Back to Journals » International Journal of Nanomedicine » Volume 15
Anti-Biofouling Coatings on the Tooth Surface and Hydroxyapatite
Authors Zhou L , Wong HM , Li QL
Received 8 September 2020
Accepted for publication 21 October 2020
Published 13 November 2020 Volume 2020:15 Pages 8963—8982
DOI https://doi.org/10.2147/IJN.S281014
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Thomas Webster
Li Zhou,1 Hai Ming Wong,1 Quan Li Li2
1Department of Paediatric Dentistry, Faculty of Dentistry, The University of Hong Kong, Hong Kong, SAR 999077, People’s Republic of China; 2Key Laboratory of Oral Diseases Research of Anhui Province, College and Hospital of Stomatology, Anhui Medical University, Hefei 230000, People’s Republic of China
Correspondence: Hai Ming Wong
Faculty of Dentistry, The University of Hong Kong, 34 Hospital Road, Hong Kong, SAR 999077, People’s Republic of China
Tel +852 28590261
Fax +852 25593803
Email [email protected]
Quan Li Li
College and Hospital of Stomatology, Anhui Medical University, No. 69, Meishan Road, Hefei 230000, People’s Republic of China
Email [email protected]
Abstract: Dental plaque is one type of biofouling on the tooth surface that consists of a diverse population of microorganisms and extracellular matrix and causes oral diseases and even systematic diseases. Numerous studies have focused on preventing bacteria and proteins on tooth surfaces, especially with anti-biofouling coatings. Anti-biofouling coatings can be stable and sustainable over the long term on the tooth surface in the complex oral environment. In this review, numerous anti-biofouling coatings on the tooth surface and hydroxyapatite (as the main component of dental hard tissue) were summarized based on their mechanisms, which include three major strategies: antiprotein and antibacterial adhesion through chemical modification, contact killing through the modification of antimicrobial agents, and antibacterial agent release. The first strategy of coatings can resist the adsorption of proteins and bacteria. However, these coatings use passive strategies and cannot kill bacteria. The second strategy can interact with the cell membrane of bacteria to cause bacterial death. Due to the possibility of delivering a high antibacterial agent concentration locally, the third strategy is recommended and will be the trend of local drug use in dentistry in the future.
Keywords: anti-biofouling coating, antibacterial agent, hydroxyapatite, tooth surface, binding
Introduction
The definition of fouling is the accumulation of undesirable elements on material surfaces to hinder the normal functions of the material.1 Biofouling is a dynamic procedure that concerns a series of complex-forming steps and biological structures, including living organisms and their by-products (extracellular matrix of polymeric substances).1,2 Biofouling is a serious problem in many fields, such as biomedical devices, biosensors, drug delivery, biomedical implants, protective clothing, food packaging and marine equipment.2,3 It affects the performance and application of all host materials. To retain the main function of the host materials, there are numerous studies targeting anti-biofouling strategies, especially preventing the bacterial colonization of biomedical surfaces. Since a thin coating can provide an anti-biofouling surface without affecting the host materials, anti-biofouling coatings have become a very active and practical field of research and have progressively replaced the traditional antibiotic tactics.4
In the oral cavity environment, a mass of microbes and proteins are present on the oral mucosa, tongue surface, dental restorations and tooth surfaces, especially close to the gingival margin. Microorganisms on the oral mucosa and the tongue surface are easily removed because epithelial tissue and surface microorganisms shed all the time. However, microbial masses, saliva, and food debris adhering on the tooth surfaces, namely, dental plaque, are difficult to remove because of the lack of metabolic shedding for dental hard tissue, especially on areas that lack self-cleaning functionality. Dental plaque consists of a diverse population of microorganisms (one gram of wet plaque contains approximately 2 × 1011 bacteria) and an extracellular matrix of polymers (principally made up of glucans and fructans of microbial origin) from the host and microbial community.5,6 The formation of dental plaque on the tooth surface is followed by four stages: 1) acquired pellicle formation; 2) bacterial initial adhesion; 3) coaggregation to attached colonizers; and 4) multiplication, development and detachment of biofilm.6–8 Dental plaque as one type of dental biofouling is complex, and it is also the main etiologic factor that causes oral diseases, such as dental caries and periodontal diseases.7 At present, studies have confirmed that there is a relationship between systematic diseases and periodontal diseases, such as obesity, cardiovascular disease, diabetes, adverse pregnancy outcomes and Alzheimer’s disease.9 Therefore, there are many ways to prevent dental plaque buildup, including brushing, flossing, using mouthwash and professional teeth cleaning; many studies have investigated methods of cleaning bacteria and protein on teeth by antibiotics, surfactant agents, fluoride varnishes and anti-biofouling coatings. Considering that dental plaque is one kind of biofouling agent, designing an anti-biofouling coating on the tooth surface has attracted many researchers in recent years. A durable anti-biofouling coating on the tooth surface must resist the masticatory force, dissolution by excessive saliva and other complex physicochemical factors from the oral environment, and such a coating represents a great challenge in the field of the dentistry and biomaterials.
In this review, numerous anti-biofouling coatings on the tooth surface and hydroxyapatite (HA) (as the main component of dental hard tissue) were reviewed (Figure 1). They are summarized based on their different mechanisms, which include three major strategies: antiprotein and antibacterial adhesion through chemical modification, contact killing through the modification of antimicrobial agents, and antibacterial agent release (Table 1).
 |  |  |  |
Table 1 Major Strategies, Categories, Chemicals, Findings and Probable Mechanisms of Anti-Biofouling Coatings |
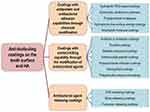 |
Figure 1 Anti-biofouling coatings on the tooth surface and HA. |
Coatings with Antiprotein and Antibacterial Adhesion Capabilities Through Chemical Modification
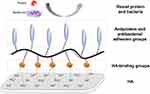
The use of chemical surface modifications as nonspecific methods for coatings that can resist protein adsorption, such as PEG and zwitterion, have demonstrated great antiadhesion properties in vitro and are generally regarded as the standard approach for antiadhesive coatings. Figure 2 shows the common mechanism schema of this strategy, which consists of two parts: HA-binding groups (adsorbing onto HA) and antiprotein and antibacterial adhesion groups (resisting protein and bacteria adhesion).
Coatings with Contact-Killing Capability Through the Modification of Antimicrobial Agents
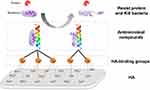
In these coatings, antimicrobial compounds are anchored to the material surface. Adhered bacteria are believed to be killed due to the disruption of their cell membrane by the attached compounds, which reach across the microbial membrane.4 Figure 3 shows the common mechanism schema of this strategy, which consists of two parts: HA-binding groups and antimicrobial compounds (killing bacteria).
Antibacterial Agent-Releasing Coatings
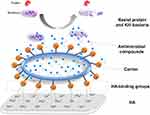
These copolymer coatings kill both adhered and adjacent planktonic bacteria by releasing loaded antibacterial compounds over time. The release of incorporated antibacterial agents is achieved by diffusion into an aqueous medium, erosion/degradation, or hydrolysis of covalent bonds.10 Compared with contact-killing coatings, antibacterial agent-releasing coatings offer the possibility of delivering a high antibacterial agent concentration locally without exceeding systemic toxicity or eco-toxicity limits. In addition, they minimize drug resistances and systemic repercussions. Figure 4 shows the common mechanism schema of this strategy, which consists of three parts: HA-binding groups, carriers (encapsulating antimicrobial compounds) and antimicrobial compounds (killing bacteria).
