Back to Journals » Clinical Ophthalmology » Volume 9
Anterior ischemic optic neuropathy due to biopsy-proven giant cell arteritis in Thai patients
Authors Attaseth T , Vanikieti K, Poonyathalang A , Preechawat P, Jindahra P, Wattanatranon D
Received 14 February 2015
Accepted for publication 7 April 2015
Published 12 June 2015 Volume 2015:9 Pages 1071—1075
DOI https://doi.org/10.2147/OPTH.S82898
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Taweevat Attaseth,1 Kavin Vanikieti,1 Anuchit Poonyathalang,1 Pisit Preechawat,1 Panitha Jindahra,2 Duangkamon Wattanatranon3
1Department of Ophthalmology, 2Department of Medicine, 3Department of Pathology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Abstract: Giant cell arteritis is a systemic granulomatous vasculitis affecting medium to large arteries. An arteritic anterior ischemic optic neuropathy is the most common cause of permanent visual loss. Giant cell arteritis is very rare among Asians. We report six patients with biopsy-proven arteritic anterior ischemic optic neuropathy. Demographic data, clinical manifestations, laboratory findings, treatment, and visual outcome are described in detail and compared with Caucasian patients. We found no differences in any clinical features except for sex preference. Moreover, perioptic nerve sheath enhancement was observed in half of our patients.
Keywords: giant cell arteritis, temporal artery biopsy, perioptic nerve sheath enhancement, choroidal ischemia, Asian
Introduction
Giant cell arteritis (GCA) is a vasculitis of medium to large sized arteries, it can interfere with arterial flow to the optic nerve head causing arteritic anterior ischemic optic neuropathy (A-AION). It is the most common form of systemic vasculitis affecting elderly persons, which commonly occurs in the Caucasian population.1 Overall annual incidence of GCA is 15–25 per 100,000 in American and North European people over the age of 50 years and significantly higher in women.2 The disease seems to be infrequent in Asians, but the data are sparse. In a study from Japan, the prevalence is extremely low at 1.47 per 100,000 population.3 The incidence of GCA is unknown in Thailand and only a few case reports have been published.4 Although GCA is a well-known cause of A-AION, there is not sufficient information about Thai patients probably due to its rarity.
Patients and methods
Medical records of all patients with anterior ischemic optic neuropathy (AION) seen in Neuro-Ophthalmology Unit at Department of Ophthalmology, Ramathibodi Hospital from January 2005 through October 2014 were reviewed. Only patients with AION and biopsy-proven GCA were included in this study. The diagnosis of GCA requires three or more of the following criteria:5 1) age of onset greater than 50 years; 2) new onset headache; 3) temporal artery abnormality on examination (tender or reduced pulsation); 4) elevated erythrocyte sedimentation rate (ESR) >50 mm/hr; 5) abnormal temporal arterial biopsy, showing necrotizing vasculitis with predominant mononuclear cell infiltration or granulomatous inflammation. Patients were evaluated in a standard fashion and underwent a systematic comprehensive neuro-ophthalmic history and examination, including dilating funduscopic examination, formal visual field testing by Goldmann perimetry or Humphrey Automated Field Analyzer 30-2, fundus fluorescein angiography, and indocyanine green angiography. Each patient underwent a thorough work-up evaluating for other causes of optic neuropathy and other systemic vasculitis, including blood tests, cerebrospinal fluid analysis and magnetic resonance imaging (MRI) of brain and orbit. ESR and CRP were tested in all cases.
Results
Demographic data
During the period 2005–2014, there were 236 patients diagnosed with AION. Temporal artery biopsies were performed in 20 patients with clinical suspicion of GCA and positive biopsy results were found in only six patients (2.5%). Summarized information of all patients are shown in Table 1. Four patients (67%) were male. Mean age at onset was 72.5 years, ranging from 63 to 81 years.
Clinical presentation
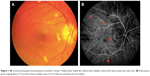
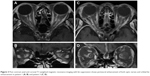
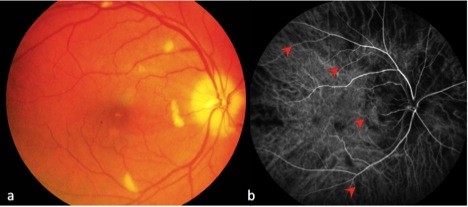
Of the six patients with biopsy-proven GCA, two had bilateral, simultaneous onset of A-AION and four had unilateral involvement (eight eyes in total). In the unilateral A-AION group, two patients developed contralateral visual loss from cilioretinal artery occlusion 2 weeks before the onset of A-AION. All eyes had severe visual loss (20/200 or worse) at initial presentation. No light perception was detected in three eyes. The mean duration of visual loss was 8.2 days, ranging from 1 day to 17 days. Optic discs appeared diffusely swollen and pale in all patients (Figure 1). Neither peri-papillary hemorrhages nor exudates were found. Retinal cotton wool spots were identified in three eyes (two patients). One patient experienced ipsilateral transient monocular visual loss prior to the onset of A-AION. Systemic symptoms were present before and at the time of visual loss in three patients (50%). These included headache and at least one of the following symptoms: scalp tenderness, jaw claudication, fever and general malaise, and anorexia and weight loss. Abnormal superficial temporal arteries were found during examination in three cases. No patients complained of diplopia. Fundus fluorescein angiography and indocyanine green angiography were performed in four patients. Delayed choroidal filling time was demonstrated in all, whereas, choroidal non-perfusion was found in one patient (Figure 2). MRIs of orbit and brain were performed in five patients. Abnormal MRI findings were shown in three patients (Figure 3). Perineural enhancement of the intraorbital part of the optic nerves was observed in these three patients. Their optic nerve signals were normal. Stranding of bilateral intraorbital fat was also identified. Diffuse weighted image sequences performed in two patients with abnormal MRI findings were unremarkable. There were no imaging signs of cerebral vasculitis in any of the patients. The mean ESR was 95 mm/hr (75–102 mm/hr). CRP was high in four patients, (17–122 mg/L). There were four patients with anemia and two patients with thrombocytosis.
