Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Antidiabetic and Anti-hyperlipidemic Effects of the Crude Hydromethanol Extract of Hagenia abyssinica (Rosaceae) Leaves in Streptozotocin-Induced Diabetic Mice
Authors Kifle ZD , Belayneh YM
Received 30 August 2020
Accepted for publication 14 October 2020
Published 29 October 2020 Volume 2020:13 Pages 4085—4094
DOI https://doi.org/10.2147/DMSO.S279475
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Prof. Dr. Antonio Brunetti
Zemene Demelash Kifle,1 Yaschilal Muche Belayneh2
1Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Pharmacy, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
Correspondence: Zemene Demelash Kifle
Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar 196, Ethiopia
Tel +251918026724
Email [email protected]
Background: The leaves of Hagenia abyssinica (Rosaceae) have been used traditionally for the management of diabetes mellitus. Thus, this study aimed to evaluate the antidiabetic and anti-hyperlipidemic activity of Hagenia abyssinica leaves crude extract in streptozotocin-induced diabetic mice.
Methods: Antidiabetic and anti-hyperlipidemic activity of the crude extract of Hagenia abyssinica was studied in streptozotocin-induced diabetic mice. The effects of the extract on fasting blood glucose level, body weight, and serum lipid profiles were analyzed. One-way ANOVA followed by Tukey’s post hoc test was used for data analysis and p< 0.05 was considered as statistically significant.
Results: Hagenia abyssinica leaves crude extract showed significant (p< 0.05-p< 0.001) blood-glucose-lowering activity. Moreover, the crude extract of H. abyssinica reduced the fasting blood glucose level by 23.21%, 38.20%, 43.53%, and 58.99%, respectively, for CE100, CE 200, CE 400, and GLC 5 mg/kg on the 14th day of treatment. After diabetic mice were treated with H. abyssinica (100, 200 and 400 mg/kg) for 14 days, there was a significant decrease in serum total cholesterol, very-low-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and serum triglyceride and a significant increase in body weight, and HDL-cholesterol level as compared to diabetic control.
Conclusion: The present findings revealed that H. abyssinica leaves could be useful for the management of diabetes mellitus and other abnormalities related to this metabolic disorder. Thus, the present study may support the traditional use of H. abyssinica for diabetes mellitus treatment.
Keywords: Hagenia abyssinica, diabetes mellitus, streptozotocin
Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by elevated blood glucose levels as a result of the impaired secretion of insulin/insulin insensitivity.1 The progression of DM has been growing worldwide from 108 million by 1980 to 463 million by 2019 with 1.6 million deaths in 2016 accredited to them worldwide, estimated to increase to 578 million (10.2%) by 2030 and 700 million by 2045.2 According to the WHO, about 80% of the population is using herbal medicines in the aim of treating several diseases,3 and gaining growing attention in global healthcare debates.4
Presently, the existing pharmacotherapy for DM includes insulin and different oral antidiabetic agents for instance sulfonylureas, α-glucosidase inhibitors, thiazolidinediones, some others. These drugs have been used as monotherapy or in combination to control the elevated glycemia. However, these medications are linked to different adverse effects.5 So, the development of antidiabetic drugs from natural plant sources with minimal side effects is increased. Plants have played a major role in the introduction of new therapeutic agents. A medicinal plant, Galega officinalis led to the discovery and synthesis of metformin.6 The phytoconstituents derived from medicinal plants that have anti-diabetic activity contain polysaccharides, alkaloids, peptidoglycan, guanidine, glycosides, carbohydrates, galactomannan gum, terpenoids, hypoglycans, amino acids, glycopeptides, inorganic ions, and steroids. These touch numerous metabolic cascades, which directly or indirectly affect the level of blood glucose in the human body.7 Anti-hyperglycemic, anti-dyslipidemia, and anti-renal failure effects of these plants are attributed to their ability to restore the function of pancreatic tissues by causing an increase in insulin output or inhibit the intestinal absorption of glucose or to the facilitation of metabolites in insulin-dependent processes.8 Thus, herbs and phytochemicals play a major role in the discovery of new therapeutic agents and have received attention as sources of antioxidants, hypoglycemic, and antihyperlipidemic agents.6
Hagenia abyssinica (Bruce) J. F. Gmel (Figure 1), commonly known as ‘kosso’ and ‘African rosewood’, belongs to the monospecific genus Hagenia of the family Rosaceae.6,9 The ecological distribution of this plant starts in northern Ethiopia and ends in southern Zimbabwe and also occurs in Kenya, Tanzania, Uganda, Sudan, Congo, Malawi, Burundi, and Rwanda.9 Different parts of H. abyssinica have been used for the treatment of diarrhea, tongue infection, ulcer, and other diseases in Ethiopian rural communities.10,11 The leaf part is used to treat diarrhea, typhoid, cough, livestock disease (mixed with Juniperus procera), cancer, hypertension, bone fracture, allergic, and wound.12,13
 |
Figure 1 Photo of Hagenia abyssinica tree from the site of collection. |
The flower crude extract and solvent fractions of Hagenia abyssinica showed significant α–amylase inhibitory activity, scavenging diphenyl-2-picrylhydrazyl (DPPH) and improving serum lipid profile levels. In addition, the flower crude extract and solvent fractions of Hagenia abyssinica were also effective in lowering blood glucose levels in diabetic and normoglycemic mice.14 Traditionally, the leaf part of H. abyssinica has been used in the treatment of DM in Ethiopia. An ethnobotanical survey which was carried out in Ethiopia reported that the leaf of the plant is taken orally to treat DM.15–17 However, there is no previous study in the antidiabetic and anti-hyperlipidemic activities of the leaf extract of H. abyssinica in streptozotocin-induced diabetic mice. Thus, the objective of this study is to evaluate the anti-hyperglycemic, and anti-hyperlipidemic activity of the repeated doses of the H. abyssinica leaf extract in streptozotocin-induced diabetic mice.
