decrease future cardiovascular events.
Treatment of aortic stiffness as it relates to hypertension
The old adage in medicine is that prevention is better than cure. Any treatment for hypertension, particularly in the young, should address concerns regarding subsequent aortic stiffness, systolic hypertension, and the difficulty in controlling systolic blood pressure as the patient ages. Several studies have evaluated commonly used antihypertensives and their effects on aortic stiffness. The CAFE trial22 demonstrated that not all antihypertensive agents have similar effects with regard to lowering central blood pressure. In that study, perindopril and amlodipine were found to be superior in lowering central blood pressure and pulse pressure than the combination of atenolol and bendroflumethiazide, even though the brachial pressure reduction was similar in both groups. It is hypothesized that it was this difference that led to better outcomes in the former group in the CAFÉ study. In REASON (Preterax in Regression of Arterial Stiffness in a Controlled Double-Blind Study),32 a very low-dose combination of indapamide (0.625 mg) and perindopril (2 mg) were compared against atenolol (50 mg). Reduction in central blood pressure over the ensuing 12 months was significantly greater on angiotensin-converting enzyme inhibitor/diuretic therapy as compared with beta-blocker therapy. Further, a subset analysis of this study concluded that patients with elevated central blood pressure had a higher left ventricular mass index. Another study known as EXPLOR (Amlodipine/Valsartan on Central Aortic Blood Pressure in Uncontrolled Essential Hypertension With Amlodipine 5 mg) compared amlodipine/valsartan therapy against amlodipine/atenolol, and concluded that the former combination achieved a significantly greater reduction in augmentation index and central blood pressure.33 The ATLAAST (Aliskiren/hydrochlorothiazide and amlodipine monotherapy in African American patients with stage 2 hypertension) trial34 compared the efficacy of aliskiren/hydrochlorothiazide therapy in an African American population diagnosed with stage II hypertension and concluded that renin inhibitor/diuretic-based therapy achieved a substantial reduction in central blood pressure as compared with amlodipine monotherapy. Another meta-analysis by Ong et al included 15 randomized, double-blind, parallel-group trials performed over 7 years and showed that angiotensin-converting enzyme inhibitors were superior to calcium channel blockers and placebo in reducing aortic stiffness.35 Nonetheless, nonpharmacologic therapies, eg, aerobic exercise training36 and treatment with continuous positive airways pressure for sleep apnea,37 have been shown to reduce central blood pressure as well.
Conclusion
Aortic stiffness and central blood pressure are strong predictors of adverse cardiovascular outcomes. Therapies targeting reduction in central blood pressure rather than peripheral blood pressure should be instituted in high risk patients.
Disclosure
The authors report no conflicts of interest in this work.
References
Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107(22):2864–2869. | |
Young T. The Croonian Lecture: on the functions of the heart and arteries. Philos Trans R Soc Lond. 1809;99:1–31. | |
Nichols WW, O’Rourke M. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. 4th ed. London, UK: Edward Arnold; 1998. | |
Izzo JL Jr. Arterial stiffness and the systolic hypertension syndrome. Curr Opin Cardiol. 2004;19(4):341–352. | |
O’Rourke MF, Pauca A, Jiang XJ. Pulse wave analysis. Br J Clin Pharmacol. 2001;51(6):507–522. | |
Nelson MR, Stepanek J, Cevette M, Covalciuc M, Hurst RT, Tajik AJ. Noninvasive measurement of central vascular pressures with arterial tonometry: clinical revival of the pulse pressure waveform? Mayo Clinic Proc. 2010;85(5):460–472. | |
Zambanini A, Cunningham SL, Parker KH, Khir AW, McG Thom SA, Hughes AD. Wave-energy patterns in carotid, brachial, and radial arteries: a noninvasive approach using wave-intensity analysis. Am J Physiol Heart Circ Physiol. 2005;289(1):H270–H276. | |
Karamanoglu M, O’Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993;14(2):160–167. | |
Sharman JE, Lim R, Qasem AM, et al. Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension. 2006;47(6):1203–1208. | |
Weber T, Wassertheurer S, Rammer M, et al. Validation of a brachial cuff-based method for estimating central systolic blood pressure. Hypertension. 2011;58(5):825–832. | |
