Back to Journals » International Journal of Nanomedicine » Volume 17
Antibacterial Effect of Zirconia Nanoparticles on Polyethyl Methacrylate Resin for Provisional Crowns
Authors Kim HS , Jang W , Im YG, Lim HP
Received 12 July 2022
Accepted for publication 9 December 2022
Published 21 December 2022 Volume 2022:17 Pages 6551—6560
DOI https://doi.org/10.2147/IJN.S382053
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Israel (Rudi) Rubinstein
Hee-Seon Kim,1,* Woohyung Jang,1,* Yeong-Gwan Im,2 Hyun-Pil Lim1
1Department of Prosthodontics, School of Dentistry, Chonnam National University, Gwangju, 61186, Republic of Korea; 2Department of Oral Medicine, School of Dentistry, Chonnam National University, Gwangju, 61186, Republic of Korea
*These authors contributed equally to this work
Correspondence: Hyun-Pil Lim, Department of Prosthodontics, School of Dentistry, Chonnam National University, Gwangju, 61186, Republic of Korea, Tel +82-62-530-5577, Email [email protected] Yeong-Gwan Im, Department of Oral Medicine, School of Dentistry, Chonnam National University, Gwangju, 61186, Republic of Korea, Tel +82-62-530-5578, Email [email protected]
Objective: Mid-to-long term use of provisional crowns in the oral cavity is associated with bacterial adhesion and biofilm formation, thus necessitating provisional crowns exhibiting antibacterial properties to prevent the occurrence of gingivitis and periodontal disease. This study aimed to evaluate the antibacterial effect of zirconia nanoparticle-containing polyethyl methacrylate (PEMA) resin for provisional restorations.
Methods: Zirconia nanoparticles were added to the monomer of PEMA resin for provisional restorations, and the mixture was stirred for 2 h using a magnetic stirrer. Disk-shaped specimens were prepared by mixing the polymer with the nanoparticle solution. The control group contained pure PEMA resin samples, and the experimental groups Group Z2, Group Z4, and Group Z8 included PEMA resin specimens containing 2, 4, and 8% w/v zirconia nanoparticles, respectively. After analyzing the sample surfaces, the antibacterial effect of the specimens was evaluated using Streptococcus mutans. Statistical analysis was performed using Tukey’s test and Mann–Whitney U-test, according to the normality result in the Shapiro–Wilk test.
Results: FE-SEM and EDX analyses revealed the successful addition of zirconia nanoparticles. Results showed no significant difference in the measured values for surface roughness and contact angle between the experimental and control groups; however, adhesion and biofilm thickness of S. mutans were significantly decreased in Group Z2, Group Z4, and Group Z8 compared to the control group (P < 0.05, P < 0.01, P < 0.01, respectively).
Conclusion: The addition of zirconia nanoparticles can lower the incidence of adhesion and biofilm thickness of S. mutans on PEMA resin used for provisional crowns. Thus, incorporating zirconia nanoparticles in repair materials for provisional crowns and PEMA resin can produce an antibacterial effect and prevent gingivitis, periodontal disease, and dental caries.
Keywords: provisional crowns, polyethyl methacrylate (PEMA) resin, zirconia nanoparticles, antibacterial effect, Streptococcus mutans
Introduction
The purpose of a provisional crown is to ensure the preservation of tooth function, proper phonetics, dental esthetics, and soft tissue health while maintaining tissue compatibility until the fabrication of definitive restorations.1,2 Mid-to-long term use of provisional crowns often leads to denaturization, initiating plaque deposition on surfaces.3 Plaque offers habitats for bacteria, resulting in gingivitis, periodontal disease, and dental caries.4 As provisional crowns have higher surface roughness and lower marginal adaptability, they may accumulate more biofilms than permanent prostheses.5 Successful provisional crowns should be resistant to biofilm adhesion; however, most studies have focused on physical aspects, including fracture strength and flexural strength (according to manufacturing methods),6,7 and evaluating the accuracy of 3D-printed provisional crowns fabricated using different materials.8,9 Studies on antibacterial substances, such as chlorhexidine and vancomycin, applied to provisional crowns have limitations owing to the risk of antibiotic resistance, creating challenges in clinical practice.10–12 Therefore, research on developing provisional crowns resistant to biofilm adhesion is needed. Thus far, no study has attempted to incorporate antibacterial substances into resins used for provisional crowns to suppress gingivitis, periodontal disease, and dental caries.