Methods
Drugs, Reagents, and Instruments
The following drugs, reagents, and instruments were used in the study, glibenclamide (Sanofi-aventis, France), citric acid (Lab tech chemicals, India), Tri-sodium citrate dihydrate (Blulux Laboratories, India), glucose solution (Reyoung pharmaceuticals, China), Streptozotocin (Fisco Research laboratories, India), deep freezer (Labfreez instrument group, Germany), Hot air oven (Medit-Medizin Technik, Germany), automated chemistry analyzer (Shenzhen Mindray Bio-medical Electronics Co., Ltd, China), i-QARE DS-W® blood glucose meter and strips (Alliance international, Taiwan), Lyophilizer (Labfreez, China), pH meter (Bante Instruments, UK), desiccators, digital electronic balance (EPH-400 Abron Exports).
Plant Materials
The Fresh leaves of H. abyssinia were collected in February 2019. The botanical identification and authentication of the plant materials were performed and the voucher specimen was deposited in the Herbarium of Biology Department, Faculty of Natural and Computational Science, University of Gondar.
Preparation of Plant Extract
The leaf of the plant was thoroughly washed with distilled water to remove dirt and then dried under shade at room temperature (25–27°C) with optimal ventilation. The dried plant materials were ground into coarse powder by the electrical mill. Then, the coarse powdered plant materials (1.05 kg powdered leaves) were macerated separately in 80% methanol for 72 hours, and then the extracts were filtered by using Whatman filter paper No.1. The marc was re-macerated two times with fresh solvent, each for 72 hours, and the filtrates obtained from the successive maceration were concentrated under reduced pressure using a rotary evaporator (Hamato, Japan) followed by hot air oven (Medit-Medizin Technik, Germany) set at 40°C. The semi-dried residues were frozen in the refrigerator overnight and then, dried using a lyophilizer (Labfreez, China) to completely remove the solvent residue. Then, the dried leaf extract was kept separately in a desiccator until used for the experiment.18,19
Preliminary Phytochemical Screening of Leaves Crude Extracts
The crude extract was screened for the presence or absence of secondary metabolites such as tannins, alkaloids, saponins, flavonoids, triterpenoid, phenols, steroids, glycosides, and anthraquinones using standard procedures. Detection of terpenoids (Salkowski’s test): The dried 80% methanolic extract (100 mg) was dissolved in 5mL of distilled water. Five milliliters of plant extract solution were mixed in 2 mL of chloroform, followed by the careful addition of 3 mL concentrated H2SO4. A layer of the reddish-brown coloration formed at the interface indicates a positive result for the presence of terpenoids. Detection of saponins (Froth test): To 0.25 g of the crude extract, 5 mL of distilled water was added in a test tube. Then, the solution was shaken vigorously for 2 minutes and observed for a stable persistent froth. The formation of froth indicates the presence of saponins. Detection of flavonoids (NaOH test): About 0.3g of crude extract was dissolved in 2mL distilled water then three drops of 20% sodium hydroxide solution were added. The yellow color was formed which turn to colorless on the addition of three drops of 20% hydrochloric acid which indicates the presence of flavonoids. Detection of tannins (Braemer’s Test): About 0.25g crude extract was stirred with 10mL of distilled water in a test tube and filtered with filter paper (Wathman No. 1). Then a few drops of 2% ferric chloride were added to the clear filtrate. Then, the filtrate was observed when it gives a green precipitate which indicates the presence of tannins. Detection of steroids (Liebermann–Burchardt test): 0.5g of methanolic crude extract was dissolved in 2mL of distilled water. 2 mL chloroform was added to the solution and an equal volume of concentrated H2SO4 was added to the test tube carefully. A red color formed in the lower chloroform layer indicates the presence of steroids. Detection of alkaloids (Wagner’s Test): Formation of a reddish-brown color when three drops of Wagner’s reagent were added to 10 mg of crude extract dissolved in distilled water confirms the presence of alkaloids. Detection of phenolic compounds (ferric chloride test): 10mg of crude extract was dissolved in 1mL distilled water and to this 0.5 mL of neutral 5% ferric chloride solution was added. The formation of blue-green color indicates the presence of phenolic compounds. Detection of glycosides (Keller-kiliani test): Half a gram of dried extract was placed into a test tube. About 20 mL of distilled water was added, and after 24 hr, the extract was filtered using a Whatman No. 1 filter paper. Thereafter, 5 mL of the extract was treated with 2 mL of concentrated glacial acetic acid and two drops of 0.1% ferric chloride solution. The mixture was then poured into a test tube of 1mL concentrated H2SO4. The formation of a brown ring at the interface indicates the presence of glycosides. Detection of anthraquinone (Borntrager’s test): 3mL of the plant extract was dissolved by 3mL of benzene and filtered with Wathman No 1 filter paper. Then 2mL of 10% ammonium hydroxide was added. The formation of a purple ring indicates the presence of anthraquinones.20–22