Laugesen E, Rossen NB, Peters CD, et al. Assessment of central blood pressure in patients with type 2 diabetes: a comparison between Sphygmocor and invasively measured values. Am J Hypertens. 2014;27(2):169–176. | |
Jiang B, Liu B, McNeill KL, Chowienczyk PJ. Measurement of pulse wave velocity using pulse wave Doppler ultrasound: comparison with arterial tonometry. Ultrasound Med Biol. 2008;34(3):509–512. | |
Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. | |
Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39(1):10–15. | |
Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106(16):2085–2090. | |
Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003;63(5):1852–1860. | |
Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21(12):2046–2050. | |
Cheng HM, Chuang SY, Sung SH, et al. Derivation and validation of diagnostic thresholds for central blood pressure measurements based on long-term cardiovascular risks. J Am Coll Cardiol. 2013;62(19):1780–1787. | |
McEniery CM, Yasmin, McDonnell B, et al. Central pressure: variability and impact of cardiovascular risk factors: the Anglo-Cardiff Collaborative Trial II. Hypertension. 2008;51(6):1476–1482. | |
Fukuda D, Yoshiyama M, Shimada K, et al. Relation between aortic stiffness and coronary flow reserve in patients with coronary artery disease. Heart. 2006;92(6):759–762. | |
Devereux RB, Pickering TG, Harshfield GA, et al. Left ventricular hypertrophy in patients with hypertension: importance of blood pressure response to regularly recurring stress. Circulation. 1983;68(3):470–476. | |
Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113(9):1213–1225. | |
Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50(1):197–203. | |
Manisty C, Mayet J, Tapp RJ, et al. Wave reflection predicts cardiovascular events in hypertensive individuals independent of blood pressure and other cardiovascular risk factors: an ASCOT (Anglo-Scandinavian Cardiac Outcome Trial) substudy. J Am Coll Cardiol. 2010;56(1):24–30. | |
Pereira T, Maldonado J, Pereira L, Conde J. [Aortic stiffness is an independent predictor of stroke in hypertensive patients]. Arq Bras Cardiol. 2013;100(5):437–443. Portuguese. | |
Henskens LH, Kroon AA, van Oostenbrugge RJ, et al. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension. 2008;52(6):1120–1126. | |
Poels MM, van Oijen M, Mattace-Raso FU, et al. Arterial stiffness, cognitive decline, and risk of dementia: the Rotterdam study. Stroke. 2007;38(3):888–892. | |
Zeki Al Hazzouri A, Newman AB, Simonsick E, et al. Pulse wave velocity and cognitive decline in elders: the Health, Aging, and Body Composition study. Stroke. 2013;44(2):388–393. | |
Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107(1):139–146. | |
Cardoso CR, Ferreira MT, Leite NC, Salles GF. Prognostic impact of aortic stiffness in high-risk type 2 diabetic patients: the Rio de Janeiro Type 2 Diabetes Cohort Study. Diabetes Care. 2013;36(11):3772–3778. | |
Mansour AS, Yannoutsos A, Majahalme N, et al. Aortic stiffness and cardiovascular risk in type 2 diabetes. J Hypertens. 2013;31(8):1584–1592. | |
Protogerou A, Blacher J, Stergiou GS, Achimastos A, Safar ME. Blood pressure response under chronic antihypertensive drug therapy: the role of aortic stiffness in the REASON (Preterax in Regression of Arterial Stiffness in a Controlled Double-Blind) study. J Am Coll Cardiol. 2009;53(5):445–451. | |
Boutouyrie P, Achouba A, Trunet P, Laurent S, Group ET. Amlodipine-valsartan combination decreases central systolic blood pressure more effectively than the amlodipine-atenolol combination: the EXPLOR study. Hypertension. 2010;55(6):1314–1322. | |
Ferdinand KC, Pool J, Weitzman R, Purkayastha D, Townsend R. Peripheral and central blood pressure responses of combination aliskiren/hydrochlorothiazide and amlodipine monotherapy in African American patients with stage 2 hypertension: the ATLAAST trial. J Clin Hypertens (Greenwich). 2011;13(5):366–375. | |
Ong KT, Delerme S, Pannier B, et al. Aortic stiffness is reduced beyond blood pressure lowering by short-term and long-term antihypertensive treatment: a meta-analysis of individual data in 294 patients. J Hypertens. 2011;29(6):1034–1042. | |
Kakiyama T, Matsuda M, Koseki S. Effect of physical activity on the distensibility of the aortic wall in healthy males. Angiology. 1998;49(9):749–757. | |
Phillips C, Hedner J, Berend N, Grunstein R. Diurnal and obstructive sleep apnea influences on arterial stiffness and central blood pressure in men. Sleep. 2005;28(5):604–609. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.