Back to Journals » Clinical Ophthalmology » Volume 15
Anophthalmic Socket Syndrome: Prevalence, Impact and Management Strategies
Authors Quaranta-Leoni FM , Fiorino MG, Quaranta-Leoni F, Di Marino M
Received 24 June 2021
Accepted for publication 21 July 2021
Published 6 August 2021 Volume 2021:15 Pages 3267—3281
DOI https://doi.org/10.2147/OPTH.S325652
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Francesco M Quaranta-Leoni,1,2 Maria Grazia Fiorino,1 Flavia Quaranta-Leoni,3 Matteo Di Marino1
1Orbital and Adnexal Service, Villa Tiberia Hospital – GVM Care & Research, Rome, 00137, Italy; 2Oftalmoplastica Roma, Rome, 00197, Italy; 3Catholic University of the Sacred Heart, Rome, 00168, Italy
Correspondence: Francesco M Quaranta-Leoni Email [email protected]
Abstract: Anophthalmic socket syndrome determines functional deficits and facial deformities, and may lead to poor psychological outcomes. This review aims to comprehensively evaluate the features of the syndrome, based on literature review and authors’ clinical and surgical experience. An electronic database (PubMed,MEDLINE and Google Scholar) search of all articles written in English and non-English language with abstract translated to English on anophthalmic socket syndrome was performed. Data reviewed included demographics, presentations, investigations, management, complications and outcomes. Different types of orbital implants were evaluated; the management of implant exposure was examined; different orbital volume enhancement procedures such as secondary implantation, subperiosteal implants and the use of fillers in anophthalmic patients were described; the problems related to socket contraction were outlined; the treatment options for chronic anophthalmic socket pain and phantom eye syndrome were assessed; the most recent advances in the management of congenital anophthalmia were described. Current clinical evidence does not support a specific orbital implant; late exposure of porous implants may be due to pegging, which currently is seldom used; filler absorption in the orbit appears to be faster than in the dermis, and repeated treatments could be a potential source of inflammation; socket contraction results in significant functional and psychological disability, and management is challenging. Patients affected by anophthalmic socket pain and phantom eye syndrome need specific counseling. It is auspicable to use a standardized protocol to treat children affected by clinical congenital anophthalmia; dermis fat graft is a suitable option in these patients as it helps continued socket expansion. Dermis fat graft can also address the volume deficit in case of explantation of exposed implants and in contracted sockets in both children and adults. Appropriate clinical care is essential, as adequate prosthesis wearing improves the quality of life of anophthalmic patients.
Keywords: orbital implants, socket surgery, contracted socket, phantom eye syndrome, congenital anophthalmia
Introduction
The loss of an eye after enucleation or evisceration determines a functional deficit and facial deformity, but may also lead to poor psychological outcomes, mainly following malignancy or unexpected trauma.1
However, both clinical experience and research suggest that the severity of a disfiguring condition does not necessarily predict distress, and that the main factor associated with patients’ adjustment to this condition is their psychological attitude.2–4 For this reason, prosthetic and surgical management of anophthalmic patients should carefully consider the patient’s perceived quality of life together with the objective clinical findings.
The aims of the current review were to analyze the features of the anophthalmic socket syndrome, to examine the management of implant exposure, to describe different orbital volume enhancement procedures, to outline the problems related to socket contraction, to evaluate the treatment options for chronic anophthalmic socket pain and Phantom Eye Syndrome, and to outline the most recent advances in the management of congenital anophthalmia.
Method of Literature Search
The authors performed an electronic database (PubMed, MEDLINE and Google Scholar) search of all articles published in English and non-English language with abstract translated to English on Anophthalmic Socket Syndrome.
A comprehensive literature search strategy was performed up to May 2021 and included articles published between 1980 and 2021. Search included a combination of the following terms: enucleation, evisceration, post enucleation socket syndrome, socket surgery, orbital implants, ocular prosthesis, contracted socket, congenital anophthalmia, custom-made conformers, hydrogel expanders, orbital expansion, dermis-fat graft, mucous membrane graft, secondary orbital implant, subperiosteal implant, fillers in the anophthalmic socket, socket pain, Phantom Eye Syndrome.
Pertinent cross references were obtained from the resultant studies and relevant articles were read and reviewed. Retrospective interventional and observational case series, prospective randomized comparative study, prospective trials, prospective observational cohort studies, prospective noncomparative interventional and observational case series, retrospective consecutive case series, noninterventional case series, nonrandomized clinical trials, noncomparative interventional case series, chart reviews, reviews, case reports, cohort studies, comparative studies, clinical trials, case–control studies, surgical techniques, comments, discussions, letters to editor and book chapters were included. Data reviewed included demographics, presentations, investigations, management, complications and outcomes. No restrictions regarding sex, age, follow-up time, setting or number of participants were applied.
Search and screening of articles were performed by the Authors; titles and abstracts were examined, and duplicates and irrelevant reports were removed. 127 articles were selected, and definitions of the last 15 years were predominantly considered.