Experimental Animals
Healthy Male Swiss albino mice (weighing 20–28 g and age of 6–10 weeks) were purchased from the animal house of the Ethiopian Public Health Institute, Addis Ababa, Ethiopia. The animals were kept in polypropylene cages, maintained under standard condition (12 hours light and 12 hours dark cycle), and were allowed free access to a pellet diet and water ad libtum. After randomized grouping and before initiation of the experiment, animals were acclimatized to the laboratory conditions. Animal handling and care were carried out throughout the experiment according to international laboratory animal use and care guidelines.23
Acute Toxicity Study
Based on the limit test standard of the Organization for Economic Cooperation and Development (OECD) No 425 Guideline,24 an acute oral toxicity test was carried out for leaf crude extract of H. abyssinica. One female Swiss albino mouse fasted for 4 hours on the first day of the test then; 2 gm/kg of the extracts was given orally by oral gavage and was observed strictly for physical or behavioral changes. The animals were housed separately and observed for the manifestation of gross behavioral and physical toxicities like changes in the skin, urination, lacrimation, reduction in feeding activity, excitation, paw licking, increased respiratory rate, decreased motor activity, diarrhea, weight loss, and paralysis for one day and give special attention during the first 4 hours. Based on the results from the first mouse, the other four females’ mice were recruited and fasted for 4 hours and then give 2 gm/kg a single dose and were observed strictly in the same manner. The observation was continued for a total of 2 weeks for any signs of toxicity.24
Grouping and Dosing of Animals
In the diabetic mice model, after the mice were randomly grouped a total of 6 groups were used for the experiment, of which the first five groups were diabetic mice and the last one group was normal mice (each group contains six mice). The first group one served as a diabetic control and was given distilled water; the next group two served as a positive control and was given glibenclamide 5 mg/kg; the coming three groups (group three, four, and five) were given the three doses (100 mg/kg, 200 mg/kg and 400 mg/kg, respectively) of H. abyssinica leaf crude extract; and the last group six (normal mice) served as a negative control and was given distilled water.25,26 The distilled water, the standard drug, and each dose of the crude extract were administered once daily for fourteen days. Before the administration of distilled water, standard drug, and each dose of the crude extract, the fasting body weight, and blood glucose level were determined as a baseline. Then, after overnight fasting (fourteen hours) similar measurement of the body weight and BGL were done on the 7th and 14th day of administration of distilled water, the standard drug, and each dose of the crude extract.27,28 Finally, on the 15th day, following overnight fasting (fourteen hours) of all groups of mice blood samples were collected in all mice and the serum lipid profile levels were determined.29,30
As per the OECD guideline, the extract doses to be administered are determined based on the acute toxicity study and the volume of administration is 1 mL/100 g of body weight of the mouse.24 The middle dose was one-tenth of the limit dose (200 mg/kg), the higher dose was twice the middle dose (400 mg/kg), and the lower dose was calculated as half of the middle dose (100 mg/kg). Glibenclamide (5mg/kg) was selected as a standard drug for the study based on earlier studies.26
Induction of Experimental Diabetes
After overnight fasting of all groups of male mice, the body weight and the BGL were measured before diabetes mellitus induction by streptozotocin solution. Then, the streptozotocin solution at a dose of 150 mg/kg, pH 4.5 was injected through the intraperitoneal route. After diabetes induction, five percent glucose was given to all groups of mice after 6 hours of the streptozotocin solution administration (to avoid death secondary to hypoglycemic shock). Seventy-two hours later, the mice were screened for diabetes mellitus. Those mice having >200 mg/dl blood glucose levels were contained within the experiment.31
Statistical Analysis
The data that was obtained from the experiments were expressed as mean ± SEM. Statistical analysis was done using statistical package for social sciences (SPSS) version 24. Between and within-group analyses were carried out by using one-way ANOVA, subsequently Tukey’s multiple comparison tests. Finally, the findings were considered significant when p-value < 0.05.
Results
The Percentage Yield of Plant Material Extraction
A total of 153 g of dried hydro-methanolic crude leaf extract of H. abyssinica was harvested at the end of the extraction process. The extraction yield was found to be 14.6% (w/w).