Anti-Biofouling Coating Strategies on the Tooth Surface and HA
Antiprotein and Antibacterial Adhesion Through Chemical Modification
Hydrophilic PEG-Based Coatings
Polyethylene glycol (PEG)-based coatings have been extensively used to prevent nonspecific protein and cell adhesion due to their hydrophilic property, large space repulsion, and excellent biocompatibility. The low value of interfacial energy between PEG and water leads to the formation of a highly hydrated layer barrier that hinders the access of protein molecules.1,49 In addition, PEG chains can be adhered to substance surfaces based on their physical or chemical adsorption, covalent attachment, and block/graft copolymerization.2 However, their low surface densities and sensitivity to oxidative damage might result in the loss of antifouling capabilities for long-term applications.50–52 Therefore, a number of modified PEG polymers have been investigated. Many studies have focused on PEG-based coatings for the tooth surface and HA surface.3,11–13
Because of the hydrophilic property of PEG, the affinity of certain polymers to the HA surface was designed by grafting tooth-binding or HA-binding moieties to PEG. Chen et al designed a novel oral hygiene drug-free product pyrophosphate (PPi)-PEG copolymer.11 PPi as a tooth-binding anchor can rapidly bind to the HA surface. Copolymers of different molecular weights (MWs) and PEG building blocks (PEG 2000 or PEG 600) with different PPi contents were synthesized. The results showed that PPi-PEG could effectively bind to HA within two minutes. The PPi-PEG copolymer with a high MW and high pyrophosphate content showed the best efficacy in inhibiting salivary protein and Streptococcus mutans (S. mutans) adhesion. The possible mechanisms were that the PPi groups of PPi-PEG can effectively occupy the calcium site of the HA surface instead of the carboxyl groups of salivary proteins and the neutral hydrophilic layer PEG polymer on the HA surface could effectively reduce the hydrophobic interactions of salivary protein and bacteria with the HA surface.
Bikker et al reported that PEG molecules conjugated with the HA-binding moiety of salivary agglutinin glycoprotein (SAG).12 By evaluating peptides covering the complete consensus sequence of SAG, only the peptide P3 (DDSWDTNDANVVCRQLGA) was found to bind significantly to HA. Compared with the uncoated surface in vitro, the P3 coating itself showed bacterial-repelling characteristics, which was likely because P3 exhibited a net-negative charge that potentially repelled the negatively charged bacterial membrane and membrane-anchored structures, such as lipopolysaccharide and lipoteichoic acid. In addition, in comparison with the P3 coating, due to covalently linking with the hydrophilic and bacterial-repellent-moiety PEG, the P3-PEG coating increased the antifouling activity by up to 40% and 30% against S. mutans and Staphylococcus epidermidis, respectively.
PEG derivatives could also be utilized by free radical polymerization with HA-binding moieties. Cui et al designed copolymers containing polyethylene glycol methyl ether methacrylate (PEGMA) and ethylene glycol methacrylate phosphate (Phosmer).13 They interacted via free radical polymerization with the different feed ratios of monomers in an aqueous medium. Phosmer bound with calcium ions (Ca2+) from HA by electrostatic interactions, covalent bonding and hydrogen bonding, while PEGMA as a hydrophilic polymer brush in the distant end of HA to inhibit bacterial adhesion (Figure 5). Eventually, copolymers containing 40−60% PEGMA segments were shown to be very effective against S. epidermidis adhesion.
 |
Figure 5 Copolymer containing PEGMA (Blue) and Phosmer (Pink). PEGMA as a hydrophilic polymer brushes in the distant end of HA inhibits bacterial adhesion, while Phosmer binds with Ca2+ from HA. Reproduced with permission from Cui X, Koujima Y, Seto Het al Inhibition of bacterial adhesion on hydroxyapatite model teeth by surface modification with PEGMA-Phosmer copolymers. ACS Biomater. Sci. Eng. 2016;2(2):205–212.13 Copyright 2016, American Chemical Society. |
Hydrophilic Zwitterionic Polymers
Zwitterionic polymers are super hydrophilic materials because of the thick hydration layer formed around zwitterionic polar groups that can inhibit protein adsorption; therefore, they are a promising antifouling material.49,53 Zwitterionic structures have an equal amount of positive and negative domains within a single molecule and remain overall electrically neutral.53 For this reason, zwitterionic materials have been widely used in the biomedical field, such as on the HA surface. The classical cations are quaternized ammonium, and the classical anions are sulfonates, carboxylates, and phosphonates. There are three typical zwitterionic groups: sulfobetaine methacrylate (SBMA), carboxybetaine methacrylate (CBMA), and 2-methacryloyloxyethyl phosphorylcholine (MPC).15
Kang et al synthesized a 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer to prevent oral bacterial adhesion on the tooth surface.14 However, MPC is difficult to sustain on material surfaces in aqueous media because of its extreme hydrophilicity. A 2-methacryloyloxyethyl phosphate (MOEP) monomer had Ca2+-binding moieties that bound with HA. MOEP was polymerized with MPC by free radical polymerization. The zwitterionic anti-biofouling coating MPC-ran-MOEP (abbreviated as PMP) was created to coat the HA surface. After the anti-biofouling data analysis, the anti-biofouling coating with 50% MPC (PMP50) had the best inhibition effect against bovine serum albumin (BSA), NIH-3T3 mouse fibroblasts cell and S. mutans adhesion.
Then, this research team generated zwitterionized HA surfaces by immersing HA in zwitterionic polymer solutions to provide an antibacterial HA surface, and they used the three monomers phosphorylcholine (PC), sulfobetaine (SB), and carboxybetaine (CB) to copolymerize with a methacrylic monomer containing a Ca2+-binding moiety by the free radical polymerization method.15 The copolymer containing the Ca2+-binding moiety and a hydroxyl group was synthesized as the control. After a water contact angle analysis and X-ray photoelectron spectroscopy (XPS) analysis, the zwitterionic functional groups were stably immobilized on the HA surface. The zwitterionized HA surface showed significantly decreased protein adsorption, no S. mutans adhesion after 6 h and the lowest level of bacterial adhesion after 24 h compared with the control group.
In addition, Venault and coworkers reported a zwitterionic sulfobetaine-grafted polyethyleneimine polymer PEI-g-SBMA.16 Polyethyleneimine (PEI) is the positively charged polymer backbone and SBMA macromonomer is the antifouling side functional group that acts as a fouling brush. PEI-g-SBMA efficiently coats onto HA due to the establishment of electrostatic interactions between the negatively charged SBMA and Ca2+ of HA. Because of the surface positively charged PEI and the hydrophilicity of the zwitterionic polymer, PEI-g-SBMA protected HA discs from positively charged bacteria (Stenotrophomonas maltophilia) and S. mutans attachment. In addition, PEI-g-SBMA inhibited up to 70% of bacteria adhesion considering that the rough tooth surface physically promotes the attachment of proteins and bacteria.
Polyelectrolyte Multilayers
Polyelectrolyte multilayers (PEMs) is a physisorption method that was designed for antibacterial applications via alternately depositing oppositely charged polyelectrolytes on a charged surface using ionic cross-linking interaction. Highly swollen and hydrated multilayers improved the polyelectrolytes surface hydrophilicity and softness and resulted in their good antiadhesive properties. Lee and coworkers studied the effect of anionic and cationic polymers to inhibit bacteria attachment on the tooth surface.17 Cationic polymer 75% guanidine-functionalized polyallylamine (PAA-G75) and anionic polymers Gantrez and sodium hyaluronate (NaHa) were chosen because of their potential antibacterial properties. All polymers showed strong single species adsorption of polyelectrolytes on HA surfaces. When double layers with two oppositely charged polymers adsorbed on the HA, such as adsorption of cationic PAA-G75 polymer followed by adsorption of the oppositely charged polymer Gantrez, the coating thickness decreased due to the electrostatic cross-linking of anionic and cationic polymers. There was a significant period of rearrangement to form a stable Gantrez/PAA-G75 layer via electrostatic cross-linking between anionic and cationic polymers. When HA surfaces were pretreated with pellicle layer or artificial saliva layer, the adsorbed amount of PAA-G75 on/into the pellicle layer was 2 times larger than that absorbed on/into artificial saliva layer. An electrostatic interaction was observed between the polymer and saliva and/or oppositely charged polymers that stabilize the coatings on HA. These stable coatings as potential antibacterial agents were used by controlling bacterial accumulation on the surface. Through the antibacterial activity test of polymers on the HA surface, the PAA-G75-coated HA surfaces showed a significantly lower bacterial viability than the untreated HA, Gantrez-coated HA surfaces and binary polymer-coated HA disks. The order of surface deposition for the cationic polymer impacted its antibacterial performance, which showed that the Gantrez/PAA-G75-coated surface possessed significantly more antibacterial activity than the PAA-G75/Gantrez-coated surface, untreated surface, and Gantrez-coated controls.