Nanoparticles are materials that occur in nature as minerals, clays, and microbial products. The reactivity between nanoparticles and biomolecules is affected by several factors, including particle size and shape and manufacturing methods.13 As a nanoplatform commonly studied in materials and medicine, nanoparticles can deliver various biomolecules, including DNA, miRNA, siRNA, and ribonucleoprotein. These delivery systems are considered non-cytotoxic vectors with biocompatibility.14 Recently, nanocomposites, including nano-biocomposites, nanoTiO2, and chitosan-nanocomposites, have been widely studied and reported in various fields, such as medical science, pharmaceuticals, and food industries.15
Besides excellent antimicrobial properties and biocompatibility, zirconia nanoparticles have high abrasion resistance and strength with less ability to induce bone resorption or immune response in vivo. Therefore, they have been actively studied as surface treatment materials.16,17 Since zirconia has acceptable esthetic properties and biocompatibility,18,19 studies have focused on zirconia applications in dentistry.20,21 Methods, such as blasting and dip coating, have been used to process resins with zirconia nanocomposites.22 Dip coating is a method in which materials to be coated are vertically immersed into slurries and then slowly lifted at a constant speed; the thickness of coating layers may depend on the viscosity of slurries and lifting speed. However, highly viscous slurries do not run down smoothly, resulting in uneven surfaces in the formed coating layer and defects due to residual droplets left behind at the lower edge of the coating layer.23 Recently, the physical and mechanical properties of PMMA/zirconia nanoparticle composites used for dentures have shown remarkable improvement with reduced adhesion of Candida albicans.24,25 However, no experiments have attempted to change the physical composition by adding antibacterial materials to resins for provisional crowns using Streptococcus mutans, a Gram-positive aerobic bacterium that forms the initial biofilm.
This study aimed to evaluate the antibacterial effect of polyethyl methacrylate (PEMA) resin containing zirconia nanoparticles for provisional restorations. The first null hypothesis was that adding zirconia nanoparticles does not affect the antibacterial effect of PEMA resin used for provisional restorations. The second null hypothesis was that the concentration of zirconia nanoparticles does not affect the antibacterial effect.
Materials and Methods
Experimental Materials
Samples
Zirconia nanoparticles (ZrO2 45~55 nm, 99% Uninano Ltd., Houston, USA) were added at a concentration of 2, 4, or 8% w/v to the monomer of PEMA resin (Snap Parkell, Inc., NY, USA) for preparing monomer solutions with nanoparticles, followed by stirring for 24 h using a magnetic stirrer (MS-300HS, AHN Lab Ltd).
Replicable disk-shaped specimens were prepared (diameter, 15 mm; thickness, 2.5 mm) by mixing the monomer solutions with nanoparticles and then using a mold fabricated with polyvinyl siloxane putty material (CharmFlex, Dentkist Ltd., Seongnam, Korea). Allowed to room temperature dry before specimens fabricated was removed in mold.
Subsequently, the specimens were polished under running water using a grinder (LaboPol-5, Struers Co., Rotherham, UK) and #800 grit SIC paper to obtain a uniform surface. All specimen surfaces were washed with 70% ethanol.
The control group (Control) contained pure PEMA resin samples, whereas the experimental groups named Group Z2, Group Z4, and Group Z8 contained PEMA resin specimens with 2, 4, and 8% w/v of zirconia nanoparticles, respectively (Table 1).
 |
Table 1 Treatment Groups in the Experiment |
Assessment of Surface Characteristics
Zirconia nanoparticles was particle size analysis via transmission electron microscope (TEM, Tecnai-F20 ST, FEI). The zirconia nanoparticle powder was dispersed in ethanol, sonicated for 5 min, and deposited onto the TEM grid film.