Preliminary Phytochemical Screening
Preliminary phytochemical screening of the crude leaf extract of H. abyssinica showed the presence of phenols, triterpenoid, flavonoids, saponins, and anthraquinones (Table 1).
 |
Table 1 Phytochemical Screening of Leaves Extract of H. abyssinica |
Anti-Hyperglycemic Activity of the Repeated Doses of H. abyssinica Extract
The antidiabetic effect of the crude extract of H. abyssinica on fasting blood glucose levels of streptozotocin-induced diabetic mice was measured weekly. Succeeding the induction of DM by STZ, all groups of diabetic mice showed a significant (p < 0.001) difference in blood glucose level as compared to normal mice across all time intervals. Though, all groups of diabetic mice didn`t exhibit a significant difference in baseline fasting blood glucose levels as compared to the negative control group. Administration of the three doses (100 mg/kg, 200 mg/kg, and 400 mg/kg) of the crude extract of H. abyssinica showed a significant (p < 0.05, p < 0.01, and p < 0.001, respectively) reduction in blood glucose level as compared to the diabetic control group on the 7th day of treatment. Likewise, the three doses (100 mg/kg, 200 mg/kg, and 400 mg/kg) of the crude extract of H. abyssinica showed a significant (p < 0.01, p < 0.001, and p < 0.001, respectively) reduction in blood glucose level as compared to the diabetic control group on the 14th day of treatment. Similarly, GLC 5 mg/kg treated group showed a significant (p < 0.001) reduction in blood glucose level as compared to the diabetic control group on the 7th and 14th days of treatment.
Within a group analysis revealed that all doses (100 mg/kg, 200 mg/kg, and 400 mg/kg) of the crude extract of H. abyssinica showed a significant (p < 0.01, p < 0.001, and p < 0.001, respectively) reduction in the blood glucose level on the 7th and 14th days as compared to the baseline blood glucose level. Likewise, the standard drug significantly (P < 0.001) reduced the blood glucose level on the 7th and 14th days as compared to the baseline blood glucose level. As summarized in Figure 2, repeated administration of the crude extract of H. abyssinica at doses of 100 mg/kg, 200 mg/kg, and 400 mg/kg showed a maximum reduction in fasting blood glucose level at the 14th days, 23.21%, 38.20%, 43.53%, and 58.99%, respectively, for CE100, CE200, CE400, and GLC 5 mg/kg (Table 2).
 |
Table 2 Antihyperglycemic Activity of Repeated Daily Doses of the Crude Extract in Diabetic Mice |
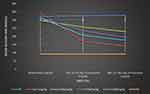 |
Figure 2 Effect of repeated daily doses of Hagenia abyssinica on blood glucose level of diabetic mice. |
Effect of the Repeated Daily Doses of H. abyssinica Extract on the Bodyweight
The effect of the repeated daily doses of the crude extract of H. abyssinica on the body weight is presented in Table 3. Earlier to the indication of DM by streptozotocin, all groups of mice didn`t show a significant difference in body weight. However, streptozotocin showed a significant reduction in the body weight of the diabetic control group on the 7th and 14th days of treatment as compared to the normal control group. Succeeding treatment of diabetic mice with the crude extract of H. abyssinica for two weeks prevented weight loss in streptozotocin-induced diabetic mice as compared to diabetic control. Only CE 400 mg/kg prevented weight loss significantly (P < 0.01) compared with the diabetic control group after the 7th days of treatment. However, CE 100 mg/kg and CE 200 mg/kg didn`t prevent weight loss significantly following the 7th day of treatment. Similarly, the middle and the higher doses of the crude extract of H. abyssinica (CE 200 mg/kg (P < 0.01) and CE 400 mg/kg (P < 0.001)) and GLC 5 mg/kg (P < 0.001) improved weight loss significantly as compared to the diabetic control group following 14 days of administration. Within a group analysis revealed that the middle (200 mg/kg) and the higher (400 mg/kg) doses, the crude extract of H. abyssinica showed a significant (P < 0.05) increment on the body weight on the 14th days as compared to the baseline bodyweight. Similarly, GLC 5 mg/kg significantly (P < 0.01) increased the body weight on the 14th day as compared to the baseline body weight (Table 3).
 |
Table 3 Effect of Repeated Daily Doses of the Crude Extract on Body Weight of Diabetic Mice |
Effect of Repeated Daily Doses of H. abyssinica Extract on Serum Lipid Level
In the current finding, statistically significant (p<0.001) increment in STC, STG, VLDL-c, and LDL-c with a progressive decrease in HDL-c was observed within the diabetic control group compared with the normal control group.
