Back to Journals » Journal of Experimental Pharmacology » Volume 15
Anthocyanin-Containing Purple Sweet Potato (Ipomoea batatas L.) Synbiotic Yogurt Inhibited 3T3-L1 Adipogenesis by Suppressing White Adipocyte-Specific Genes
Authors Ariyanto EF , Shalannandia WA , Lantika UA, Fakih TM , Ramadhan DSF, Gumilar AN, Permana FK, Rahmah AN, Atik N , Khairani AF
Received 26 January 2023
Accepted for publication 8 May 2023
Published 22 May 2023 Volume 2023:15 Pages 217—230
DOI https://doi.org/10.2147/JEP.S405433
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Eko Fuji Ariyanto,1 Widad Aghnia Shalannandia,2 Uci Ary Lantika,3 Taufik Muhammad Fakih,4 Dwi Syah Fitra Ramadhan,5 Arini Nurisydayanti Gumilar,6 Farhan Khalil Permana,6 Anisa Nadia Rahmah,6 Nur Atik,2 Astrid Feinisa Khairani2
1Division of Biochemistry and Molecular Biology, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Jatinangor, West Java, Indonesia; 2Division of Cell Biology, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Jatinangor, West Java, Indonesia; 3Department of Biomedical Sciences, Faculty of Medicine, Universitas Islam Bandung, Bandung, West Java, Indonesia; 4Department of Pharmacy, Faculty of Mathematics and Natural Sciences, Universitas Islam Bandung, Bandung, West Java, Indonesia; 5Politeknik Kesehatan Kementerian Kesehatan Makassar, Makassar, Indonesia; 6Undergraduate Program Medical Doctor, Faculty of Medicine, Universitas Padjadjaran, Jatinangor, West Java, Indonesia
Correspondence: Astrid Feinisa Khairani, Division of Cell Biology, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Jalan Raya Bandung – Sumedang Km 21, Jatinangor, West Java, 45363, Indonesia, Tel +62-22-7795594, Email [email protected]
Purpose: We unravel the effect of anthocyanin-containing purple sweet potato synbiotic yogurt (PSPY) on 3T3-L1 adipocyte differentiation and its fundamental molecular mechanisms.
Methods: Molecular docking simulation was performed to observe and identify the affinity and interaction between bioactive compounds and targeted proteins. MDI (isobutylmethylxanthine, dexamethasone, and insulin)-containing medium, a cocktail that stimulates adipogenesis, was used in this study. The toxic effect possibility of the yogurt product was evaluated using 3-[4, 5-dimethylthiazol-2-yl]-2.5 diphenyl tetrazolium bromide (MTT) assay. A 0.25%, 0.5%, 1%, and 5% (v/v) plain or purple sweet potato yogurt supernatant was given to 3T3-L1 preadipocyte culture medium from 24 h after seeding until day 11 of MDI-induced differentiation. The mRNA expression and lipid accumulation were analyzed using RT-qPCR and Oil red O staining, respectively, on day 11 after differentiation induction.
Results: In silico study suggested that anthocyanin-derived compounds have the potential to inhibit peroxisome proliferator activated receptor gamma (PPAR-γ), a master regulator for white adipogenesis. Anthocyanin-containing PSPY significantly suppressed the expression of Pparg, Adipoq, Slc2a4, and Pgc1a. PSPY significantly suppressed Pparg with 1% and 5% concentrations, while with a concentration of 0.25%, PSPY significantly suppressed Adipoq expression as compared to control. Significant inhibition of Slc2a4 and Pgc1a was observed starting from a 0.25% concentration of PSPY. The suppression of adipogenic genes was also observed with the treatment of plain yogurt; however, the effects were relatively lower than the PSPY. The group treated with 1% and 5% of PSPY also showed inhibition effects on lipid accumulation.
Conclusion: This study demonstrated PSPY inhibition effect on white adipocyte differentiation through suppression of Pparg and its downstream genes, Adipoq and Slc2a4, indicating the potential of this yogurt as a functional food for obesity management and prevention.
Keywords: adipocyte differentiation, anthocyanin, Pparg, purple sweet potato synbiotic yogurt
Introduction
Obesity is a global health problem whose prevalence increases from year to year.1 It is characterized by excessive fat deposition due to an imbalance between energy intake and energy expenditure.2 Obesity is accompanied by chronic low-grade inflammation, which is known to be an essential risk factor for the development of several metabolic diseases such as type 2 diabetes mellitus, coronary heart disease, cerebrovascular disease, and hypertension.3,4 To treat and prevent obesity, some strategies are required to change the lifestyle by improving physical activity and consuming healthy foods and a balanced diet.