Anophthalmic Socket Syndrome
The goals of anophthalmic socket surgery include achieving satisfactory eyelid contour, volume and lining with adequate fornices; transmitting good motility from the implant to the prosthesis; achieving comfort and reasonable symmetry.
Socket discharge and reduced peripheral visual field are the most common complaints of the anophthalmic patients,5 associated with fear of poor cosmetic appearance, related to volume deficit, lid malpositions and reduced motility of the artificial eye.6 Several factors may be responsible for socket inflammation and mucous discharge, as the cleaning regime and the age of the prosthesis.
Daily removal and cleaning of the prosthesis is usually not required and it may disturb the micro-environment of the anophthalmic socket, necessary for the lubrication of the prosthesis. The action of removing and inserting the prosthesis could irritate the conjunctiva and disturb the physiological bacterial flora, that plays a crucial role in the socket homeostasis. As the severity of discharge is related to inflammation, the use of anti-inflammatory eye drops can lead to an improvement of symptoms.7
Polymethyl methacrylate (PMMA) is currently the most commonly used material for prosthetic eyes worldwide, as the potential breakage of cryolite glass may represent a concern. Cryolite glass is still commonly used in Germany, Austria and Switzerland; although the mean reported rate breakage of these prostheses is low, protective glasses are recommended at all time.8
Watering, crusting, and discharge show no significant differences among patients wearing different types of prosthesis.9 However, patients wearing a cryolite glass prosthesis older than 9 months appear to benefit from a prosthesis replacement, as mechanical irritation increases with age-related hydrolytic surface changes of the prosthesis.7 PMMA prostheses are heavier than cryolite glass prostheses of the same volume, and this might have a role in the development of the post-enucleation socket syndrome.10
It is generally accepted that evisceration provides better prosthetic motility and implant stability than enucleation, that is reserved for suspected intraocular malignancy and when there is insufficient sclera to adequately cover the implant.11 Evisceration is also feasible in cases of endophthalmitis and acutely inflamed eyes, as complications such as implant exposure and extrusion rate have been quoted as acceptable.12,13
Previous research has emphasized the importance of emotional outcomes after removal of the eye. Female single anophthalmic patients with a low level of education and young people overall appear to need careful psychological assessment and specific intervention to avoid undesirable consequences, mainly if the anophthalmic condition is related to trauma.5,14–16 Older patients suffer less from the impact of eye removal, as they feel to be accepted by their environment more than young people.17
In any case, the success of the rehabilitation of anophthalmic patients is related to appropriate clinical care of patients and ability to improve their quality of life.5 Anxiety and depression are often underdiagnosed in anophthalmic patients and a psychometric screening is mandatory in their routine clinical evaluation, that should be carried out as a multidisciplinary approach by ophthalmic plastic surgeons, ocularists, general practitioners and psychologists.18–20
Orbital Implants and Management of Implant Exposure
An ideal orbital implant should adequately restore the orbital volume, be biocompatible, have a suitable mechanical resistance, be easily covered by soft vascularized tissue, be cost effective and ensure satisfactory motility of the prosthesis.6,21 Modern orbital implants add peculiar values, such as in situ mouldability or antibacterial and angiogenetic properties.22 However, there are still uncertainties about the roles of integrated (hydroxyapatite (HA), porous polyethylene (PP), bioceramic composites and non-integrated (polymethylmethacrylate (PMMA)/acrylic and silicone) orbital implants.23
PMMA implants are widely used, being economic, easy to place and to remove and giving satisfactory clinical outcomes. Cost–benefit analysis is important when considering the selection of care options and there are reports in the literature suggesting that there are few advantages to the use of porous implants, compared to nonporous implants, when these implants are properly placed in the orbit.24–26 Acrylic implants are useful when treating children, as ease of removal should be considered for the possible need of implant exchange with a larger one with time.27 Moreover, in a study conducted on patients after enucleation for uveal melanoma no major differences between hydroxyapatite and acrylic implants in surgical outcomes and patient satisfaction were noted.28
Wrapping of the implant is important in case of enucleation or secondary implantation, as wrapped orbital implants facilitate muscle suturing, leading to a better motility.29,30 Different wrapping materials have been proposed, such as donor sclera, porcine collagen, fascia lata, bovine pericardium, human rectus abdominal sheath, and posterior auricular muscle.6 Polyglactin mesh wrap minimizes some disadvantages of the autologous tissues, such as inflammation, scarring and infection, and has shown better resistance to the melting of the early phase of other materials, favoring vascularization of the implant.31
However, a large case series of enucleations with primary insertion of unwrapped HA orbital implants showed a low exposure rate, indicating that that absence of wrapping material around these implants may not compromise surgical outcomes and has the benefit of reduced surgical time.32
Porous polyethylene implants apparently have a reduced exposure rate compared to bioceramic implants.33 Late exposures of porous orbital implants have been noted during long-term follow-ups and this may be associated to pegging procedures.34
It is well documented that an adequate surgical technique is essential to avoid implant exposure. In enucleation surgery, end-to-end muscle suturing can reduce the risk of implant exposure by protecting the conjunctiva and promoting fibrovascular ingrowth, with the possible drawback of reduced motility of the prothesis due to muscle fadenisation.32 A retrospective study on unwrapped implants compared the end-to-end recti suturing technique with the orthotropic muscle suturing and demonstrated that the risk of exposure with the orthotropic suturing technique was 8.11 times greater.35
Closure of Tenon’s capsule and conjunctiva in separate layers has been recommended, to give less tension on the wound, reducing the risk of anterior surface breakdown.36 However, according to Verhoekx and coll. no difference was found in the frequency of spheric acrylic implant exposure or extrusion in patients who underwent eye removal with single-layer closure of Tenon’s capsule and conjunctiva compared with patients treated with separate closure of these two layers.37
A temporary tarsorrhaphy helps reducing conjunctival edema and possible conjunctival breakdown, and it increases conformer stability.38 It can be left it in place for 3 to 5 days following evisceration and secondary ball implantation, and up to 3 weeks following a dermis-fat graft.