Hydrophobic Low-Surface Energy Coatings
Yin and coworkers created a slippery liquid-infused porous surface (SLIPS) on the enamel surface to prevent dental plaque.18 After 37% phosphoric acid etching and rinsing with water, the enamel surface was coated by the hydrophobic low-surface energy heptadecafluoro-1,1,2,2-tetra-hydrodecyltrichlorosilane. After ethyl alcohol washing, the enamel surface was coated by fluorocarbon lubricants (Fluorinert FC-70). The SLIPS enamel surface significantly prevented the adsorption of salivary protein of mucin and S. mutans by in vitro and animal studies. Acid etching can generate a rough enamel surface to improve wicking of the lubricating liquid for the first step of SLIPS. Then, hydrophobic low-surface energy heptadecafluoro-1,1,2,2-tetra-hydrodecyltrichlorosilane was used to match the infiltrated lubricant. The trichlorosilane residual groups reacted with the hydroxyl groups of HA, while the polyfluoroalkyl tail showed hydrophobicity at the distant end of the enamel surface. In the last step, the lubricant FC-70 was used because it is immiscible with ambient fluid due to its hydrophobicity. Polyfluoroalkyl silane facilitated the infusion of hydrophobic FC-70 into the rough hydrophilic enamel surface and was very important. Salivary protein and dental bacteria could not easily adhere to the SLIPS on the enamel surface.
Amphiphilic Molecule (AM) Coatings
Amphiphilic molecules (AMs) possess both hydrophobic and hydrophilic blocks. Due to the AMs’ dual nature, the adherence of proteins or glycoproteins to the substrate surface becomes energetically unfavorable via either hydrophobic or hydrophilic interactions to decrease the interactions of the organism with the surface.3 Marine and coworkers created multifunctional antiattachment AM coatings on HA with a sugar backbone, hydrophobic arms, a PEG tail, and a carboxylate or phosphate anchor.19 In this study, the PEG tail also acted as a polymer brush to inhibit bacterial attachment, and the carboxylate and phosphate groups were chosen for binding Ca2+ with the HA surface. The carboxylates M12P5 and M6P5, 2-aminoethylphosphate P-M12P5, P-M6P5, P-T12P5 and P-T6P5 were synthesized. After evaluation, the adsorption data suggested that P-M12P5 with an increased hydrophobicity displayed stronger adsorption and retention than P-M6P5. After the rinsing step in QCM-D, 65% of P-M12P5 was retained compared to 47% of M12P5. The polymers with phosphates had stronger chelating properties with HA. A comparison of P-M12P5 with P-T12P5 showed that decreasing the degree of branching led to weaker adsorption and retention. P-M12P5 and M12P5 both demonstrated the highest retention after rinsing and the highest antiattachment efficacy against mixed Actinomyces viscosus and S. mutans present in the oral cavity. In summary, AMs with a higher degree of branching and hydrophobicity displayed the greatest adsorption, retention, and antiattachment. AM polymer pretreatment of enamel can be an effective preventative measure to address bacterial adhesion.
Contact Killing Through the Modification of Antimicrobial Agents
Antibiotic or Antiseptic Coatings
Antibiotics have been locally used in the oral cavity in the past 50 years. Järvinen et al studied the effect of chlorhexidine (CHX) and six common antibiotics (amoxicillin, cefuroxime, penicillin, sulfamethoxazole-trimethoprim, tetracycline, and erythromycin) to S. mutans.20 S. mutans was susceptible to all common antimicrobial agents, especially CHX. Many antibiotics have been mixed with HA as a drug delivery system for bone grafts, such as gentamicin and vancomycin.54 Ciprofloxacin was observed to adsorb on HA because of the micro-porous nature of HA, and it inhibits Staphylococcus aureus, Staphylococcus epidermidis and Escherichia coli.55 CHX is a cationic antimicrobial that can inhibit a considerable variety of gram-positive and gram-negative bacteria as well as fungi and has different formulations, namely, CHX digluconate, CHX dihydrochloride and CHX diacetate.56,57 CHX can adsorb onto HA possibly by the precipitation of insoluble salts, eg, CHX·H3PO4 and/or CHX·2HC1, and possibly by the interaction between the biguanide groups of CHX and negatively charged sites of the HA surface.58,59 Furthermore, the antibacterial activity will not be affected after CHX adsorbs onto the HA surface. CHX binds to negatively charged regions in the cytoplasmic membrane of microorganisms and causes the leakage of intracellular components.60
Triclosan is used in the oral cavity because of its broad-spectrum antimicrobial property, and it is in the chlorinated diphenyl ether class of antibacterial compounds. However, poor water solubility and low retention (high initial concentration of triclosan decreased with time) reduced its antibacterial effects in the oral cavity. Chen and coworkers designed a drug delivery system using triclosan-loaded tooth-binding micelles, which can improve the water solubility of triclosan and maintain a certain concentration on the tooth surface for the prevention and treatment of dental caries, and they successively designed three different micelles from triclosan-loaded alendronate (ALN)-pluronic copolymers, triclosan-loaded di-phosphoserine-pluronic (DPS-P123) copolymers, to triclosan-loaded pyrophosphate-pluronic P123 copolymers (PPi-P123).21,22 Pluronic was used as the hydrophobic core to increase drug solubility. The mineral binding moieties (ALN, DPS and PPi) conjugated to the two ends of the pluronic copolymer have a high affinity with HA to enhance the retention of triclosan on the tooth surface. Although the ALN moiety shows good binding with HA, DPS and PPi were better substitutes in terms of safety because a certain relationship was observed between ALN and osteonecrosis of the jaw.61 Inspired by the strong HA affinity of two phosphoserine residues from statherin, DPS was chosen and conjugated to pluronic.22 DPS binds with the HA crystal by electrovalent bonds [phosphate ions (PO43-) of DPS and Ca2+ ion of HA] and intermolecular hydrogen bonds.22 PPi was chosen and tested because of its high affinity to HA, good biodegradability and wide application.22,62 Compared with DPS, PPi showed a slightly higher affinity with HA. The probable reason was the slightly higher efficiency of PPi for occupying the HA surface binding site compared with DPS. Because the binding sites on the HA surface were occupied by these mineral binding moieties (ALN, PPi or DPS), salivary proteins and oral bacteria could not easily adhere to the surface.21,22
Fluoride Coatings
Fluoride is a well-studied and widely used agent for the prevention of dental caries. The suspected mechanisms for the cariostatic effect include inhibitory effects on biofilm acid production, disruption of intracellular pH regulation, inhibition of extracellular polysaccharide production, inhibition of enamel demineralization and enhancement of remineralization.23,63 Sodium fluoride (NaF) is mentioned more frequently among the fluoride compounds, which also include tin difluoride (SnF2), sodium monofluorophosphate (Na2PO3F), amine fluoride, and acidulated phosphate fluoride. Fluoride is integrated into our daily lives through various fluoride application methods, such as water fluoridation, toothpaste, mouthwash, fluoride varnish, and fluoride gel. Regarding the effects on the cariogenic bacteria, the effect of a 1-min fluoride treatment with concentrations ≥300 ppm significantly reduced the acidogenicity, aciduricity, and extracellular polysaccharide formation of 46-hour-old S. mutans biofilms.23 The mechanisms underlying the inhibition of bacterial acid production of fluoride may be related to the inhibition of enolase and a proton-translocating ATPase and the enhancement of intracellular acidification.25 In addition, Loskill et al found that the adherence of S. mutans decreased after HA pellets were immersed in NaF solution for 5 min.64 The suspected mechanisms were the variations in zeta potential and surface energy of HA by the fluoride treatment.