Yogurt is one of the functional foods that have the potential to improve human health, in which the lactic acid bacteria that ferment milk can act as probiotics. Probiotics consumption can modulate gut microbiota and give some benefits for health, such as maintaining intestinal permeability to prevent inflammation, transforming dietary phytochemicals into bioactive molecules, and producing antioxidants.5,6 It also has antimicrobial, immunomodulatory, and hepatoprotective effects and reduces adipogenesis and lipogenesis by suppressing insulin signaling.5–8
Yogurt, which contains probiotics and prebiotics, is known as synbiotic yogurt.9 Lactic acid bacteria, a probiotic, use prebiotic substances to provide nutrition for their growth and proliferation.10 Purple sweet potato (Ipomoea batatas L.) is rich in fermentable carbohydrates. Therefore, it can be used to fortify yogurt and act as a prebiotic.11 In addition to carbohydrates, purple sweet potato also contains other nutrients such as carotenoids, proteins, lipids, vitamins, minerals, dietary fibers, and many anthocyanins, a class of flavonoid compounds.12 Many studies have revealed that anthocyanins in purple sweet potato have beneficial health effects such as antioxidative, antiaging, immunomodulatory, antihyperglycemic, hepatoprotective, antimicrobial, and anti-obesity effects.13–16 Furthermore, purple sweet potato yogurt was reported to have relatively higher antioxidant activity compared to plain yogurt.17
A previous study by Khairani et al reported that purple sweet potato yogurt decreased visceral fat and liver weight in high-fat diet mice models and improved lipid profiles, including total cholesterol, triglycerides, and LDL level.18 However, the molecular mechanisms explaining how purple sweet potato yogurt exerts anti-obesity effects are still poorly understood. This study aimed to investigate the potential effects of purple sweet potato yogurt on adipocyte differentiation and the underlying molecular mechanisms using 3T3-L1 preadipocytes, the most commonly used cell line in elaborating the molecular mechanisms of adipocyte differentiation in vitro.
Materials and Methods
Molecular Docking Analysis
Protein Preparation
The proteins used in this simulation were PPAR-g, ADIPOQ, PGC-1α, and GLUT4. Crystallized protein structures were downloaded from Protein Data Bank Website (https://rcsb.org), while GLUT4 was modeled using the SWISS-MODEL Server.19 Protein structures were obtained and prepared using Discovery Studio 2017 and AutoDock 1.5.6. The preparation was carried out by removing water molecules and natural ligands, followed by adding polar hydrogen atoms and calculating the Kollman partial charge.20
Molecular Modeling of the Test Compounds and Protein Active Site Identification
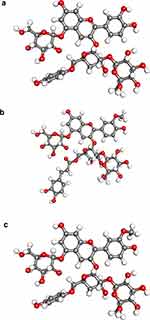
Test compounds used in this study were Cyanidin-3-caffeoyl-p-hydroxy benzoyl sophoroside-5-glucoside, Peonidin-3-caffeoyl sophoroside-5-glucoside, and Peonidin-3-caffeoyl-p-hydroxy benzoyl sophoroside-5-glucoside that were included in anthocyanin-derived compounds. Molecular structures of the compound were drawn using GaussView Software and optimized geometrically using Gaussian 03W Software.21 The semi-empirical method was chosen with Austin Model 1 as the basic set. The optimized structures with modification of their partial charges were used as an input for molecular docking simulation.
The macromolecular enzymes were prepared and processed for binding site identification, evaluation, and exploration process to check their biological activity using BIOVIA Discovery Studio 2020 Software.22
Molecular Docking Simulation and Analysis
Molecular docking simulation was performed using MGLTools 1.5.6 equipped with AutoDock4 to observe and identify the affinity and interaction between bioactive compounds and proteins. The gap between the enzyme and the bioactive compound surface was limited to 0.375 Å as a maximum radius. All simulations were carried out by 52 × 50 × 50 grid box size21 and continued using the Lamarckian Genetic Algorithm method with 100 confirmations.23
Molecular interactions between protein and test compounds were identified and evaluated based on Gibbs’ binding value.21 The ligands’ docking conformation was determined by selecting the compounds with the highest affinity. Amino acid residues that play a role in the molecular interactions were then observed using BIOVIA Discovery Studio 2020 Software.22
Preparation of Purple Sweet Potato Synbiotic Yogurt
Lactic acid bacteria (L. bulgaricus ATCC-11842 and S. thermophilus FNCC-0015) were prepared to be used as probiotics. These probiotics were provided and prepared in Advanced Biomedical Laboratory, Division of Microbiology and Parasitology Laboratory, Faculty of Medicine, Universitas Padjadjaran, Indonesia. Firstly, the bacteria were cultured in medium I containing 10% skim milk in DeMan, Rogosa, and Sharpe (MRS) broth medium. Following that, 10% (v/v) of the cultured product from the medium I was subcultured into medium II (whole, skim milk) to make the starter yogurt. Finally, the starter yogurt was used to make the plain skim milk yogurt, and 3% of purple sweet potato flour was mixed to make the purple sweet potato yogurt.