Between-group analysis showed that the middle (200 mg/kg) and the higher (400 mg/kg) doses of the crude extract of H. abyssinica showed a statically significant improvement in serum total cholesterol (p<0.001). Similarly, all tested doses (100 mg/kg, 200 mg/kg and 400 mg/kg) of the crude extract revealed a statically significant improvement in serum lipid profiles: serum triglyceride (p<0.05, p<0.001, p<0.001, respectively); high-density lipoprotein cholesterol (p<0.05, p<0.01, p<0.001, respectively); very-low-density lipoprotein cholesterol (p<0.01, p<0.001, p<0.001, respectively); and low-density lipoprotein cholesterol (p<0.05, p<0.01, p<0.001, respectively) as compared to the diabetic control. Therefore, treatment of diabetic mice with the crude extract of H. abyssinica and the standard drug significantly and dose-dependently improved serum TC, TG, and LDL-c, and VLDL-c levels as compared to the diabetic control group. Although a significant increase in serum HDL-cholesterol level was shown in the crude extract of H. abyssinica treated groups as compared to the diabetic control (Table 4).
 |
Table 4 Effect of Repeated Daily Doses of the Crude Extract on Serum Lipid Level of Diabetic Mice |
Discussion
Diabetes mellitus can be described as a disorder of multiple etiologies with abnormalities in protein, carbohydrate, and lipid metabolism. Abnormalities in lipid profile, cardiovascular, renal function, and glucose metabolism are the main risk factors for DM.32 The use of the leaf part of H. abyssinica for the management of DM has been reported in different ethnobotanical surveys. In the present study, to establish the scientific basis for the utility of H. abyssinica in the treatment of diabetes, evaluation of the antihyperglycemic and anti-hyperlipidemic activity of the crude methanolic leaves extract was done in STZ-induced diabetic mice.
Streptozotocin-induced diabetic mice are one of the most commonly used animal models of diabetes mellitus.33 It is well known for its selective pancreatic islet β-cell cytotoxicity and has been extensively used to induce DM in experimental mice model. Glibenclamide is often used as a standard antidiabetic drug in streptozotocin-induced diabetes to compare the activity of variety of medicinal plants with antidiabetic activities.34
In the acute oral toxicity study, administering crude extract at a single dose of 2000 mg/kg body weight orally did not cause any major toxicity and death of mice during observation. This study revealed that the LD50 is greater than 2000 mg/kg. This result supports the study which presents the tough evidence of the non-toxic outcome of the plant.35
The increase in fasting BGL is an important characteristic feature of DM.36 In the present study, there were elevations in fasting blood glucose levels in diabetic-treated mice. Though, the crude extract of H. abyssinica reduced fasting blood glucose levels in diabetic mice. The fasting blood glucose level was decreased by 13.89% (p<0.05); 25.67% (p<0.01); and 34.06% (p<0.001) at 100, 200, and 400mg/kg doses of the extract, respectively, on the 7th day of treatment. Similarly, the fasting blood glucose level was decreased by 23.21% (p<0.01); 38.20% (p<0.001); and 43.53% (p<0.001) at 100, 200, and 400mg/kg doses of the extract, respectively, on the 14th day of treatment. Likewise, the standard drug (GLC5mg/kg) reduced the fasting blood glucose level by 51.28% (p<0.001), and 58.99% (p<0.001), respectively, on the 7th and 14th days of treatment. These findings of a reduction in fasting blood glucose levels are in agreement with previous studies.14,37 Hence, when the concentrations of H. abyssinica crude extract increased, the fasting blood glucose level was shown to decrease. The glycemic control was nearly similar between GLC 5 mg/kg and H. abyssinica crude extract treatment. Therefore, the increment of the H. abyssinica crude extract doses may further provide a similar result as the standard drug. The current result showed the potential antihyperglycemic activity of the crude extract. Among, the possible explanations for the present result, the antihyperglycemic effect of H. abyssinica crude extract could be due to restoration of insulin response via the presence of antihyperglycemic, “insulin-releasing” and “insulin-like” activity.38 It was also suggested that the anti-hyperglycemic effects of the H. abyssinica crude extract might be because of the high level of fiber that interferes with carbohydrate absorption, increased peripheral uptake of glucose, improved sensitivity of insulin receptor, and regenerative activity of H. abyssinica crude extract on pancreatic tissue.39
Slight body weight Loss was observed in streptozotocin-induced diabetic mice and almost normalized by treatment with H. abyssinica crude extract. Dehydration and loss of body weight have been associated with diabetes mellitus.36 In diabetic mice, increased water intake and decreased body weight were observed. This indicates a polydipsic condition and loss of weight due to the excessive breakdown of tissue proteins.40 The decrease in body weight in diabetic mice could be due to dehydration and catabolism of fats,41 as well as proteins, which might lead to muscle wasting.42 Oral administration of H. abyssinica crude extract for 14 consecutive days to diabetic mice lessened their water intake and improved body weight. These effects could be due to better control of the hyperglycemic state in diabetic mice. Decreased fasting blood sugar improves body weight in streptozotocin-induced diabetic mice.43,44