Metallic Compound Coatings
Metallic compounds, such as copper, zinc and silver compounds, have been used as antibacterial agents for a long time. In dentistry, silver compounds are used widely. As early as the 1840s, silver nitrate (AgNO3) was used as a caries prevention agent, a cavity sterilizing agent and a dentine desensitizer.24 Later, silver was applied with fluoride, such as silver fluoride (AgF) and silver diamine fluoride (SDF), to produce a combined anticaries effect. SDF is a colorless heavy-metal halide coordination complex solution containing silver, fluoride, and ammonia, and it is applied to prevent and arrest caries in children.65 The fluoride and silver released from SDF-treated tooth surfaces inhibited the metabolic activity (acid production) of S. mutans cells.25 Silver can inhibit bacterial metabolism by binding to bacterial proteins, inducing protein coagulation, and inactivating enzymes. However, the clinical application of silver fluoride compounds has been limited due to the black staining associated with caries lesions. The mechanism of silver compounds might be linked to its interaction with dental tissue and its antibacterial ability for cariogenic bacteria.24 Probable chemical reactions occurred between silver compounds and the major dental component HA. For example, AgNO3 reacts with HA to form Ag3PO4 which might act as a reservoir of PO43-.24 In addition, the antibacterial effects of silver ions include the destruction of the cell wall structure; denaturation of cytoplasmic enzymes and inhibition of microbic DNA replication.24
Antimicrobial Peptides Coatings
Numerous natural host-defense peptides, which are also named antimicrobial peptides (AMPs), belong to the innate immune system of animals and plants. Due to their advantages, which include antimicrobial activities against broad-spectrum gram-negative and gram-positive bacteria and fungi, high antibacterial efficacy at low concentration, low bacterial resistance and cytocompatibility to mammalian cells, AMPs have attracted much attention in research and clinical medicine for the prevention of microorganisms.66–68 Many natural and mimic AMP molecules have been used in the oral cavity to prevent oral pathogens. To overcome the decreasing concentration of AMPs from saliva dilution and degradation, the methods of modifying AMPs as coatings on the tooth surface to prevent microorganisms were developed.26–30
Huang et al designed a HA-binding antimicrobial peptide (HBAMP) by the bio-conjugate method that contains a HA-binding part (HBP7, NNHYLPR) and a synthetic antimicrobial peptide part (KSLW, KKVVFWVKFK).26 KSLW has a broad range of antimicrobial activity. HBP7 was isolated by a bio-panning phage display random heptapeptide library, and it exhibited a specific affinity to the enamel surface. Laboratory tests showed that the HA-binding antimicrobial peptide HBP7-KSLW has more effective and stable antibacterial activity and inhibits biofilm formation on the contact interface after adhering to the tooth surface. The electrostatic interaction between HBP7 and HA plays a critical role in the binding process. Figure 6 shows the schematic mechanisms of HBAMP. HBAMP may damage the bacterial cell membrane by disrupting the lipid bilayer, translocating into the cell interior, and interacting with intracellular targets, which results in the regulation of certain genes that control the growth, transition, and formation of biofilms. HBAMP has become a promising antimicrobial peptide coating.
Townsend and coworkers designed one dual AMP coating by two mechanisms: covalent attachment and electrostatic attachment to HA surface.27 The dual effective coating achieved stability on the HA surface over 12 months and inhibited gram-positive and gram-negative bacteria. There were three treatments in this study. In the first group, HA pellets were incubated via the electrostatic attachment of the peptide (5(6)-carboxyfluorescein (5(6)-CF))–RRRRRRGALAGRRRRRRGALAGEEEEEEE (eAMP). In the second group, thiol-functionalized HA (tHA) pellets were incubated via the covalent attachment of the peptide (cAMP) (5(6)-CF)–RRRRRRGALAGRRRRRRGALAG after incubating in N, N-dimethylformamide (DMF) and succinimidyl trans-4-(maleimidylmethyl) cyclohexane-1-carboxylate (SMCC). In the third group, tHA pellets were incubated successively in DMF, SMCC, the cAMP for 12 h, and the eAMP 30 min, and it was named the dual coating (dAMP). eAMP is a broad-spectrum AMP designed via sequence matching from human α- and β-defensins, and it was bound to HA by the electrostatic interaction and inhibited bacterial growth in solution. cAMP formed a covalent bond with the HA surface that would not allow the peptide to desorb from the surface. As cAMP was grafted directly to the surface without linker spacer units, the HA surface provided a large amount of steric hindrance to prevent enzymes from reaching the regions and inhibit the formation of biofilm. However, neither eAMP or cAMP alone can inhibit both the growth of planktonic bacteria and biofilm formation of bacteria. After antimicrobial efficacy evaluations, the permanent coating of cAMP and extended release of eAMP were mixed to form dAMP, which combined surface binding and antimicrobial activity to achieve short-term sterilization and long-term antimicrobial activity at the surface.
Based on the antimicrobial activity of the AMP (KRWWKWWRRC) and the surface binding property of the peptide (SKHKGGKHKGGKHKG), Chen et al designed an anchor-AMP that exhibited excellent antimicrobial activity onto the surface of biomaterials, including titanium, gold, polymethyl methacrylate (PMMA) and HA.28 KRWWKWWRRC is a broad-spectrum antimicrobial peptide against gram-negative and gram-positive bacteria and shows a low immunological reaction, rapid bactericidal rate and low bacterial resistance.69 SKHKGGKHKGGKHKG was a surface binding peptide primarily composed of histidine and lysine. This peptide can directly assemble onto several biomaterials’ surfaces and showed a strong affinity over a range of pH and salt concentrations.70 After 5–7 days incubation in vivo, the anchor-AMP still showed strong affinity and excellent antimicrobial activity.
Yang and coworkers designed and compared two novel antimicrobial peptides that contained immunomodulatory peptide and HA-binding parts.29 The immunomodulatory peptide VRLIVAVRIWRR-NH2 (IDR-1018) could inhibit oral biofilm formation and improve [(p)ppGpp] degradation.71,72 Two HA-binding heptapeptides were screened by a bio-panning phage display random heptapeptide library with a specific affinity to the tooth surface. The peptide IDR-1018 was modified into CAGHHPLMC-VRLIVAVRIWRR-NH2 (SHABP) and CAQAFGPNC-VRLIVAVRIWRR-NH2 (MHABP). After effective inhibition tests of bacteria and biofilms, SHABP with higher HA-binding affinity presented higher antimicrobial activity and stronger biofilm reduction than MHABP with medium HA-binding affinity and unmodified peptide 1018.
To resolve bacterial colonization on implanted biomaterials, Gou et al studied a salivary statherin protein (SSP) inspired poly (amidoamine) dendrimer (SSP-PAMAM-NH2).30 Peptide sequence DDDEEKC of SSP has a strong ability to adsorb on HA while PAMAM-NH2 possesses effective antibacterial activity due to its numerous peripheral amino groups. Additionally, SSP-PAMAM-NH2 is a zwitterionic polymer that includes cationic amino groups and anionic carboxylic groups, and it could form aggregates by intermolecular electrostatic interactions to promote its adsorption on HA. After 4 weeks of incubation, SSP-PAMAM-NH2-coated HA disks still sustained stable antibacterial activity.
A family of peptides (histatin family) has their own affinity with HA because this family exists in acquired enamel pellicles.66 Histatin peptides are salivary cationic histidine-rich proteins secreted by parotid glands and submandibular glands, and they have broad-spectrum antimicrobial and antifungal properties.31,35,66 There are three major members in the histatin family: histatin-1 (38 amino acids; Mw: 4929 Da), histatin-3 (32 amino acids; Mw: 4063 Da) and histatin-5 (DSHAKRHHGYKRKFHEKHHSHRGY, 24 amino acids; Mw: 3037 Da).35 Histatin-5 is the main proteolytic product of histatin-3.35 Histatin-5 was reported to have the most anticandidal activity compared with histatin-1 and histatin-3.73 The activity of histatin-5 against the fungus Candida albicans was initiated through cell wall binding followed by translocation and intracellular targeting.36 In P-113 (AKRHHGYKRKFH), a 12-amino-acid fragment of histatin-5, two cationic lysine residues at positions 2 and 10 were reported as important for transport into the cytosol.32 The activity of histatin-5 against the gram-positive bacterium S. mutans was also through interacting with the peptidoglycan layer and anionic teichoic acids, and then penetrating the plasma membrane.38 Yin and coworkers investigated and compared the HA adsorption characteristics of histatin-1, recombinant histatin-1 without phosphate, histatin-3, histatin-5 and P113.74 The results showed that histatin-1 presented higher HA adsorption affinity than recombinant histatin-1 because of the phosphorylated amino acid and the highest HA adsorption affinity among five peptides. In addition, P113 showed a higher binding constant of HA adsorption compared with that of histatin-3 and histatin-5. There were seven histidine, three lysine and three arginine residues in histatin-5, which gave histatin-5 its positive charge in neutral pH environments. Research showed that the strong molecular interaction between histatin-5 and HA was due to the electrostatic attraction of the histatin-5 positive charge to HA.75,76 Molecular dynamics simulations showed that the binding mechanism between histatin 5 and HA was the negatively charged amino acid residue Asp2 and the positively charged amino acid residues Lys6, Arg13, Lys14 and Lys18.37 Because of the antifungal activity and the affinity ability to HA of histatin-5, Candida albicans strain colonization was significantly inhibited on the histatin-5-coated HA surface, even after 1440 min.39
Polysaccharide-Chitosan Coatings
Chitosan is a natural polysaccharide derived from chitin by N-deacetylation. The glucosamine backbone gives chitosan a poly-cationic character. Chitosan composite coatings, such as chitosan-bioactive glass nanoparticles can be deposited by cathodic electrophoretic deposition and improve cell adhesion and spreading, degradation properties, and tissue compatibility.77–80 Chitosan could prevent organic acid damage on the tooth surface by buffering the oral pH value, preventing the adhesion of several pathogens, and stimulating the ordered regeneration of oral soft tissues and bone tissues.