To confirm the addition of zirconia nanoparticles and their surface structure, platinum was coated on the surface using a sputter coater (E-1030, Hitachi Co. Ltd, Tokyo, Japan) in a vacuum for 60s, and the surface was observed using field emission scanning electron microscopy (FE-SEM; S-4700, Hitachi Co. Ltd, Tokyo, Japan). The elemental composition was analyzed using energy-dispersive X-ray spectroscopy (EDX; S-4700, Hitachi Co. Ltd, Tokyo, Japan).
The shape and surface roughness of the specimens were observed using a nanosurface 3D optical profiler (3DOP; NV-E1000, Nano System Inc, Daegu, Korea) (n = 3). The degree of surface hydrophilicity was measured using a contact angle analyzer (video-based contact angle measuring device, Phoenix 300, SEO Co. Ltd, Suwon, Korea) (n = 3).
Assessment of Bacterial Activity
Bacterial Cultures
S. mutans (KCOM 1504, Korean Collection for Oral Microbiology, Gwangju, Korea), a gram-positive bacterium known to be involved in the early stage of biofilm formation, was obtained from the Korean Collection for Oral Microbiology (KCOM). The S. mutans strain in brain heart infusion medium (BHI; Becton, Dickinson and Company, Sparks, MD) was cultured at 37 °C in a culture chamber (LIB-150M, Daihan Labtech Ltd. Namyangju, Korea).
Bacterial Inoculation
All specimens were inoculated with S. mutans at a concentration of 1.5 × 107 CFU/mL and cultured for 24 h. After culturing the bacteria, the culture medium floating on the specimens was removed and washed twice with phosphate-buffered saline (SPL Life Sciences Co, Ltd, Incheon, Korea).
Bacterial Adhesion Assessment
Crystal Violet Staining Assay
Bacteria adhering to the specimens were stained using 500 μL of 0.3% crystal violet solution for 10 min. The specimens were washed thrice with PBS to remove the crystal violet solution and dried for 15 min. Next, 500 μL of desalting solution (80% ethyl alcohol + 20% acetone) was dispensed on each specimen, the plate was tightly sealed to prevent volatilization of the solution, and then the solution was stirred for 1 h.
Subsequently, 200 μL of each sample was added to a 96-well plate (SPL Life Sciences Co, Ltd, Incheon, Korea), and the absorbance was measured using a microplate reader (VersaMax ELISA Microplate Reader, Molecular Device, San Jose, CA) at a wavelength of 595 nm (n = 10).
Fluorescent Nucleic Acid Staining Assessment
Bacterial adhesion was visualized using the LIVE/DEAD BacLight Bacterial Viability Kit (SYTO 9, Molecular Probes Europe BV, Leiden, Netherlands). After culturing, bacteria floating in PBS and the remaining culture medium were washed; 200 μL of fluorescence reagent (SYTO 9, dye: propidium iodide: dH2O = 1.5 μL:1.5 μL:1.0 mL) was dispensed to each specimen at room temperature for 15 min, and the plate was covered with aluminum foil to block light for staining the specimens. The remaining staining solution was washed again with PBS, and bacteria adhered to the specimens were observed using a confocal laser scanning microscope (Leica TCS SP5 AOBS/tandem, Leica Microsystems, Mannheim, Germany) (n = 3).
Statistical Analysis
Statistical analysis was performed using SPSS 21.0 (SPSS Inc., Chicago, USA) when normality was met in the Shapiro–Wilk test. One-way ANOVA was used to test parameters, and Tukey’s test was used as a post hoc test. The Kruskal–Wallis test, a non-parametric test, was used when normality was not met, and the Mann–Whitney U-test was used as a post-hoc test (P < 0.05).