Treatment and visual outcome
High dose of intravenous methylprednisolone (1 g per day for 3 days) and subsequent long-term oral corticosteroid were given in all cases. Oral steroid was slowly tapered on the basis of symptoms, ESR, and CRP. Follow-up period ranged from 5 months to 120 months. The disc edema in all eyes resolved within 6 weeks after treatment. Final visual acuity remained unchanged in most of the patients (five eyes, 63%). Three eyes (37%) showed visual acuity improvement and two eyes achieved a final visual acuity of 20/50. All patients eventually developed diffuse optic atrophy in the affected eyes.
Discussion
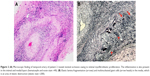
This study showed that the clinical manifestations of GCA in Thai patients were similar to those previously described except for the sex preference (Table 2).1,2,6,7 All patients aged over 60 years, presented with a rapid onset of severe visual loss and developed typical pallid “chalky white” disc edema. They all had a significantly elevated ESR and most of them had high CRP levels. Systemic symptoms, anemia, and/or thrombocytosis were found in approximately half of the patients. Although the visual prognosis is very poor in A-AION, a study revealed some recovery of vision in one third of cases.1 The result of our study confirms the potential of visual recovery after corticosteroid treatment. In the past, orbital MRI was not done in typical GCA cases as it is mainly diagnosed on clinical grounds. However, there is increasing evidence of abnormal enhancement of the optic nerve in GCA using T1-weighted images with gadolinium.8–10 The optic nerve sheath involvement is prominent in those cases. Biopsy of the optic nerve sheath showed inflammation of the perineural vasculature with multinucleated giant cells and disruption of the internal elastic lamina.9 Likewise, we showed perineural enhancement of the optic nerve in three out of six patients. The mechanism of selective enhancement of optic nerve sheath is still not known but the theory of blood optic nerve barrier disruption has been proposed.10
Conclusion
Arteritic AION caused by GCA is not common in Thailand. The clinical features and visual outcome in Thai patients are not different from those described in Asian and Caucasian populations. Enhancement of the perioptic nerve sheath can be detected in A-AION. It might be useful in GCA diagnosis particularly in difficult cases. Further studies are required to evaluate its clinical application.
Acknowledgment
This paper was presented at the North American Neuro-Ophthalmology Society 41st Annual Meeting as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in program information of the meeting: http://www.nanosweb.org/files/2015%20Poster%20Table%20of%20ContentsFULLI.pdf.
Disclosure
The authors report no conflicts of interest in this work.
References
Rahman W, Rahman FZ. Giant cell (temporal) arteritis: an overview and update. Surv Ophthalmol. 2005;50(5):415–428. | ||
Kale N, Eggenberger E. Diagnosis and management of giant cell arteritis: a review. Curr Opin Ophthalmol. 2010;21(6):417–422. | ||
Kobayashi S, Yano T, Matsumoto Y, et al. Clinical and epidemiologic analysis of giant cell (temporal) arteritis from a nationwide survey in 1998 in Japan: the first government-supported nationwide survey. Arthritis Rheum. 2003;49(4):594–598. | ||
Aui-Aree N, Tungsinmunkong K, Hirunpat S, Ratanasukon M, Wangsupadilok B. A variety of atypical manifestations in giant cell arteritis. J Med Assoc Thai. 2010;93(5):629–632. | ||
Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33(8):1122–1128. | ||
Liu GT, Glaser JS, Schatz NJ, Smith JL. Visual morbidity in giant cell arteritis. Ophthalmology. 1994;101(11):1779–1785. | ||
Cheng CK, Lee CC, Huang KH, Wu TE, Peng PH. Giant cell (Temporal) arteritis with anterior ischemic optic neuropathy: a biopsy-proven case in Taiwan. J Formos Med Assoc. 2010;109(7):550–554. | ||
Lee AG, Eggenberger ER, Kaufman DI, Manrique C. Optic nerve enhancement on magnetic resonance imaging in arteritic ischemic optic neuropathy. J Neuroophthalmol. 1999;19(4):235–237. | ||
Morgenstern KE, Ellis BD, Schochet SS, Linberg JV. Bilateral optic nerve sheath enhancement from giant cell arteritis. J Rheumatol. 2003;30(3):625–627. | ||
Liu KC, Chesnutt DA. Perineural optic nerve enhancement on magnetic resonance imaging in giant cell arteritis. J Neuroophthalmol. 2013;33(3):279–281. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.