Tarsi et al reported the properties of low-molecular-weight chitosan, N-carboxymethyl chitosan and imidazolyl chitosan in preventing S. mutans attachment to HA beads.40 When saliva-coated or uncoated HA beads were treated with any of the chitosans, a decrease in S. mutans adsorption was observed, and it ranged from 47% to 66%, and even minor amounts of modified chitosans prevented S. mutans adsorption to HA. The probable mechanisms of the antiadherence activity were bacterial surface modifications, altered expression levels of bacterial surface ligands, and chitosan adsorption to host surfaces.
Sano et al studied the influence of chitosan on the adsorption of Streptococcus sobrinus 6715 to saliva-treated HA (S-HA).41 The chitosan samples with different molecular masses (0.8–6 kDa) and degrees of deacetylation (10–95%) were prepared for the study. The inhibition of adsorption of S. sobrinus 6715 to S-HA correlated positively with the molecular mass of chitosan, and the optimal degree of deacetylation was 50–60% for maximum inhibition of bacterial binding to S-HA. Chitosan also reduced the magnitude of the zeta potential and surface hydrophobicity of the oral bacteria. An electrostatic interaction occurred between the positively charged chitosan and the negatively charged bacterial cell surface. When chitosan chains were enough long to bind more than one cell, bridges between bacterial cells were formed so that bacteria could not adsorb onto S-HA. As a polycationic agent, chitosan prevented bacteria adsorption onto S-HA by adsorbing onto cell surfaces and bridging together into aggregates. Moreover, increasing concentrations of chitosan induced a successive decrease in cell hydrophobicity due to alterations of the bacterial cell surface hydrophobic expression.
Bae et al studied a newly water-soluble reduced chitosan for the prevention of S. mutans on teeth using the classical 4-day plaque regrowth design in a clinical trial.42 S. mutans exposed to chitosan exhibited a substantial delay in growth compared with the cells exposed to distilled water. The water-soluble reduced chitosan exhibited significant antibacterial activity and plaque reducing activity. The probable mechanism for the antimicrobial activity of chitosan may be an ionic interaction between the cations (amino groups of chitosan) and the anions (phospholipids and carboxylic acids in the bacterial cell wall).42,81
Sphingolipid Coatings
Cukkemane et al explored the antiadherence properties on HA surfaces and the bactericidal activity of sphingolipids, including sphingosine, phytosphingosine (PHS), and sphinganine, as anti-biofouling agents.43 Sphingolipids occur in human cells, and studies have shown that sphingolipids have bactericidal activity (including against gram-positive and gram-negative bacteria) and candidacidal activity.82–84 In addition, sphingolipids easily adhere to negatively charged HA surfaces because of their positively charged groups.85 All of them were effective against planktonic films and disrupted biofilms of S. mutans. Compared with an untreated HA surface, sphinganine showed the strongest antiadherence ability by 1000-fold, and phytosphingosine and sphingosine showed eight- and five-fold antiadherence abilities, respectively.43 The probable mechanisms were that the hydrophobic tail of sphinganine prevented its penetration through the hydrophilic extracellular polysaccharide matrix of the biofilm. The additional hydroxyl group in PHS may cause additional hydrogen bond formation with the polysaccharide matrix, thereby slowing down diffusion through this layer.
Polyphenolic Compound Coatings
Tannic acid (TA) is a hydrosoluble antibacterial polyphenolic substance from fruits and green tea, and it is used widely in the biomedical field.44,86 The main antimicrobial mechanisms of TA and other polyphenols are destabilizing the cytoplasmic membrane, increasing the permeability of the cell membrane, changing protein-to-lipid ratios in the membrane, and inhibiting cell wall synthesis.44,87,88 Because of TA does not present adsorption with HA, Yang and coworkers designed an anchor that included three salivary acquired pellicles (SAPs) and short sequence DDDEEKC to modify TA (SAP3-TA) by click reaction to bind with the HA surface.44 The first six peptide sequence DpSpSEEK in statherin forms a helical structure and can closely adsorb on HA.89 In addition, the fragment DDDEEK of the Asp (D) residue-substituted phosphoserine also showed excellent adsorption capacity onto the HA surface.90 Therefore, three DDDEEKC peptide sequences were used to modify one TA molecule. After SAP3-TA coating, the HA surfaces became super-hydrophilic surfaces, which can resist both protein adhesion and bacterial biofilm formation.
Antibacterial Agent-Releasing Coatings
CHX-Releasing Coatings
There are numerous pharmaceutical applications that inhibit dental biofilms, such as toothpaste, mouthwashes, and gels. The main disadvantage of these applications is the low sustainable effect of the antimicrobial agents on the tooth surface because of salivary rinsing. Sustained-release varnish (SRV) has been applied in dentistry to decrease cariogenic bacteria.91,92 Steinberg et al designed a sustained-release CHX varnish that mixed CHX diacetate with ethyl cellulose (EC) and polyethylene glycol (PEG) in 100 mL of absolute 100% ethanol.45 EC is a biodegradable, biocompatible, hydrophobic and stable polymer, while PEG is a hydrophilic polymer. When EC and PEG were blended, they became a channeling agent to conduct controlled drug release due to their appropriate physicochemical properties. After the application of the sustained-release CHX varnish, the duration of the drug was prolonged in the oral cavity, and it eventually affected the formation and maturation of the dental plaque biofilm. Beyth et al conducted a clinical trial on orthodontic patients by the sustained-release CHX varnish.93 This varnish decreased S. mutans levels and was useful in preventing caries lesions.
Silver-Releasing Coatings
Recently, silver nanoparticles (AgNPs) have been introduced for infection control in dentistry and the management of oral biofilms without a tooth staining problem. AgNP dentine coatings had a strong antibacterial effect (complete bacterial growth inhibition and over 95% bactericidal).46 In terms of antibacterial activity, AgNPs could attach and anchor to the surface of cells, causing structural changes and damage, thereby affecting the permeability, causing pits and gaps, depressing the activity of respiratory chain enzymes, and finally leading to cell death.94 Moreover, these nanoparticles can catalyze the production of oxygen radicals that oxidize essential cell compounds. Then, the active oxygen diffuses into the surrounding environment to inhibit microbes. Additionally, AgNPs could provide longer-lasting protection against bacteria than AgNO3. The possible mechanism might be the binding affinity between AgNPs and the negatively charged phosphate groups in HA crystallites, which imparts greater time for the bacterium-NP interaction.95 In addition, the shape of AgNPs, such as triangular AgNPs, which contain more reactive planes than traditionally used rods, spheres, or Ag ions, was reported to affect its functions and promotes its toxicity to microbes.96 The strong anti-biofilm activity and the sustainable efficacy suggested that AgNP coatings can create a resilient antimicrobial front to protect teeth from bacterial colonization.
Zhang and coworkers synthesized a new block copolymer containing bisphosphonate, pyridine oligomers, and Ag ions.47 This polymer’s bisphosphonate group as an anchor interacted with HA. Ag ions were reduced into Ag nanoparticles by exposure to NaBH4, and then the Ag nanoparticles coordinated with the pyridine oligomers groups performed antibacterial properties. Eventually, HA surfaces were modified with polymer layers of approximate 4 nm and the well-dispersed Ag nanoparticles of 5–15 nm in diameter. From the outcomes of the experiment, this block copolymer coating (with Ag nanoparticles) revealed a strong antibacterial ability to Lactobacillus plantarum (L. plantarum) after immobilization onto the HA surface.