Results
Surface Characteristics
The average particle size of zirconia nanoparticle powder measured using TEM was 55±3 nm (Figure 1). FE-SEM results showed that zirconia nanoparticles were spread on the surface of the specimens of Group Z2 (Figure 1B), Group Z4 (Figure 1C), and Group Z8 (Figure 1D), unlike Control (Figure 1A) with an even polished surface (Figure 2). Elemental composition analysis using EDX revealed that the zirconia ratio increased with increased concentration of added zirconia nanoparticles (Figure 3). The surface roughness values, as determined using 3D-OP, for Control, Group Z2, Group Z4, and Group Z8 were 0.29 ± 0.11, 0.24 ± 0.05, 0.11 ± 0.12, and 0.08 ± 0.03 μm, respectively (Figure 4) (n=3). The contact angle, measured using a contact angle analyzer, was 77.69° ± 0.9° for Control, 75.78° ± 1.3° for Group Z2, 72.9° ± 1.2° for Group Z4, and 70.85° ± 1.4° for Group Z8 (Figure 5) (n=3).
 |
Figure 1 Transmission electron microscope (TEM) images of zirconia nanoparticles. |
 |
Figure 4 Three-dimensional surface morphology and roughness of polyethyl methacrylate resin samples in (A) Control group; (B) Group Z2; (C) Group Z4; (D) Group Z8 (n = 3). |
 |
Figure 5 Water contact angle measurements for polyethyl methacrylate resin samples in (A) Control group; (B) Group Z2; (C) Group Z4; (D) Group Z8 (n = 3). |
Inhibition of Biofilm Formation
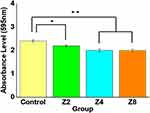
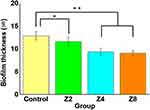
Antibacterial assay results showed that the adhesion of S. mutans was significantly reduced in Group Z2, Group Z4, and Group Z8 compared to Control (P < 0.05, P < 0.01, P < 0.01, respectively) (Figure 6) (n=10). The biofilm thickness was 13.41 ± 0.23 μm in Control and 10.90 ± 0.07, 8.98 ± 0.5, and 8.68 ± 0.9 μm in Group Z2, Group Z4, and Group Z8, respectively, indicating that S. mutans adhesion significantly decreased in the experimental groups compared to Control (P < 0.05, P < 0.01, P < 0.01, respectively) (Figure 7) (n=3).
The LIVE/DEAD BacLight Bacterial Viability Kit was used to visualize live and dead bacteria indicated by green and red color, respectively (Figure 8). Green fluorescence was observed on the surface of the Control specimen, indicating the presence of numerous live bacteria (Figure 8A). In contrast, Group Z2, Group Z4, and Group Z8 specimens had only a small number of adherent bacteria on the surface, with few live bacteria; their surfaces mostly showed red fluorescence, indicating numerous dead bacteria (Figure 8B–D).
Discussion
This study found that the adhesion and biofilm thickness of S. mutans were significantly reduced in provisional crowns fabricated using PEMA resin with zirconia nanoparticles. Therefore, the first null hypothesis was rejected. In Group Z4 and Group Z8, the adhesion and biofilm thickness of S. mutans were significantly decreased compared to Control. Therefore, the second null hypothesis was also rejected.
Although zirconia does not chemically bind with bones, it is widely used in dentistry owing to its high bioaffinity, stress resistance, and biocompatibility. Zirconia does not cause corrosion, induce inflammatory responses, or trigger allergies in vivo.26,27 Reports show that zirconia has an antibacterial effect even in the oral cavity.28 Bacterial adhesion in the oral cavity involves four stages: bacterial movement to the surface, early reversible/irreversible adhesion events, adhesion through specific interactions, and colony biofilm formation. In a healthy state, bacterial adhesion to and removal from the oral surface exists in a dynamic equilibrium, but increased bacterial accumulation may cause disease. The major factors related to the degree of adhesion include surface roughness and contact angle.29 An increase in surface roughness and contact angle significantly increases bacterial counts and binding strength, and interaction between these two factors further increases these effects.30 This study evaluated surface roughness, contact angle, and antibacterial effects of provisional crowns fabricated using PEMA resin with zirconia nanoparticles.