For yogurt treatment on 3T3-L1 preadipocytes, 10–12 mL of purple sweet potato yogurt was placed in a 15 mL conical tube and was centrifuged at 8000 rpm for 10 min. The supernatant of the yogurt was filtered using a 0.2 μm syringe filter and disposable syringe twice. The filtered supernatant-containing conical tube was wrapped in aluminum foil and stored in a 4°C storage cabinet until used in the following steps.
Culture and Differentiation of 3T3-L1 Cells and Yogurt Treatment
The 3T3-L1 cells, a murine fibroblast/pre-adipocyte cell line, were obtained from the German Diabetes Centre, Düsseldorf, and the Institute of Pharmacology and Toxicology, University Hospital of Bonn. The 3T3-L1 preadipocytes were cultured in high-glucose Dulbecco’s modified eagle medium (DMEM, Sigma, USA) containing 10% fetal bovine serum (FBS, Sigma, USA) and 1% penicillin-streptomycin at 37°C, 5% CO2. The cell line was cultured in 12-well plates with a seeding amount of 12 × 104 cells/well until 100% confluent. The confluent cells were further cultured in DMEM for 48 h. Afterward, the cells were induced by an MDI differentiation cocktail consisting of 0.5mM methyl-isobutyl-xanthine (Sigma, USA), 1 μM dexamethasone (Sigma, USA), and 10μg/mL insulin (Sigma, USA), which was determined as day 0 of the experiment.
After culturing the cells in MDI-containing DMEM for 48 h, on day 3, the medium was then replaced with 10μg/mL insulin-containing DMEM. Further, to optimize glucose uptake and lipogenesis during the differentiation process, the cells were cultured with the same medium (10 μg/mL insulin-containing DMEM) until day 10. The medium replacement was conducted every 48 h. Yogurt treatment was given to the cells by adding 0.25%, 0.5%, 1%, and 5% (v/v) of plain yogurt supernatant and purple sweet potato yogurt supernatant into the medium starting from 24 h after cell seeding until day 11 throughout the experiment. Staining of lipid droplets in the cells and RNA extraction for RT-qPCR analysis were performed on day 11.
MTT Assay
The possibility of the toxic effect of purple sweet potato yogurt and plain yogurt on 3T3-L1 preadipocytes was done using 3-[4,5-dimethylthiazol-2-yl]-2.5 diphenyl tetrazolium bromide (MTT) assay as previously described.24 The cells were seeded in 96-well plates, incubated for 24 h, and treated with 0.25%, 0.5%, 1%, and 5% (v/v) of purple sweet potato yogurt supernatant or plain yogurt supernatant for 72 h. MTT reagents were then added to the cells for 4 h, and the reaction was stopped using DMSO. The plate was then shaken to dilute the crystal formazan before measuring the absorbance using a microplate reader at a wavelength of 550 nm. The percentage of cell death was calculated based on the absorbance value of the sample (cell treated with yogurt), control, and blank.
Quantitative RT-qPCR Analysis
Quantitative RT-qPCR was used to measure the mRNA expression of genes analyzed in this study. Total RNA was isolated from the cells using the Quick-RNA™ cDNA Synthesis Kit (Bioline Reagents Ltd., UK). Afterward, quantitative RT-PCR was performed using SensiFast™ SYBR® No-ROX Kit according to the manufacturer’s instructions. The polymerase activation was set at 95°C for 2 min, followed by 40 cycles of denaturation at 95°C for 5 s and annealing/extension at 60–65°C for 20 s. All mRNA expression was normalized with Ppib mRNA expression. The primers used in this study are listed in Table 1.25
 |
Table 1 Primer Sequences for RT-qPCR Analysis |
Oil Red O Staining
The cells were stained on day 11 of differentiation with Oil Red O (ORO, Sigma, USA) as previously described (Wakabayashi, 2009). Briefly, the cells were washed with phosphate-buffered saline (PBS, Sigma, USA) and fixed with 10% formaldehyde in PBS for 10 min. After washing the cells twice in PBS, the cells were stained for 15–20 min in freshly diluted ORO solution (0.18% (wt/vol) ORO in 60% isopropanol). After removing the stain, the cells were washed with PBS twice, and the images were taken under a microscope (Olympus CK40) with 100x and 200x magnifications.
Statistical Analysis
Statistical analysis in this study was performed using GraphPad Prism ver. 9 (GraphPad Software, Inc., La Jolla, CA) based on one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons. The results were expressed as mean ± standard deviation (SD). Differences between groups were considered statistically significant at p<0.05.