Furanone-Releasing Coatings
Kang et al designed poly (butyl methacrylate-co-methacryloyloxyethyl phosphate)-coated poly (lactide-co-glycolide) (PLGA/PBMP) microparticles.48 Poly (lactide-co-glycolide) (PLGA) parts were chosen as the carrier of furanone C-30 (against antibiotic-resistant bacteria) to achieve a sufficient sustained release of drug concentrations, and PBMP contains Ca2+-binding phosphomonoester groups to adhere to HA (Figure 7).14,15,48,97,98 Furanone C-30 as a halogenated furanone antibacterial compound can effectively inhibit bacterial quorum sensing (QS) by interfering with N-acyl homoserine lactone or luxS signals, which were used to coordinate bacterial population behavior during infection and biofilm formation.99,100 In addition, furanones were often used to encapsulate lactide polymer-based microparticles or nanoparticles.101 The swelling and degradation of PLGA polymers would induce furanone release from encapsulated PLGA/PBMP. PLGA/PBMP-encapsulated furanone C-30 effectively prevented the growth of S. mutans and biofilm formation on the HA surface up to 100 h compared with the uncoated PLGA microparticles. This antibacterial therapy maintained an effective drug concentration that inhibited biofilm formation on the material surface to avoid antibiotic resistance in the long term, which is called QS inhibitor-based therapy.48,97,102
Conclusions and Future Prospects
We have reviewed the main approaches for developing anti-biofouling coatings on the tooth surface and HA over the past decades. There are three main strategies. For those types of coatings with antiprotein and antibacterial adhesion capabilities through chemical modification, hydrophilic PEG-based coatings and zwitterionic polymer coatings are the most commonly used. Although these coatings can resist the adsorption of proteins and bacteria, microbes cannot be killed by the passive strategies. Coatings with contact-killing capability through the modification of antimicrobial agents can bind with the bacterial cell membrane, penetrate into the plasma membrane, and translocate and interact with intracellular DNA. However, antibiotic or antiseptic coatings, fluoride coatings, and metallic compound coatings present toxicity and side effects. Recently, AMPs are recommended because of their broad-spectrum antimicrobial activity, low bacterial resistance, and cytocompatibility. In addition, antibacterial agent-releasing coatings are designed to be carriers of bactericidal agents, which can be used for prolonged periods without possible side effects at non-targeted sites in the body. These controlled drug-releasing coatings will be the trend of local drug use in future.
Acknowledgment
This work was supported by funding from the National Natural Science Foundation of China (No. 81571018).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Magin CM, Cooper SP, Brennan AB. Non-toxic antifouling strategies. Materials Today. 2010;13(4):36–44. doi:10.1016/S1369-7021(10)70058-4
2. Liu S, Guo W. Anti‐biofouling and healable materials: preparation, mechanisms, and biomedical applications. Adv Funct Mater. 2018;28:1800596. doi:10.1002/adfm.201800596
3. Banerjee I, Pangule RC, Kane RS. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv Mater. 2011;23(6):690–718.
4. Cloutier M, Mantovani D, Rosei F. Antibacterial coatings: challenges, perspectives, and opportunities. Trends Biotechnol. 2015;33(11):637–652. doi:10.1016/j.tibtech.2015.09.002
5. Arathi R, eds. Principles and Practice of Pedodontics.
6. Marsh PD. Dental plaque as a microbial biofilm. Caries Res. 2004;38(3):204–211. doi:10.1159/000077756
7. Hojo K, Nagaoka S, Ohshima T, et al. Bacterial interactions in dental biofilm development. J Dent Res. 2009;88:982–990. doi:10.1177/0022034509346811
8. Allison D, Gilbert P, Lappin-Scott HM, eds. Community Structure and Co-Operation in Biofilms. Cambridge University Press; 2000.
9. Gurenlian JR. The role of dental plaque biofilm in oral health. J Dent Hyg. 2007;81(5):1–11.
10. Campoccia D, Montanaro L, Arciola CR. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 2013;34(34):8533–8554. doi:10.1016/j.biomaterials.2013.07.089
11. Chen F, Jia Z, Rice K, et al. The development of drug-free therapy for prevention of Dental Caries. Pharm Res. 2014;31(11):3031–3037. doi:10.1007/s11095-014-1396-1
12. Bikker FJ, Cukkemane N, Nazmi K, et al. Identification of the hydroxyapatite‐binding domain of salivary agglutinin. Eur J Oral Sci. 2013;121(1):7–12. doi:10.1111/eos.12013
13. Cui X, Koujima Y, Seto H, et al. Inhibition of bacterial adhesion on hydroxyapatite model teeth by surface modification with PEGMA-Phosmer copolymers. ACS Biomater Sci Eng. 2016;2(2):205–212. doi:10.1021/acsbiomaterials.5b00349
14. Kang S, Lee M, Kang M, et al. Development of anti-biofouling interface on hydroxyapatite surface by coating zwitterionic MPC polymer containing calcium-binding moieties to prevent oral bacterial adhesion. Acta Biomater. 2016;40:70–77. doi:10.1016/j.actbio.2016.03.006
15. Lee M, Kim H, Seo J, et al. Surface zwitterionization: effective method for preventing oral bacterial biofilm formation on hydroxyapatite surfaces. Appl Surf Sci. 2018;427:517–524. doi:10.1016/j.apsusc.2017.08.067
16. Venault A, Yang H-S, Chiang Y-C, et al. Bacterial resistance control on mineral surfaces of hydroxyapatite and human teeth via surface charge-driven antifouling coatings. ACS Appl Mater Inter. 2014;6:3201–3210. doi:10.1021/am404780w
17. Lee H, Myers C, Zaide L, et al. Competitive adsorption of polyelectrolytes onto and into pellicle-coated hydroxyapatite investigated by QCM-D and force spectroscopy. ACS Appl Mater Inter. 2017;9(15):13079–13091. doi:10.1021/acsami.7b02774
18. Yin J, Mei ML, Li Q, et al. Self-cleaning and antibiofouling enamel surface by slippery liquid-infused technique. Sci Rep. 2016;6(1):25924. doi:10.1038/srep25924
19. Marine J, Myers CP, Picquet GA, et al. Reduction of bacterial attachment on hydroxyapatite surfaces: using hydrophobicity and chemical functionality to enhance surface retention and prevent attachment. Colloid Surface B. 2018;167:531–537. doi:10.1016/j.colsurfb.2018.04.045
20. Järvinen H, Tenovuo J, Huovinen P. In vitro susceptibility of Streptococcus mutans to chlorhexidine and six other antimicrobial agents. Antimicrob Agents Chemother. 1993;37(5):1158–1159. doi:10.1128/AAC.37.5.1158
21. Chen F, Rice K, Liu X-M, et al. Triclosan-loaded tooth-binding micelles for prevention and treatment of dental biofilm. Pharm Res. 2010;27(11):2356–2364. doi:10.1007/s11095-010-0119-5
22. Chen F, Jia Z, Rice KC, et al. The development of dentotropic micelles with biodegradable tooth-binding moieties. Pharm Res. 2013;30(11):1–10. doi:10.1007/s11095-013-1105-5
23. Pandit S, Cai J-N, Jung J-E, et al. Effect of 1-minute fluoride treatment on potential virulence and viability of a cariogenic biofilm. Caries Res. 2015;49(4):449–457. doi:10.1159/000434731
24. Peng JJY, Botelho MG, Matinlinna JP. Silver compounds used in dentistry for caries management: A review. J Dent. 2012;40(7):531–541. doi:10.1016/j.jdent.2012.03.009
25. Ishiguro T, Mayanagi G, Azumi M, et al. Sodium fluoride and silver diamine fluoride-coated tooth surfaces inhibit bacterial acid production at the bacteria/tooth interface. J Dent. 2019;84:30–35. doi:10.1016/j.jdent.2018.12.017
26. Huang Z, Shi X, Mao J, et al. Design of a hydroxyapatite-binding antimicrobial peptide with improved retention and antibacterial efficacy for oral pathogen control. Sci Rep. 2016;6:1. doi:10.1038/srep38410
27. Townsend L, Williams RL, Anuforom O, et al. Antimicrobial peptide coatings for hydroxyapatite: electrostatic and covalent attachment of antimicrobial peptides to surfaces. J R Soc Interface. 2017;14:126. doi:10.1098/rsif.2016.0657
28. Chen J, Zhu Y, Song Y, et al. Preparation of an antimicrobial surface by direct assembly of antimicrobial peptide with its surface binding activity. J Mater Chem B. 2017;5(13):2407–2415. doi:10.1039/C6TB03337G
29. Yang Y, Xia L, Haapasalo M, et al. A novel hydroxyapatite-binding antimicrobial peptide against oral biofilms. Clin Oral Investig. 2019;23(6):2705–2712. doi:10.1007/s00784-018-2701-x
30. Gou Y, He L, Xu X, et al. Bio-inspired peptide decorated dendrimers for a robust antibacterial coating on hydroxyapatite. Polym Chem. 2017;8(29):4264–4279. doi:10.1039/C7PY00811B
31. Kavanagh K, Dowd S. Histatins: antimicrobial peptides with therapeutic potential. J Pharm Pharmacol. 2004;56:285–289. doi:10.1211/0022357022971
32. Huo L, Zhang K, Ling J. Antimicrobial and DNA-binding activities of the peptide fragments of human lactoferrin and histatin 5 against Streptococcus mutans. Arch Oral Biol. 2011;56(9):869–876. doi:10.1016/j.archoralbio.2011.02.004
33. Wirginia K, Anna J, Jakub P, et al. Effect of histatin-5 and lysozyme on the ability of Streptococcus mutans to form biofilms in in vitro conditions. Postepy Hig Med Dosw. 2015;69:1056–1066.