The findings indicated that repaired PEMA resin reinforced with zirconia nanoparticles significantly reduced bacterial adhesion. The addition of zirconia nanoparticles to repaired resin resulted in a significant decrease in S. mutans adhesion, and the maximum reduction occurred with the addition of 8% of zirconia nanoparticles. This decrease in bacterial adhesion may be attributed to the bacterial activity of zirconia nanoparticles or its potential effect in improving surface properties. Reports show that zirconia nanoparticles exhibit an antibacterial effect by inhibiting biofilm formation and preventing the cytoplasmic accumulation of bacteria.31 In agreement with the findings of Kumaresan et al,32 zirconia nanoparticles in this study almost showed significant bacterial inhibition. Zirconia nanoparticles may actively inhibit the growth of gram-positive bacteria by interfering with cell function and causing deformation in gram-positive bacterial hyphae. Consistent with these findings, adding zirconia nanoparticles was associated with an increased antibacterial effect in this study.
The present study indicated that bacterial adhesion was significantly reduced compared to the control group only in Group Z4 and Group Z8. Antibacterial activity was significantly affected by surface roughness. An increase in surface roughness increased bacterial adhesion by providing shelter within surface irregularities, and surface roughness less than 0.2 μm showed an antibacterial effect.33,34 Gad et al35 reported that adding zirconia nanoparticles to various types of PMMA resins reduced surface roughness. Compared to the control group, SEM analysis showed that adding zirconia nanoparticles changed the surface of the tested specimens by filling the pores. Zirconia nanoparticles formed clusters on the surface of specimens. The reduced surface roughness may have resulted from the small size of the particles, which reduced the inter-particle distance, resulting in close contact between the nanoparticles at lower concentrations. Some studies showed that zirconia nanoparticles filled polymeric chain spaces and spaces on the surface; proper bonding to the polymer matrix resulted in smooth surfaces, preventing S. mutans adhesion.36 The present study also showed that a less than 0.2 μm reduction in surface roughness in Group Z4 and Group Z8 increased the antibacterial effect.
The addition of zirconia nanoparticles to PEMA resin showed a significant change in the contact angles of the specimens containing zirconia nanoparticles compared to the control group. This result verges from several studies that reported changes in the PEMA resin contact angle with the addition of nanofillers.37 Studies show that adding zirconia nanoparticles to PEMA resin increases resin hydrophilicity and reduces the potential for bacterial adhesion.38 Several studies tested the effect of the contact angle on S. mutans adhesion and reported varied findings.39 Some authors found that the contact angle significantly affected S. mutans adhesion to resin.40 One of the major factors affecting the adhesion of S. mutans is the surface hydrophobicity of specimens.41 The surface of S. mutans is hydrophobic in nature,42 and in particular, more bacteria adhere to hydrophobic surfaces than hydrophilic surfaces.43 Therefore, increasing the hydrophilicity of the dental restoration surface may be an effective way to reduce the adhesion of S. mutans. In this study, the contact angle showed a tendency to decrease with the increased addition of zirconia nanoparticles to the specimens. It seems that the addition of zirconia nanoparticles reduced the surface contact angle and increased surface hydrophilicity, decreasing the adhesion of S. mutans. Thus, the present study showed that adding zirconia nanoparticles to PEMA resin could reduce bacterial adhesion and biofilm thickness.
A limitation of this study is that additional studies are needed to increase the antibacterial properties while maintaining appropriate physical and mechanical properties for the clinical use of polyethyl methacrylate (PEMA) resin with zirconia nanoparticles. Moreover, the specimens were tested under conditions that did not simulate the oral environment. Therefore, the recommendations of this study require additional in vivo and clinical research on provisional crowns with the addition of zirconia nanoparticles in a simulated oral environment.