Results
Anthocyanin-Derived Compounds Potentially Inhibit PPAR-γ
Ligand and Protein Preparation
In the molecular docking analysis, the three test compounds, namely Cyanidin-3-caffeoyl-p-hydroxy benzoyl sophoroside-5-glucoside, Peonidin-3-caffeoyl sophoroside-5-glucoside, and Peonidin-3-caffeoyl-p-hydroxy benzoyl sophoroside-5-glucoside were successfully imaged and optimized, as shown in Figure 1. The three compounds’ structural descriptions were taken from a previous study.26 Further, the protein structure was successfully prepared and visualized, as shown in Figure 2.
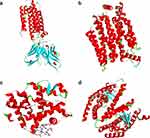 |
Figure 2 The 3D protein structures of ADIPOQ (a), GLUT4 (b), PGC-1α (c), and PPAR-γ (d). |
Molecular Docking
The test compounds simulated in this study are the main active compounds in the purple sweet potato, which are reported to reduce blood sugar levels.27,28 In this study, the molecular docking of proteins related to adipogenesis was simulated to see whether the decrease in blood sugar levels was related to their activity on adipogenesis and precisely to determine the affinity strength. Each test compound was successfully docked to the receptor’s active site by producing 100 conformations for each compound.
The tabulation of conformational energy for each compound against the protein is presented in Table 2. The compound with the lowest conformational energy, which indicated the highest affinity, was selected.29 The simulation results show that the compound Peonidin-3-caffeoyl-p-hydroxy benzoyl sophoroside-5-glucoside has a significantly higher affinity with PPAR-γ compared to other compound-protein combinations.
 |
Table 2 Tabulation of Conformational Energy of Each Compound Against the Protein |
Interaction analysis was carried out to see the molecular binding mode of the compound with the lowest energy in each protein, as shown in Figure 3. Interaction analysis of each protein-compound complex shows interaction in red that is commonly referred to as unfavorable interactions developed a steric collision between amino acid side chains with chemical compounds, where this interaction indicates poor activity.30 However, there is a difference in the amount between each complex. The protein-compound complex with the least unfavorable interaction was PPAR-γ protein with only four unfavorable interactions.31 Meanwhile, the other proteins showed relatively more unfavorable interactions ranging between 7 and 11 interactions (Figure 3).
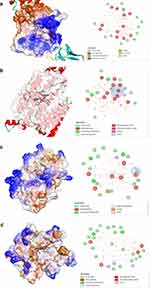 |
Figure 3 Analysis of compound interaction with the lowest binding energy in ADIPOQ (a), GLUT4 (b), PGC-1α (c), and PPAR-γ (d) proteins. |
Purple Sweet Potato Synbiotic Yogurt Does Not Affect 3T3-L1 Viability
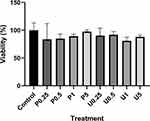
The MTT assay aimed to confirm whether plain yogurt and purple sweet potato yogurt can affect 3T3-L1 growth and viability. The result shows that the treatment of 0.25%, 0.5%, 1%, and 5% (v/v) of plain yogurt supernatant (indicated as P0.25, P0.5, P1, and P5, respectively, in Figure 4) and PSPY supernatant (indicated as U0.25, U0.5, U1, and U5, respectively, in Figure 4) only affect less than 20% cell viability, suggesting that plain yogurt and PSPY up to the concentration of 5% are not toxic to 3T3-L1 preadipocytes.32
Purple Sweet Potato Synbiotic Yogurt Inhibits White Adipocyte-Specific Genes During 3T3-L1 Adipocyte Differentiation
The mRNA expression of Pparg, Adipoq, Slc2a4, and Pgc1a was measured to investigate PSPY’s capability to suppress white adipocyte-specific genes during 3T3-L1 adipocyte differentiation. Measurement of samples under the treatment of 0.25%, 0.5%, 1%, and 5% of plain yogurt supernatant (indicated as P0.25, P0.5, P1, and P5, respectively, in Figure 5) and PSPY supernatant (indicated as U0.25, U0.5, U1, and U5, respectively, in Figure 5) was conducted on day 11 of differentiation period.
The result showed that PSPY (indicated by U in Figure 5A) inhibited Pparg expression in a dose-dependent manner. Significant inhibition was observed in the concentration of 1% and 5% (Figure 5A). On the other hand, plain yogurt (indicated by P in Figure 2a) also inhibits Pparg expression in the concentration of 0.5%, 1%, and 5%. However, the degree of inhibition was not in line with the increase in concentration (Figure 5A).
Further, the RT-qPCR result on Adipoq expression demonstrates the inhibitory effect of PSPY (indicated by U in Figure 5B) and plain yogurt (indicated by P in Figure 5B) on this gene in a dose-dependent manner, as compared with control. Interestingly, the degree of inhibition on Adipoq mRNA expression caused by PSPY treatment was higher than those caused by plain yogurt treatment (Figure 5B). With a concentration of 0.25%, PSPY treatment yields significant inhibition of Adipoq expression compared with control (p<0.01). Meanwhile, plain yogurt treatment with 0.25% concentration does not significantly inhibit Adipoq expression (p>0.05). Furthermore, for the concentration of 0.25% and 0.5%, the degree of inhibition caused by PSPY is about two-fold higher than those caused by plain yogurt. The statistical comparison between two yogurts at the same concentration does not show significant differences (Figure 5B). These findings imply that purple sweet potato synbiotic yogurt exerts a stronger inhibitory effect on Adipoq expression than plain yogurt.