Lipid profile is crucial in the diagnosis and treatment of several cardiovascular diseases and control of DM.45 Several studies have conveyed that cardiovascular complications linked with DM are due to abnormality in lipid metabolism.46 The results of this study showed a significant increment of lipid profiles in streptozotocin-induced diabetic mice such as TC, TG, VLDL-c, and LDL-c, all this combined with a decrease in HDL-c. The administration of H. abyssinica crude extract exhibited a significant improvement in lipid profile. Administration of H. abyssinica crude extract significantly increased the HDL-cholesterol in diabetic mice. The reductions of TG, TC, and LDL level by the extract of H. abyssinica might be due to the involvement of the polyphenolic part of the extract in preventing the formation of AGEs in diabetic mice.47 The extract of H. abyssinica fiber may delay the absorption of glucose and fatty acids, therefore providing fewer substrate for the production of triglycerides.48 In addition, the leaf extract of H. abyssinica may have inhibitory effects on pancreatic cholesterol esterase and pancreatic lipase which may contribute to anti-hyperlipidemic activities in the diabetic mice. This finding is in line with previous similar studies.37,49
Secondary metabolites play a significant role in the biological activities of medicinal plants such as hypoglycemic, antidiabetic, antimicrobial, anti-leprosy activities, anti-inflammatory, anti-malarial, anticarcinogenic, anti-cholinergic, and antioxidant.50 In the present study, the crude extract of H. abyssinica contains phytoconstituents such as phenols, triterpenoid, flavonoids, saponins, and anthraquinones. Flavonoids and tannin were some of the renowned compounds that were isolated from the extract are capable of reducing the elevated blood glucose level.51–53 Saponin is indicated to use as antioxidant, antihyperglycemic, antihyperlipidemic and renoprotective, while tannin possesses antioxidant and renoprotective activity.54,55 Polyphenolic compounds that are isolated from the extract are capable of reducing the TG, TC, and LDL levels through inhibition of the formation of AGEs in diabetic mice.47 Therefore, the significant anti-diabetic and anti-hyperlipidemic activity of the extract of H. abyssinica could be because of the presence of the above-mentioned phytoconstituents in the crude extract of H. abyssinica.
Conclusion
In this study, the crude extract of H. abyssinica showed a reduction in fasting blood glucose levels in streptozotocin-induced diabetic mice. This provides evidence that the extract of H. abyssinica improves the metabolic abnormalities related to DM and can retard the risk of complications due to chronic hyperglycemia. Therefore, the beneficial effects of H. abyssinica in diabetes seem to be attributed to the synergistic effects of its bioactive compounds such as phenols, triterpenoid, flavonoids, saponins, and anthraquinones. Additional investigations are required to identify the lead compounds and quantitative characterization of secondary metabolites existing in H. abyssinica with its molecular mechanism of action on histological analysis, PPAR, insulin sensitization, and other insulin targets based on the pathophysiology of DM.
Availability of Materials and Data
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
BGL, blood glucose level; DM, diabetes mellitus; HDL, high-density lipoprotein; LDL, low-density lipoprotein; STZ, streptozotocin; TC, total cholesterol; TG, triglycerides; VLDL, very low-density lipoprotein.
Ethics Approval and Consent to Participate
Ethical approval was obtained from the ethical review committee of the Department of pharmacy, college of medicine and health sciences, Wollo University with a Reference number of WU/236/11.
Acknowledgment
We would like to acknowledge Wollo University for providing the necessary facilities in this study.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Dekker JM, Balkau B. Counterpoint: impaired fasting glucose: the case against the new American Diabetes Association guidelines. Diabetes Care. 2006;29(5):1173–1175. doi:10.2337/dc05-2220
2. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
3. Organization WH. WHO Global Report on Traditional and Complementary Medicine 2019. World Health Organization; 2019.
4. Aslam MS, Ahmad MS. Worldwide importance of medicinal plants: current and historical perspectives. Recent Adv Biol Med. 2016;2(2016):909. doi:10.18639/RABM.2016.02.338811
5. Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414(6865):821–827. doi:10.1038/414821a
6. Aiman R. Recent research in indigenous anti-diabetic medicinal plants, An overall assessment. Indian J Physiol Pharmacol. 1970;14(2):65–76.
7. Prabhakar PK, Doble M. Mechanism of action of natural products used in the treatment of diabetes mellitus. Chin J Integr Med. 2011;17(8):563. doi:10.1007/s11655-011-0810-3
8. Fatima A, Agrawal P, Singh PP. Herbal option for diabetes: an overview. Asian Pacific J Tropical Disease. 2012;2:S536S544. doi:10.1016/S2222-1808(12)60216-3
9. Negash L. Indigenous Trees of Ethiopia: Biology, Uses and Propagation Techniques. Addis Ababa University: Department of Biology; 1995.