Conclusions
Within the limitation of this study, polyethyl methacrylate (PEMA) resins modified with zirconia nanoparticles exhibited an antibacterial effect on S. mutans by reducing surface roughness and contact angle and prevented the development of gingivitis, periodontal disease, and dental caries. Therefore, adding zirconia nanoparticles to PEMA resins for provisional prostheses can effectively reduce the adhesion of S. mutans to the surface of provisional crowns.
Funding
This work was supported by grants from the National Research Foundation of Korea (NRF), and Chonnam National University Hospital Biomedical Research Institute (No. 2020R1F1A1076982 and BCRI22050) supported by the Korean Government (MSIP).
Disclosure
The authors report no conflicts of interest in this work.
References
1. An S, Evans JL, Hamlet S, Love RM. Incorporation of antimicrobial agents in denture base resin: a systematic review. J Prosthet Dent. 2021;126:188–195. doi:10.1016/j.prosdent.2020.03.033
2. Digholkar S, Madhav V, Palaskar J. Evaluation of the flexural strength and microhardness of provisional crown and bridge materials fabricated by different methods. J Indian Prosthodont Soc. 2016;16:328–334. doi:10.4103/0972-4052.191288
3. Jubhari EH, Machmud E, Hasminar H, et al. The effect of fabrication techniques of temporary crowns on the gingiva health. J Dentomaxillofac Sci. 2019;4:133–136. doi:10.15562/jdmfs.v4i3.853
4. Laine MA. Effect of pregnancy on periodontal and dental health. Acta Odontol Scand. 2002;60(5):257–264. doi:10.1080/00016350260248210
5. Buergers R, Rosentritt M, Handel G. Bacterial adhesion of Streptococcus mutans to provisional fixed prosthodontic material. J Prosthet Dent. 2007;98:461–469. doi:10.4047/jap.2017.9.5.335
6. Kebler A, Hickel R, Ilie N. In vitro investigation of the influence of printing direction on the flexural strength, flexural modulus and fractographic analysis of 3D-printed temporary materials. Dent Mater J. 2021;40:641–649. doi:10.4012/dmj.2020-147
7. Lim JH, Lee JI. Comparative study of flexural strength of temporary restorative resin according to surface polishing and fabrication methods. J Dent Rehabil Appl Sci. 2021;37:16–22. doi:10.14368/jdras.2021.37.1.16
8. Tahayeri A, Morgan M, Fugolin AP, et al. 3D printed versus conventionally cured provisional crown and bridge dental materials. Dent Mater. 2018;34:92–200. doi:10.1016/j.dental.2017.10.003
9. Moon W, Kim S, Lim BS, Park YS, Kim RJY, Chung SH. Dimensional accuracy evaluation of temporary dental restorations with different 3D printing systems. Materials. 2021;14:1487. doi:10.3390/ma14061487
10. Makvandi P, Gu JT, Zare EN, et al. Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry. Acta Biomater. 2020;101:69–101. doi:10.1016/j.actbio.2019.09.025
11. Kamer AR, Craig RG, Niederman R, Fortea J, de Leon MJ. Periodontal disease as a possible cause for Alzheimer’s disease. Periodontol 2000. 2020;83:242–271. doi:10.1111/prd.12327
12. Yoshida RA, Gorjão R, Mayer MPA, et al. Inflammatory markers in the saliva of cerebral palsy individuals with gingivitis after periodontal treatment. Braz Oral Res. 2019;33:e033. doi:10.1590/1807-3107
13. Jogaiah S, Paidi MK, Venugopal K, et al. Phytotoxicological effects of engineered nanoparticles: an emerging nanotoxicology. Sci Total Environ. 2021;801:149809. doi:10.1016/j.scitotenv.2021.149809