Treatment of purple sweet potato yogurt (indicated by U in Figure 5C) and plain yogurt (indicated by P in Figure 5C) decreased the expression of Slc2a4, a gene-encoding glucose transporter type 4 (Glut4), in a dose-dependent manner (Figure 5C). Moreover, the degree of inhibition yielded by purple sweet potato yogurt treatment is higher than those caused by plain yogurt treatment (Figure 5C). At a concentration of 0.25%, purple sweet potato yogurt treatment produced significant inhibition of Slc2a4 expression as compared with control (p<0.01), while plain yogurt treatment did not cause significant inhibition (p>0.05). In addition, at concentrations of 0.25%, 0.5%, and 1%, the degree of inhibition exerted by purple sweet potato yogurt is about two-fold higher than those caused by plain yogurt. At the concentration of 0.25%, the comparison between the two yogurts showed a very significant difference (p<0.0001, Figure 5C). These findings suggested that purple sweet potato synbiotic yogurt has a stronger inhibitory effect on Slc2a4 expression than plain yogurt.
Similarly, Pgc1a mRNA expression during adipocyte differentiation significantly decreased in a dose-dependent manner (p<0.001) upon treatment with both purple sweet potato yogurt and plain yogurt. The higher the intervention concentration was given, the more inhibition was observed. Moreover, purple sweet potato yogurt demonstrated a stronger suppression effect on Pgc1a expression than plain yogurt at 0.25% and 0.5%. Even though the differences between the group with the same concentration were not statistically significant (Figure 5D), this result suggested that purple sweet potato synbiotic yogurt yields a stronger inhibitory effect on Pgc1a expression compared with plain yogurt.
Purple Sweet Potato Synbiotic Yogurt Inhibits Lipid Accumulation During Adipocyte Differentiation
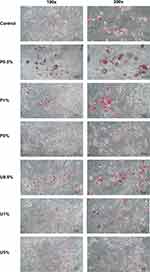
The lipid droplets in the cells were stained with Oil red O to evaluate whether inhibition of adipogenic genes by purple sweet potato yogurt also leads to the inhibition of lipid accumulation during the differentiation process. The staining process was conducted on day 11 differentiation, and the lipid droplets were observed under the microscope. The results indicated that purple sweet potato yogurt treatment at a concentration of 1% and 5% inhibits the formation of lipid droplets. Meanwhile, plain yogurt inhibited lipid accumulation in the concentration of 5% (Figure 6). This finding demonstrated that purple sweet potato synbiotic yogurt inhibited lipid accumulation during 3T3-L1 adipocyte differentiation.
Discussion
The objective of this study was to elaborate on the potential effects of purple sweet potato synbiotic yogurt on white adipocyte differentiation and the underlying molecular mechanisms. Our data showed that anthocyanin-containing purple sweet potato synbiotic yogurt inhibited the expression of white adipocyte-specific genes (Pparg, Adipoq, and Slc2a4), consequently inhibiting lipid accumulation during adipocyte differentiation. Moreover, molecular docking analysis showed that anthocyanin-derived compounds potentially inhibit PPAR-γ.
Adipocyte differentiation is a process that mostly depends on adipogenic gene expressions, including in 3T3-L1 preadipocytes. Those gene expressions will affect and can be seen in the adipocyte phenotype, such as lipid droplet accumulation at the end of the differentiation stage. Induction of Pparg, Cebpa, and their downstream genes such as Adipoq and Slc2a4 was reported to drive white adipocyte differentiation.33 Inhibition of adipocyte differentiation is one of the promising strategies for obesity management and prevention.34
Yogurt is one of the anti-obesity probiotic diets with several underlying molecular mechanisms. Conjugated linoleic acid (CLA) content in yogurt can lower Pparg expression.35 A study conducted by Guanlin et al showed a dose-dependent decrease of Pparg expression after induction by CLA.36 Lactic acid-producing bacteria as probiotic content in yogurt also support anti-adipogenic properties of yogurt. Decreases in lipid accumulation and triglyceride content due to the effect of probiotic bacteria were observed in previous research conducted by Lee et al.37 The current study showed consistent results with previous reports that yogurt treatment to 3T3-L1 preadipocytes decreased the expression of Pparg and its downstream genes such as Adipoq and Slc2a4 in a dose-dependent manner.