34. McDonald EE, Goldberg HA, Tabbara N, et al. Histatin 1 resists proteolytic degradation when adsorbed to hydroxyapatite. J Dent Res. 2011;90(2):268–272. doi:10.1177/0022034510388653
35. Khurshid Z, Najeeb S, Mali M, et al. Histatin peptides: pharmacological functions and their applications in dentistry. Saudi Pharm J. 2017;25(1):25–31. doi:10.1016/j.jsps.2016.04.027
36. Jang WS, Li XS, Sun JN, et al. The P-113 fragment of histatin 5 requires a specific peptide sequence for intracellular translocation in Candida albicans, which is independent of cell wall binding. Antimicrob Agents Chemother. 2008;52(2):497. doi:10.1128/AAC.01199-07
37. Zhou L, Wong HM, Zhang YY, et al. Constructing an antibiofouling and mineralizing bioactive tooth surface to protect against decay and promote self-healing. ACS Appl Mater Inter. 2020;12(2):3021–3031. doi:10.1021/acsami.9b19745
38. Malanovic N, Lohner K. Antimicrobial peptides targeting gram-positive bacteria. Pharmaceuticals. 2016;9:3. doi:10.3390/ph9030059
39. Vukosavljevic D, Custodio W, Del Bel Cury AA, et al. The effect of histatin 5, adsorbed on PMMA and hydroxyapatite, on Candida albicans colonization. Yeast. 2012;29(11):459–466. doi:10.1002/yea.2925
40. Tarsi R, Muzzarelli RAA, Guzmàn CA, et al. Inhibition of Streptococcus mutans adsorption to hydroxyapatite by low-molecular-weight chitosans. J Dent Res. 1997;76(2):665–672. doi:10.1177/00220345970760020701
41. Sano H, Shibasaki K-I, Matsukubo T, et al. Effect of molecular mass and degree of deacetylation of chitosan on adsorption of Streptococcus sobrinus 6715 to saliva treated hydroxyapatite. Bull Tokyo Dent Coll. 2002;43(2):75–82. doi:10.2209/tdcpublication.43.75
42. Bae K, Jun E, Lee S, et al. Effect of water-soluble reduced chitosan on Streptococcus mutans, plaque regrowth and biofilm vitality. Clin Oral Invest. 2006;10(2):102–107. doi:10.1007/s00784-006-0038-3
43. Cukkemane N, Bikker FJ, Nazmi K, et al. Anti-adherence and bactericidal activity of sphingolipids against Streptococcus mutans. Eur J Oral Sci. 2015;123(4):221–227. doi:10.1111/eos.12200
44. Yang X, Huang P, Wang H, et al. Antibacterial and anti-biofouling coating on hydroxyapatite surface based on peptide-modified tannic acid. Colloid Surface B. 2017;160:136–143. doi:10.1016/j.colsurfb.2017.09.006
45. Steinberg D, Amit U, Brayer L, et al. The effect of sustained-release varnish of chlorhexidine in plastic shells on salivary Streptococcus mutans. Clin Prevent Dent. 1991;13:9–12.
46. Besinis A, De Peralta T, Handy RD. Inhibition of biofilm formation and antibacterial properties of a silver nano-coating on human dentine. Nanotoxicology. 2014;8(7):745–754.
47. Zhang Q, Serpe M. Synthesis, characterization, and antibacterial properties of a hydroxyapatite adhesive block copolymer. Macromolecules. 2014;47(22):8018–8025. doi:10.1021/ma501997x
48. Kang M, Kim S, Kim H, et al. Calcium-binding polymer-coated poly(lactide- co-glycolide) microparticles for sustained release of quorum sensing inhibitors to prevent biofilm formation on hydroxyapatite surfaces. ACS Appl Mater Inter. 2019;11(8):7686. doi:10.1021/acsami.8b18301
49. Zhang H, Chiao M. Anti-fouling coatings of poly(dimethylsiloxane) devices for biological and biomedical applications. J Med Biol Eng. 2015;35(2):143–155. doi:10.1007/s40846-015-0029-4
50. Damodaran VB, Murthy NS. Bio-inspired strategies for designing antifouling biomaterials. Biomater Res. 2016;20(1):18. doi:10.1186/s40824-016-0064-4
51. Damodaran VB, Fee CJ, Popat KC. Prediction of protein interaction behaviour with PEG-grafted matrices using X-ray photoelectron spectroscopy. Appl Surf Sci. 2010;256(16):4894–4901. doi:10.1016/j.apsusc.2010.02.088
52. Ostuni E, Chapman RG, Holmlin RE, et al. A survey of structure-property relationships of surfaces that resist the adsorption of protein. Langmuir. 2001;17(18):5605–5620. doi:10.1021/la010384m
53. Zheng L, Sundaram H, Wei Z, et al. Applications of zwitterionic polymers. React Funct Polym. 2017;118:51–61.
54. Heijink JA, Yaszemski SM, Patel GR, et al. Local antibiotic delivery with OsteoSet®, DBX®, and Collagraft®. Clin Orthop Relat Res. 2006;451:29–33.
55. Chai F, Hornez JC, Blanchemain N, et al. Antibacterial activation of hydroxyapatite (HA) with controlled porosity by different antibiotics. Biomol Eng. 2007;24(5):510–514. doi:10.1016/j.bioeng.2007.08.001
56. McBain AJ, Bartolo RG, Catrenich CE, et al. Effects of a chlorhexidine gluconate-containing mouthwash on the vitality and antimicrobial susceptibility of in vitro oral bacterial ecosystems. Appl Environ Microbiol. 2003;69(8):4770. doi:10.1128/AEM.69.8.4770-4776.2003
57. Persson RE, Truelove EL, LeResche L, et al. Therapeutic effects of daily or weekly chlorhexidine rinsing on oral health of a geriatric population. Oral Surg Oral Med Oral Pathol Oral Radiol. 1991;72(2):184–191. doi:10.1016/0030-4220(91)90161-5
58. Misra D. Interaction of chlorhexidine digluconate with and adsorption of chlorhexidine on hydroxyapatite. J Biomed Mater Res B. 1994;28(11):1375–1381. doi:10.1002/jbm.820281116
59. CASd S, Colombo APV, Souto RM, et al. Adsorption of chlorhexidine on synthetic hydroxyapatite and in vitro biological activity. Colloid Surface B. 2011;87(2):310–318. doi:10.1016/j.colsurfb.2011.05.035
60. Gilbert P, Moore LE. Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol. 2005;99(4):703–715. doi:10.1111/j.1365-2672.2005.02664.x
61. Gupta S, Gupta H, Mandhyan D, et al. Bisphophonates related osteonecrosis of the jaw. Natl J Maxillofac Surg. 2013;4(2):151–158. doi:10.4103/0975-5950.127643
62. Shellis RP, Addy M, Rees GD. In vitro studies on the effect of sodium tripolyphosphate on the interactions of stain and salivary protein with hydroxyapatite. J Dent. 2005;33(4):313–324. doi:10.1016/j.jdent.2004.09.006
63. Oh HJ, Oh HW, Lee DW, et al. Chronologic trends in studies on fluoride mechanisms of action. J Dent Res. 2017;96(12):1353–1360. doi:10.1177/0022034517717680
64. Loskill P, Zeitz C, Grandthyll S, et al. Reduced adhesion of oral bacteria on hydroxyapatite by fluoride treatment. Langmuir. 2013;29(18):5528–5533. doi:10.1021/la4008558
65. Zhao IS, Gao SS, Hiraishi N, et al. Mechanisms of silver diamine fluoride on arresting caries: a literature review. Int Dent J. 2018;68(2):67–76.