14. Mujtaba M, Wang D, Carvalho LB, et al. Nanocarrier-mediated delivery of miRNA, RNAi, and CRISPR-Cas for plant protection: current trends and future directions. ACS Agri Sci Technol. 2021;1:417–435. doi:10.1021/acsagscitech.1c00146
15. Geetha N, Bhavya G, Abhijith P, Shekhar R, Dayananda K, Jogaiah S. Insights into nanomycoremediation: secretomics and mycogenic biopolymer nanocomposites for heavy metal detoxification. J Hazard Mater. 2021;409:124541. doi:10.1016/j.jhazmat.2020.12454116
16. Gowri S, Gandhi RR, Sundrarajan M. Structural, optical, antibacterial and antifungal properties of zirconia nanoparticles by biobased protocol. J Mater Sci Technol. 2014;30(8):782–790. doi:10.1016/j.jmst.2014.03.002
17. Hameed HK, Rahman HA. The effect of addition nano particle ZrO2 on some properties of autoclave processed heat cure acrylic denture base material. J Baghdad Coll Dent. 2015;27:1–17. doi:10.12816/0015262
18. Mallineni SK, Nuvvula S, Matinlinna JP, Yiu CK, King NM. Biocompatibility of various dental materials in contemporary dentistry: a narrative insight. J Investig Clin Dent. 2013;4(1):9–19. doi:10.1111/j.2041-1626.2012.00140.x
19. Seabra AB, Durán N. Nanotoxicology of metal oxide nanoparticles. Metals. 2015;5(2):934–975. doi:10.3390/met5020934
20. Kohorst P, Borchers L, Strempel J, et al. Low-temperature degradation of different zirconia ceramics for dental applications. Acta Biomater. 2012;8:1213–1220. doi:10.1016/j.actbio.2011.11.016
21. Ardlin BI. Transformation-toughened zirconia for dental inlays, crowns and bridges: chemical stability and effect of low-temperature aging on flexural strength and surface structure. Dent Mater. 2002;18(8):590–595. doi:10.1016/S0109-5641(01)00095-1)00095-1
22. Zafar MS, Khurshid Z, Najeeb S, Zohaib S, Rehman IU. Therapeutic applications of nanotechnology in dentistry. In: Nanostructures for Oral Medicine. Elsevier; 2017:833. doi:10.1016/B978-0-323-47720-8.00027-4
23. Eduok U, Szpunar J, Ebenso E. Synthesis and characterization of anticorrosion zirconia/acrylic nanocomposite resin coatings for steel. Prog Org Coat. 2019;137:105337. doi:10.1016/j.porgcoat.2019.105337
24. Gad MM, Rahoma A, Al-Thobity AM, ArRejaie AS. Influence of incorporation of ZrO2 nanoparticles on the repair strength of polymethyl methacrylate denture bases. Int J Nanomedicine. 2016;11:5633–5643. doi:10.2147/IJN.S120054
25. Gad MM, Al-Thobity AM, Shahin SY, Alsaqer BT, Ali AA. Inhibitory effect of zirconium oxide nanoparticles on Candida albicans adhesion to repaired polymethyl methacrylate denture bases and interim removable prostheses: a new approach for denture stomatitis prevention. Int J Nanomedicine. 2017;12:5409. doi:10.2147/IJN.S142857
26. Covacci V, Bruzzese N, Maccauro G, et al. In vitro evaluation of the mutagenic and carcinogenic power of high purity zirconia ceramic. Biomaterials. 1999;20(4):371–376. doi:10.1016/S0142-9612(98)00182-3)00182-3
27. Hannink RHJ, Kelly PM, Muddle BC. Transformation toughening in zirconia‐containing ceramics. J Am Ceram Soc. 2000;83:461–487. doi:10.1111/j.1151-2916.2000.tb01221.x
28. Scarano A, Di Carlo F, Quaranta M, Piattelli A. Bone response to zirconia ceramic implants: an experimental study in rabbits. J Oral Implantol. 2003;29:8–12. doi:10.1563/1548-1336(2003)029<0008:
29. Quirynen M, Bollen CML. The influence of surface roughness and surface‐free energy on supra‐and subgingival plaque formation in man: a review of the literature. J Clin Periodontol. 1995;22:1–14. doi:10.1111/j.1600-051X.1995.tb01765.x