Anti-adipogenic effect of probiotic yogurt can be enhanced by mixing it with prebiotics such as purple sweet potato to form a synbiotic yogurt.18 Anthocyanin and oligosaccharides are rich in purple sweet potato, so they can produce optimal effects on preventing and treating obesity.38 The oligosaccharide in purple sweet potato increases the content of probiotic bacteria to be more optimal in reducing lipid accumulation. Moreover, anthocyanins which are pretty abundant in purple sweet potatoes and are known to inhibit the expression of Pparg and Cebpa.39 It might explain why in this study, purple sweet potato yogurt had a stronger inhibition effect on adipogenic gene expression, in this case, Pparg, Adipoq, and Slc2a4 compared with plain yogurt.
Previous studies have suggested that peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha) plays an important role in the browning of adipose tissue by stimulating mitochondrial biogenesis and inducing transcription factor Ppara which subsequently stimulates the expression of brown adipocyte-specific genes such as Ucp1 and Prdm16.40,41 Interestingly, this study found that purple sweet potato yogurt and plain yogurt suppressed the mRNA expression of Pgc1a during 3T3-L1 differentiation, suggesting that brown adipogenesis might not increase as the compensation of inhibited white adipocyte differentiation. In addition to the inhibition effect of anthocyanin-containing purple sweet potato synbiotic yogurt on PPARG, this study also revealed the inhibition effect of sweet potato yogurt on Pgc1a expression. This finding supported the hypothesis that different molecular pathways regulate white and brown adipocyte differentiation.
In this experiment, the control cells, which were only treated with MDI stimulation without yogurt supplementation, accumulated lipid. The lipid accumulation occurred due to several interrelated factors, such as the abundance of glucose in the medium, increased expression of Slc2a4, which encodes and facilitates glucose transporter 4 (GLUT4) synthesis, and the presence of cAMP agonist IBMX, dexamethasone, and insulin (MDI mixture) which in turn, activated the insulin-induced adipocyte differentiation.42 Furthermore, MDI also increased the expression of Pparg and Cebpa, which accelerated adipogenesis by inducing their downstream genes such as Slc2a4 and Adipoq.40 In purple sweet potato synbiotic yogurt-treated cells, the induction of Pparg, Slc2a4, and Adipoq was inhibited, resulting in reduced lipid accumulation.
This study showed the inhibition effect of purple sweet potato synbiotic yogurt on adipocyte differentiation by suppressing Pparg and its down-regulated genes: Adipoq and Slc2a4. Although both plain and purple sweet potato yogurt showed an inhibition effect on these genes, more substantial inhibition effects were observed upon purple sweet potato yogurt supplementation.
The limitation of this study is that the inhibition effect of purple sweet potato synbiotic yogurt on the accumulation of lipid droplets, as observed in the microscopic picture in Figure 6, is not very obvious. This result might happen due to several reasons. One possible reason is that the lipid accumulation in the control cells had not reached the optimal amount; thus, the inhibition in the purple sweet potato yogurt-treated cells could not be seen clearly. It was also possible that the white adipocyte accumulation happened through several molecular pathways. Therefore, it is recommended to investigate other genes responsible for other pathways, such as the insulin signaling pathway. Furthermore, this study showed promising results in adipogenic molecular pathway, and we also suggest to proceed this investigation to the in vivo study using mice models.
Conclusion
This study reveals the inhibition effect of purple sweet potato synbiotic yogurt on white adipocyte differentiation through the suppression of PPAR-γ, suggesting the potential of this yogurt for obesity management and prevention.
Acknowledgments
This study was conducted at Microbiology, Cell Culture, and Molecular Genetics Laboratories, Faculty of Medicine, Universitas Padjadjaran, Indonesia. We would like to express our gratitude to Dr. Afiat Berbudi for the kind gift of the 3T3-L1 cell line and Tenny Putri, Nurul Qomarila, Nurul Setia Rahayu, and Erlina Widiarsih for their technical assistance.
Author Contributions
All the authors made substantial contributions to the design, conducting research, acquisition of data, and data analysis; took part in drafting the article or critically revising for important intellectual content. All authors agreed to submit to this journal, given final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding
This work was funded by Indonesian Endowment Fund for Education (Lembaga Pengelola Dana Pendidikan), Indonesia (Grant number: PRJ-51/LPDP/2019). This research was also supported by The Ministry of Education, Culture, Research, and Technology, Indonesia, and Universitas Padjadjaran, Indonesia.
Disclosure
The authors declare they have no conflicts of interest to disclose.