10. Kokwaro JO. Medicinal Plants of East Africa. East African Literature Bureau; 1976.
11. Beentje H, Adamson J, Bhanderi D. Kenya Trees. National Museums of Kenya: shrubs, and lianas; 1994.
12. Assefa B, Glatzel G, Buchmann C. Ethnomedicinal uses of Hagenia abyssinica (Bruce) JF Gmel. among rural communities of Ethiopia. J Ethnobiol Ethnomed. 2010;6(1):20. doi:10.1186/1746-4269-6-20
13. Enyew A, Asfaw Z, Kelbessa E, Nagappan R. Ethnobotanical study of traditional medicinal plants in and around Fiche District, Central Ethiopia. Current Research J Biological Sciences. 2014;6(4):154–167. doi:10.19026/crjbs.6.5515
14. Kifle ZD, Yesuf JS, Atnafie SA. Evaluation of in vitro and in vivo anti-diabetic, anti-hyperlipidemic and anti-oxidant activity of flower crude extract and solvent fractions of Hagenia abyssinica (rosaceae). J Exp Pharmacol. 2020;12:151–167. doi:10.2147/JEP.S249964
15. Lunyera J, Wang D, Maro V, et al. Traditional medicine practices among community members with diabetes mellitus in Northern Tanzania: an ethnomedical survey. BMC Complement Altern Med. 2016;16(1):282. doi:10.1186/s12906-016-1262-2
16. Mekuria AB, Belachew SA, Tegegn HG, et al. Prevalence and correlates of herbal medicine use among type 2 diabetic patients in Teaching Hospital in Ethiopia: a cross-sectional study. BMC Complement Altern Med. 2018;18(1):85. doi:10.1186/s12906-018-2147-3
17. Habte BM, Kebede T, Fenta TG, Boon H. Explanatory models of adult patients with type 2 diabetes mellitus from urban centers of central Ethiopia. BMC Res Notes. 2016;9(1):441. doi:10.1186/s13104-016-2248-3
18. Satyajit D, Sarker ZL, Alexander I. Gray. Natural Products Isolation.
19. Geleta B, Makonnen E, Debella A, Tadele A. In vivo antihypertensive and antihyperlipidemic effects of the crude extracts and fractions of Moringa stenopetala (Baker f.) Cufod. leaves in rats. Front Pharmacol. 2016;7:97. doi:10.3389/fphar.2016.00097
20. Trease GE, Evans WC. Trease and Evans' Pharmacognosy. 13th ed. London, Bailliere Tindall; 1989.
21. Pandey A, Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacognosy Phytochemistry. 2014;2:5.
22. Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Internationale Pharmaceutica Sciencia. 2011;1(1):98–106.
23. Care I, Animals U, Resources N. Guide for the care and Use of Laboratory animals. National Academies; 1985.
24. OCDE O. Acute oral toxicity: up and down procedure. OECD Guideline Testing Chemicals. 2008;425:1–2.
25. Tesfaye A, Makonnen E, Gedamu S. Hypoglycemic and anti-hyperglycemic activity of aqueous extract of Justicia Schimperiana leaves in normal and streptozotocin-induced diabetic mice. Int J Pharm Sciences Research. 2016;7(2):107–113.
26. Tamiru W, Engidawork E, Asres K. Evaluation of the effects of 80% methanolic leaf extract of Caylusea abyssinica (fresen.) fisch. & Mey. on glucose handling in normal, glucose loaded and diabetic rodents. BMC Complement Altern Med. 2012;12(1):151. doi:10.1186/1472-6882-12-151
27. Toma A, Makonnen E, Mekonnen Y, Debella A, Adisakwattana S. Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2015;15(1):242. doi:10.1186/s12906-015-0779-0
28. Sharma S, Choudhary M, Bhardwaj S, Choudhary N, Rana AC. Hypoglycemic potential of alcoholic root extract of Cassia occidentalis Linn. in streptozotocin induced diabetes in albino mice. Bulletin Faculty Pharmacy, Cairo University. 2014;52(2):211–217. doi:10.1016/j.bfopcu.2014.09.003
29. Bowe JE, Franklin ZJ, Hauge-Evans AC, King AJ, Persaud SJ, Jones PM. Metabolic phenotyping guidelines: assessing glucose homeostasis in rodent models. J Endocrinology. 2014;222(3):G13G25.
30. Ayala JE, Samuel VT, Morton GJ, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;
31. Baquer NZ, Kumar P, Taha A, Kale R, Cowsik S, McLean P. Metabolic and molecular action of Trigonella foenum-graecum (fenugreek) and trace metals in experimental diabetic tissues. J Biosci. 2011;36(2):383–396. doi:10.1007/s12038-011-9042-0
32. Graham ML, Janecek JL, Kittredge JA, Hering BJ, Schuurman H-J. The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp Med. 2011;61(4):356–360.
33. Tomlinson K. Functional consequences of streptozotocin-induced diabetes mellitus, with particular reference to the cardiovascular system. Pharmacol Rev. 1992;44:103–150.
34. Fernandes NP, Lagishetty CV, Panda VS, Naik SR. An experimental evaluation of the antidiabetic and antilipidemic properties of a standardized Momordica charantia fruit extract. BMC Complement Altern Med. 2007;7(1):29. doi:10.1186/1472-6882-7-29
35. World Health Organization. The WHO recommended classification of pesticides by hazard and guidelines to classification 2019. Geneva: World Health Organization; 2019. Available from: https://www.who.int/ipcs/publications/pesticides_hazard/en/. Accessed October 20, 2020.