30. Al-Radha ASD, Dymock D, Younes C, O’Sullivan D. Surface properties of titanium and zirconia dental implant materials and their effect on bacterial adhesion. J Dent. 2012;40:146–153. doi:10.1016/j.jdent.2011.12.006
31. Gad MM, Abualsaud R, Rahoma A, Al-Thobity AM, Akhtar S, Fouda SM. Double-layered acrylic resin denture base with nanoparticle additions: an in vitro study. J Prosthet Dent. 2020;127:174–183. doi:10.1016/j.prosdent.2020.08.021
32. Kumaresan M, Anand KV, Govindaraju K, Tamilselvan S, Kumar VG. Seaweed Sargassum wightii mediated preparation of zirconia (ZrO2) nanoparticles and their antibacterial activity against gram positive and gram negative bacteria. Microb Pathog. 2018;124:311–315. doi:10.1016/j.micpath.2018.08.060
33. Quirynen M. The clinical meaning of the surface roughness and the surface free energy of intra-oral hard substrata on the microbiology of the supra-and subgingival plaque: results of in vitro and in vivo experiments. J Dent. 1994;22:13–S16. doi:10.1016/0300-5712(94
34. Alp G, Johnston WM, Yilmaz B. Optical properties and surface roughness of prepolymerized poly (methyl methacrylate) denture base materials. J Prosthet Dent. 2019;121:347–352. doi:10.1016/j.prosdent.2018.03.001
35. Gad MM, Abualsaud R, Rahoma A, Al-Thobity AM, Al-Abidi KS, Akhtar S. Effect of zirconium oxide nanoparticles addition on the optical and tensile properties of polymethyl methacrylate denture base material. Int J Nanomedicine. 2018;13:283–292. doi:10.2147/IJN.S152571
36. Fouda SM, Gad MM, Ellakany P, et al. The effect of nanodiamonds on candida albicans adhesion and surface characteristics of PMMA denture base material-an in vitro study. J Appl Oral Sci. 2019;27. doi:10.1590/1678-7757-2018-0779
37. Njuguna J, Ansari F, Sachse S, Rodriguez VM, Siqqique S, Zhu H. Nanomaterials, nanofillers, and nanocomposites: types and properties. In: Health and Environmental Safety of Nanomaterials. Woodhead Publishing; 2021:3–37. doi:10.1016/B978-0-12-820505-1.00011-0
38. Xu XM, Costin S, Cerrada M. Polymeric materials with antimicrobial activity: from synthesis to applications. Antimicrobial Polym Dent Mater. 2013;279–304. doi:10.1039/9781782624998-00279
39. Yu P, Wang C, Zhou J, Jiang L, Xue J, Li W. Influence of surface properties on adhesion forces and attachment of Streptococcus mutans to zirconia in vitro. Biomed Res Int. 2016;2016:1–10. doi:10.1155/2016/8901253
40. Kim DH, Kwon TY. In vitro study of Streptococcus mutans adhesion on composite resin coated with three surface sealants. Restor Dent Endod. 2017;42:39–47. doi:10.5395/rde.2017.42.1.39
41. Kozmos M, Virant P, Rojko F, et al. Bacterial adhesion of Streptococcus mutans to dental material surfaces. Molecules. 2021;26:1152. doi:10.3390/molecules26041152
42. McBride BC, Song M, Krasse B, Olsson J. Biochemical and immunological differences between hydrophobic and hydrophilic strains of Streptococcus mutans. Infect Immun. 1984;44:68–75. doi:10.1128/iai.44.1.68-75.1984
43. Gottenbos B, Grijpma DW, van der Mei HC, Feijen J, Busscher HJ. Antimicrobial effects of positively charged surfaces on adhering gram-positive and gram-negative bacteria. J Antimicrob Chemother. 2001;48:7–13. doi:10.1093/jac/48.1.7
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.