References
1. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642. doi:10.1016/S0140-6736(17)32129-3/ATTACHMENT/A71C66F4-AF6A-45CC-B74B-B320544FF826/MMC1.PDF
2. González-Muniesa P, Mártinez-González MA, Hu FB, et al. Obesity. Nat Rev Dis Primers. 2017;3(1):1–18. doi:10.1038/nrdp.2017.34
3. Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–150. doi:10.1016/J.DIABRES.2014.04.006
4. Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. 2017;960:1–17. doi:10.1007/978-3-319-48382-5_1
5. Li X, Shimizu Y, Kimura I. Gut microbial metabolite short-chain fatty acids and obesity. Biosci Microbiota Food Health. 2017;36(4):135–140. doi:10.12938/bmfh.17-010
6. Choi WJ, Dong HJ, Jeong HU, Jung HH, Kim YH, Kim TH. Antiobesity effects of lactobacillus plantarum LMT1-48 accompanied by inhibition of Enterobacter cloacae in the intestine of diet-induced obese mice. J Med Food. 2019;22(6):560–566. doi:10.1089/jmf.2018.4329
7. Rocha-Ramírez LM, Pérez-Solano RA, Castañón-Alonso SL, et al. Probiotic lactobacillus strains stimulate the inflammatory response and activate human macrophages. J Immunol Res. 2017;2017:1–14. doi:10.1155/2017/4607491
8. Edwin EML, Armando MMO, Peñafiel AM, Maira RSC. Probiotics beverages: an alternative treatment for metabolic syndrome. Function Med Bever. 2019;11:459–482. doi:10.1016/B978-0-12-816397-9.00014-5
9. Mohanty D, Misra S, Mohapatra S, Sahu PS. Prebiotics and synbiotics: recent concepts in nutrition. Food Biosci. 2018;26:152–160. doi:10.1016/J.FBIO.2018.10.008
10. Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. doi:10.1038/nrgastro.2017.75
11. Guo Z, Zhao B, Li H, Miao S, Zheng B. Optimization of ultrasound-microwave synergistic extraction of prebiotic oligosaccharides from sweet potatoes (Ipomoea batatas L.). Innovat Food Sci Emerg Technol. 2019;54:51–63. doi:10.1016/j.ifset.2019.03.009
12. Wang S, Nie S, Zhu F. Chemical constituents and health effects of sweet potato. Food Res Int. 2016;89:90–116. doi:10.1016/j.foodres.2016.08.032
13. Zhang M, Pan LJ, Jiang ST, Mo YW. Protective effects of anthocyanins from purple sweet potato on acute carbon tetrachloride-induced oxidative hepatotoxicity fibrosis in mice. Food Agric Immunol. 2015;27(2):157–170. doi:10.1080/09540105.2015.1079589
14. Kim OK, Nam DE, Yoon HG, Baek SJ, Jun W, Immunomodulatory LJ. Antioxidant effects of purple sweet potato extract in LP-BM5 murine leukemia virus-induced murine acquired immune deficiency syndrome. J Med Food. 2015;18(8):882–889. doi:10.1089/jmf.2014.3274
15. Wu Q, Qu H, Jia J, et al. Characterization, antioxidant and antitumor activities of polysaccharides from purple sweet potato. Carbohydr Polym. 2015;132:31–40. doi:10.1016/j.carbpol.2015.06.045
16. Ding Y, Shen M, Wei D, et al. Study on compatible characteristics of wheat and purple sweet potato starches. Food Hydrocoll. 2020;107:105961. doi:10.1016/j.foodhyd.2020.105961
17. Darusman F, Shalannandia WA, Lantika UA, et al. In vitro antioxidant activity and nutritional value determination of yogurt fortified with purple sweet potato (Ipomoea batatas L.). J Appl Pharm Sci. 2022;12(8):148–155. doi:10.7324/japs.2022.120815
18. Khairani AF, Islami U, Syamsunarno MRA, Lantika U. Synbiotic purple sweet potato yogurt ameliorate lipid metabolism in high fat diet mice model. Biomed Pharmacol J. 2020;13(1):175–184. doi:10.13005/bpj/1874
19. Schwede T, Kopp J, Guex N, Peitsch MC. Swiss-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 2003;31(13):3381–3385. doi:10.1093/NAR/GKG520
20. Kurniawan F, Miura Y, Kartasasmita RE, Mutalib A, Yoshioka N, Tjahjono DH. In silico study, synthesis, and cytotoxic activities of porphyrin derivatives. Pharmaceuticals. 2018;11:1. doi:10.3390/ph11010008
21. Pantsar T, Poso A. Binding affinity via docking: fact and fiction. Molecules. 2018;23(8):1. doi:10.3390/MOLECULES23081899
22. Kemmish H, Fasnacht M, Yan L. Fully automated antibody structure prediction using BIOVIA tools: validation study. PLoS One. 2017;12:5. doi:10.1371/JOURNAL.PONE.0177923
23. Morris GM, Goodsell DS, Halliday RS, et al. Automated docking using a lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1639;19(14):16391662.