36. Pupim LB, Heimburger O, Qureshi AR, Ikizler T, Stenvinkel P. Accelerated lean body mass loss in incident chronic dialysis patients with diabetes mellitus. Kidney Int. 2005;68(5):2368–2374. doi:10.1111/j.1523-1755.2005.00699.x
37. Nabi SA, Kasetti RB, Sirasanagandla S, Tilak TK, Kumar MVJ, Rao CA. Antidiabetic and antihyperlipidemic activity of Piper longum root aqueous extract in STZ induced diabetic rats. BMC Complement Altern Med. 2013;13(1):37. doi:10.1186/1472-6882-13-37
38. Gray AM, Flatt PR. Insulin-releasing and insulin-like activity of the traditional anti-diabetic plant. Coriandrum Sativum (Coriander) British J Nutrition. 1999;81(3):203–209. doi:10.1017/S0007114599000392
39. Enkhmaa B, Ozturk Z, Anuurad E, Berglund L. Postprandial lipoproteins and cardiovascular disease risk in diabetes mellitus. Curr Diab Rep. 2010;10(1):61–69. doi:10.1007/s11892-009-0088-4
40. Kamalakkannan N, Prince PSM. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin‐induced diabetic wistar rats. Basic Clin Pharmacol Toxicol. 2006;98(1):97–103.
41. Hakim ZS, Patel BK, Goyal RK. Effects of chronic ramipril treatment in streptozotocin-induced diabetic rats. Indian J Physiol Pharmacol. 1997;41(4):353–360.
42. Rajkumar L, Srinivasan N, Balasubramanian K, Govindarajulu P. Increased degradation of dermal collagen in diabetic rats. Indian J Exp Biol. 1991;29(11):1081–1083.
43. Nagarajan N, Murugesh N, Kumaresan PT, Radha N, Murali A. Antidiabetic and antihyperlipidemic effects of Clemeo Felina Fitoterapia. 2005;76(34):310–315. doi:10.1016/j.fitote.2005.03.020
44. Pari L, Saravanan R. Antidiabetic effect of diasulin, a herbal drug, on blood glucose, plasma insulin and hepatic enzymes of glucose metabolism in hyperglycaemic rats. Diabetes Obes Metab. 2004;6(4):286–292. doi:10.1111/j.1462-8902.2004.0349.x
45. Akuyam S, Isah H, Ogala W. Evaluation of serum lipid profile of under-five Nigerian children. Ann Afr Med. 2007;6(3):119. doi:10.4103/1596-3519.55722
46. Qi X-Y, Chen W-J, Zhang L-Q, Xie B-J. Mogrosides extract from Siraitia grosvenori scavenges free radicals in vitro and lowers oxidative stress, serum glucose, and lipid levels in alloxan-induced diabetic mice. Nutrition Research. 2008;28(4):278–284.
47. Jia Q, Liu X, Wu X, et al. Hypoglycemic activity of a polyphenolic oligomer-rich extract of Cinnamomum parthenoxylon bark in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2009;16(8):744–750. doi:10.1016/j.phymed.2008.12.012
48. Jelodar G, Mohsen M, Shahram S. Effect of walnut leaf, coriander and pomegranate on blood glucose and histopathology of pancreas of alloxan induced diabetic rats. African J Traditional, Complementary Alternative Medicines. 2007;4(3):299–305. doi:10.4314/ajtcam.v4i3.31223
49. Subhasree N, Kamella A, Kaliappan I, Agrawal A, Dubey GP. Antidiabetic and antihyperlipidemic activities of a novel polyherbal formulation in high fat diet/streptozotocin induced diabetic rat model. Indian J Pharmacol. 2015;47(5):509. doi:10.4103/0253-7613.165200
50. Yadav M, Chatterji S, Gupta SK, Watal G. Preliminary phytochemical screening of six medicinal plants used in traditional medicine. Int J Pharm Pharm Sci. 2014;6(5):539–542.
51. Carlson TJ, King SR. From plant to patient: an ethnomedical approach to the identification of new drugs for the treatment of NIDDM. Diabetologia. 1997;40:614–617. doi:10.1007/s001250050724
52. Ragavan B, Krishnakumari S. Antidiabetic effect of T. arjuna bark extract in alloxan induced diabetic rats. Indian J Clinical Biochemistry. 2006;21(2):123. doi:10.1007/BF02912926
53. Miura T, Koike T, Ishida T. Antidiabetic activity of green tea (Thea sinensis L.) in genetically type 2 diabetic mice. J Health Science. 2005;51(6):708–710. doi:10.1248/jhs.51.708
54. Singh R, Kaur N, Kishore L, Gupta GK. Management of diabetic complications: a chemical constituents based approach. J Ethnopharmacol. 2013;150(1):51–70. doi:10.1016/j.jep.2013.08.051
55. Jani DK, Goswami S. Antidiabetic activity of cassia angustifolia vahl. and raphanus sativus linn. leaf extracts. J Traditional Complementary Medicine. 2020;10(2):124. doi:10.1016/j.jtcme.2019.03.002
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