66. Mai S, Mauger MT, Niu LN, et al. Potential applications of antimicrobial peptides and their mimics in combating caries and pulpal infections. Acta Biomater. 2017;49:16–35. doi:10.1016/j.actbio.2016.11.026
67. Khurshid Z, Naseem M, Sheikh Z, et al. Oral antimicrobial peptides: types and role in the oral cavity. Saudi Pharm J. 2016;24(5):515–524. doi:10.1016/j.jsps.2015.02.015
68. Guaní-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, et al. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol. 2010;135(1):1–11. doi:10.1016/j.clim.2009.12.004
69. Shi J, Liu Y, Wang Y, et al. Biological and immunotoxicity evaluation of antimicrobial peptide-loaded coatings using a layer-by-layer process on titanium. Sci Rep. 2015;5(1):16336. doi:10.1038/srep16336
70. Khoo X, Hamilton P, O’Toole GA, et al. Directed assembly of PEGylated-peptide coatings for infection-resistant titanium metal. J Am Chem Soc. 2009;131(31):10992–10997. doi:10.1021/ja9020827
71. Fuente-Núñez C, Reffuveille F, Haney EF, et al. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 2014;10(5):e1004152. doi:10.1371/journal.ppat.1004152
72. Wang Z, Fuente-Núñez C, Shen Y, et al. Treatment of oral multispecies biofilms by an anti-biofilm peptide. PLoS One. 2015;10(7):e0132512. doi:10.1371/journal.pone.0132512
73. Xu T, Levitz SM, Diamond RD, et al. Anticandidal activity of major human salivary histatins. Infect Immun. 1991;59(8):2549. doi:10.1128/IAI.59.8.2549-2554.1991
74. Yin A, Margolis HC, Grogan J, et al. Physical parameters of hydroxyapatite adsorption and effect on candidacidal activity of histatins. Arch Oral Biol. 2003;48(5):361–368. doi:10.1016/S0003-9969(03)00012-8
75. Vukosavljevic D, Hutter JL, Helmerhorst EJ, et al. Nanoscale adhesion forces between enamel pellicle proteins and hydroxyapatite. J Dent Res. 2014;93(5):514–519. doi:10.1177/0022034514526599
76. Siqueira WL, Margolis HC, Helmerhorst EJ, et al. Evidence of intact histatins in the in vivo acquired enamel pellicle. J Dent Res. 2010;89(6):626–630. doi:10.1177/0022034510363384
77. Patel KD, El-Fiqi A, Lee H-Y, et al. Chitosan–nanobioactive glass electrophoretic coatings with bone regenerative and drug delivering potential. J Mater Chem. 2012;22(47):24945–24956. doi:10.1039/c2jm33830k
78. Patel KD, Singh RK, Lee E-J, et al. Tailoring solubility and drug release from electrophoretic deposited chitosan–gelatin films on titanium. Surf Coat Technol. 2014;242:232–236. doi:10.1016/j.surfcoat.2013.11.049
79. Patel KD, Mahapatra C, Jin G-Z, et al. Biocompatible mesoporous nanotubular structured surface to control cell behaviors and deliver bioactive molecules. ACS Appl Mater Interfaces. 2015;7(48):26850–26859. doi:10.1021/acsami.5b09114
80. Patel KD, Singh RK, Lee J-H, et al. Electrophoretic coatings of hydroxyapatite with various nanocrystal shapes. Mater Lett. 2019;234:148–154. doi:10.1016/j.matlet.2018.09.066
81. Chen C, Chung Y. Antibacterial effect of water-soluble chitosan on representative dental pathogens Streptococcus mutans and Lactobacilli brevis. J Appl Oral Sci. 2012;20(6):620–627. doi:10.1590/S1678-77572012000600006
82. Fischer CL, Drake DR, Dawson DV, et al. Antibacterial activity of sphingoid bases and fatty acids against gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 2012;56(3):1157. doi:10.1128/AAC.05151-11
83. Tang J, Meng X, Liu H, et al. Antimicrobial activity of sphingolipids isolated from the stems of cucumber (Cucumis sativus L.). Molecules. 2010;15(12):9288–9297.
84. Veerman ECI, Valentijn-Benz M, Van’T Hof W, et al. Phytosphingosine kills Candida albicans by disrupting its cell membrane. Biol Chem. 2010;391(1):65–71. doi:10.1515/bc.2010.001
85. Valentijn-Benz M. van “T Hof W, Bikker FJ, et al. Sphingoid bases inhibit acid-induced demineralization of hydroxyapatite. Caries Res. 2015;49(1):9–17. doi:10.1159/000362096
86. Tintino SR, Oliveira-Tintino CDM, Campina FF, et al. Evaluation of the tannic acid inhibitory effect against the NorA efflux pump of Staphylococcus aureus. Microb Pathog. 2016;97:9–13. doi:10.1016/j.micpath.2016.04.003
87. Daglia M. Polyphenols as antimicrobial agents. Curr Opin Biotech. 2012;23(2):174–181. doi:10.1016/j.copbio.2011.08.007
88. Jones GA, McAllister TA, Muir AD, et al. Effects of Sainfoin (Onobrychis viciifolia Scop.) condensed tannins on growth and proteolysis by four strains of ruminal bacteria. Appl Environ Microbiol. 1994;60(4):1374–1378. doi:10.1128/AEM.60.4.1374-1378.1994
89. Long JR, Shaw WJ, Stayton PS, et al. Structure and dynamics of hydrated statherin on hydroxyapatite as determined by solid-state NMR. Biochemistry. 2001;40(51):15451–15455. doi:10.1021/bi010864c
90. Raj PA, Johnsson M, Levine MJ, et al. Salivary statherin. Dependence on sequence, charge, hydrogen bonding potency, and helical conformation for adsorption to hydroxyapatite and inhibition of mineralization. J Biol Chem. 1992;267(9):5968–5976.
91. Zyskind D, Steinberg D, Stabholz A, et al. The effect of sustained release application of chlorhexidine on salivary levels of Streptococcus mutans in partial denture wearers. J Oral Rehabil. 1990;17:61–66. doi:10.1111/j.1365-2842.1990.tb01394.x
92. Balanyk TE, Sandham HJ. Development of sustained-release antimicrobial dental varnishes effective against Streptococcus mutans in vitro. J Dent Res. 1985;64:1356–1360. doi:10.1177/00220345850640120501
93. Beyth N, Redlich M, Harari D, et al. Effect of sustained-release chlorhexidine varnish on Streptococcus mutans and Actinomyces viscosus in orthodontic patients. Am J Orthod Dentofac. 2003;123(3):345–348. doi:10.1067/mod.2003.19
94. Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346–2353. doi:10.1088/0957-4484/16/10/059
95. LeGeros RZ. Calcium Phosphates in Oral Biology and Medicine. S. Karger, New York; 1991.
96. Pal G, Dutta A, Mitra K, et al. Effect of low intensity laser interaction with human skin fibroblast cells using fiber-optic nano-probes. J Photoch Photobio B. 2007;86(3):252–261. doi:10.1016/j.jphotobiol.2006.12.001
97. He Z, Wang Q, Hu Y, et al. Use of the quorum sensing inhibitor furanone C-30 to interfere with biofilm formation by Streptococcus mutans and its luxS mutant strain. Int J Antimicrob Agents. 2012;40(1):30–35. doi:10.1016/j.ijantimicag.2012.03.016
98. Makadia H, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011;3(3):1377–1397. doi:10.3390/polym3031377
99. Laurent K, Michael GS. Communication in bacteria: an ecological and evolutionary perspective. Nat Rev Microbiol. 2006;4(4):249. doi:10.1038/nrmicro1383
100. Waters CM, Bassler BL. Quorum Sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005;21(1):319–346.
101. Cheng Y, Wu J, Gao B, et al. Fabrication and in vitro release behavior of a novel antibacterial coating containing halogenated furanone-loaded poly(L-lactic acid) nanoparticles on microarc-oxidized titanium. Int J Nanomedicine. 2012;7:5641–5652.
102. Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2012;31:2.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.