24. Bashari MH, Arsydinilhuda FZ, Ilhamsyah RS, et al. The ethanol extract of marine sponge aaptos suberitoides suppress cell viability, cell proliferation and cell migration in HER2-positive breast cancer cell line. Asian Pacific J Cancer Prevent. 2021;22(S1):25–32. doi:10.31557/APJCP.2021.22.S1.25
25. Abe Y, Fujiwara Y, Takahashi H, et al. Histone demethylase JMJD1A coordinates acute and chronic adaptation to cold stress via thermogenic phospho-switch. Nat Commun. 2018;9:1566. doi:10.1038/s41467-018-03868-8
26. Xu X, Huang M, Zou X. Docking-based inverse virtual screening: methods, applications, and challenges. Biophys Rep. 2018;4(1):1–16. doi:10.1007/s41048-017-0045-8
27. Li A, Xiao R, He S, et al. Research advances of purple sweet potato anthocyanins: extraction, identification, stability, bioactivity, application, and biotransformation. Molecules. 2019;24:21. doi:10.3390/molecules24213816
28. Amagloh FC, Yada B, Tumuhimbise GA, Amagloh FK, Kaaya AN. The potential of sweet potato as a functional food in sub-saharan Africa and its implications for health: a review. Molecules. 2021;26:10. doi:10.3390/molecules26102971
29. Hikmawati D, Fakih TM, Sutedja E, Dwiyana RF, Atik N, Ramadhan DSF. Pharmacophore-guided virtual screening and dynamic simulation of Kallikrein-5 inhibitor: discovery of potential molecules for rosacea therapy. Inform Med Unlocked. 2021;28. doi:10.1016/j.imu.2022.100844
30. Ramadhan DSF, Siharis F, Abdurrahman S, Isrul M, Fakih TM. In silico analysis of marine natural product from sponge (Clathria Sp.) for their activity as inhibitor of SARS-CoV-2 main protease. J Biomol Struct Dyn. 2021. doi:10.1080/07391102.2021.1959405
31. Pitaloka DAE, Ramadhan DSF, Arfan CL, Fakih TM, Fakih TM. Docking-based virtual screening and molecular dynamics simulations of quercetin analogs as enoyl-acyl carrier protein reductase (Inha) inhibitors of mycobacterium tuberculosis. Sci Pharm. 2021;89:2. doi:10.3390/scipharm89020020
32. International Organization for Standardization. Biological evaluation of medical devices — part 5: tests for in vitro cytotoxicity. ISO Standard No. 10993-5:2009; 2009. Available from: https://www.iso.org/standard/36406.html.
33. Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019;20(4):242–258. doi:10.1038/s41580-018-0093-z
34. Funk MI, Conde MA, Piwien-Pilipuk G, Uranga RM. Novel antiadipogenic effect of menadione in 3T3-L1 cells. Chem Biol Interact. 2021;343:109491. doi:10.1016/j.cbi.2021.109491
35. Baspinar B, Güldaş M. Traditional plain yogurt: a therapeutic food for metabolic syndrome? Crit Rev Food Sci Nutr. 2020;61(18):3129–3143. doi:10.1080/10408398.2020.1799931
36. Lin G, Wang H, Dai J, et al. Conjugated linoleic acid prevents age-induced bone loss in mice by regulating both osteoblastogenesis and adipogenesis. Biochem Biophys Res Commun. 2017;490(3):813–820. doi:10.1016/j.bbrc.2017.06.122
37. Lee CS, Park MH, Kim SH. Selection and characterization of probiotic bacteria exhibiting antiadipogenic potential in 3T3-L1 preadipocytes. Probiotics Antimicrob Proteins. 2022;14(1):72–86. doi:10.1007/S12602-021-09793-5
38. El Husna N, Novita M, Rohaya S. Anthocyanins content and antioxidant activity of fresh purple fleshed sweet potato and selected products. AGRITECH. 2013;33(3):296–302.
39. Lee B, Lee M, Lefevre M, Kim HR. Anthocyanins inhibit lipogenesis during adipocyte differentiation of 3T3-L1 preadipocytes. Plant Foods Hum Nutr. 2014;69(2):137–141. doi:10.1007/S11130-014-0407-Z
40. Wakabayashi KI, Okamura M, Tsutsumi S, et al. The peroxisome proliferator-activated receptor gamma/retinoid X receptor alpha heterodimer targets the histone modification enzyme PR-Set7/Setd8 gene and regulates adipogenesis through a positive feedback loop. Mol Cell Biol. 2009;29(13):3544–3555. doi:10.1128/MCB.01856-08
41. Bargut TCL, Souza-Mello V, Aguila MB, Mandarim-de-Lacerda CA. Browning of white adipose tissue: lessons from experimental models. Horm Mol Biol Clin Investig. 2017;31:1. doi:10.1515/hmbci-2016-0051
42. Klemm DJ, Leitner JW, Watson P, et al. Insulin-induced adipocyte differentiation: activation of creb rescues adipogenesis from the arrest caused by inhibition of prenylation. J Biol Chem. 2001;276(30):28430–28435. doi:10.1074/JBC.M103382200
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.