Management of implant exposure is still debated. Spontaneous healing takes place in a small percentage of cases, and vascularized flaps of conjunctiva may be effective for limited areas of exposures. The high failure rate after attempted repair of exposures with direct closure, scleral or mucous membrane patches could be related to the fact that the underlying cause, that is the anterior migration of the implant, remains untreated.39 Large-area implant exposures (Figure 1) may be managed by a dermis fat graft (DFG)34 or by a myoperiosteal graft, as the thick composite nature of this graft provides an adequate coverage.40,41
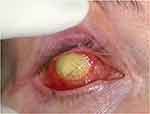 |
Figure 1 Large area of implant exposure: implant removal and DFG is advisable. Note: Image is the property of the authors. |
Porous orbital implant vascularization can be tested with contrast MRI;42 according to our experience, exposed porous implants show a reduction of fibrovascular ingrowth, that results in poor integration of the implant. The exposure allows bacterial colonization of the implant, and simultaneous explantation and DFG is a suitable option.39 Primary replacement of exposed implants, either porous and non-porous, is another possibility and it has shown a successful outcome even in cases with infection.43
Implant migration should be treated when it affects the correct placement of the prosthesis, and options include implant exchange and DFG.44,45 It has been described after evisceration with optic nerve disinsertion. This technique allows for the placement of larger orbital implants and helps in reducing the weight of the prosthesis; migration tends to be lower with porous implants, although nonporous implants can be a reasonable alternative for lower cost.46
According to a recent study, a 3D printing‑assisted custom made implant, positioned in the basin of the inferior orbital fissure, allows recentration of the implant and better prosthetic rehabilitation.47
Volume Enhancement Procedures
Secondary Implants
The purpose of secondary orbital ball implantation is to restore a proper orbital volume and to improve the motility of the prosthesis when no implant had been positioned at the time of primary surgery.48 (Figure 2)
Stable secondary ball implantation can be achieved long term, and extrusion rate appears to be lower in patients with previous evisceration than to patients with previous enucleation, as in patients who had wrapped secondary porous implants following evisceration sclera and polyglactin mesh might act as duplicate barriers between anterior surface of implants and overlying tissues.48
Axmann and Paridaens found a reduced rate of extrusion or exposure after secondary implantation in patients in whom the extraocular muscles were identified and reattached to the implant, in comparison with patients whose extraocular muscles had not been individually identified and sutured to the implant.49 However, localization of extraocular muscles may not be necessary, provided that the implant is placed deep in the orbit above the inferior rectus and lateral to the medial rectus, following careful opening of the deep tissues of the socket.48
According to another study, secondary orbital ball implantation has high complication rates and further surgery is needed in an elevate number of cases, suggesting a shift towards dermis-fat graft as preferred technique.50 A high exposure rate reported by some authors might be due to a superficial positioning of the implant, that drags orbital fat deep down the socket.51
Secondary implantation has been recently proposed through a subciliary approach, and this technique appears to be effective in correcting volume deficiency, with no risk of anterior surface breakdown and exposure.52
DFGs are less expensive than alloplastic implants and eliminate the possibility of extrusion and foreign body reactions. Dermis acts as a scaffold for conjunctival suturing and advancement, and helps to provide vascularization and to reduce the risk of fat atrophy; conjunctival fornices are preserved by suturing the conjunctival remnants to the edge of the graft and the implant can be custom-shaped to obtain deep fornices and satisfactory prosthesis motility.53
Results may vary: a recent study showed better motility following secondary DFG with respect to primary DFG implantation, although the difference was no significant.54 Another research demonstrated instead that 76% of patients undergoing a primary procedure reported good graft motility as compared to 34% of patients undergoing a secondary procedure.46 DFG can be combined with a mucous membrane graft (MMG) if conjunctiva does not allow to create adequate fornices.46
Postoperative fat atrophy should always be considered, and DFG is usually oversized of 10% to 20% to obtain an adequate orbital volume.54,55 (Figure 3)
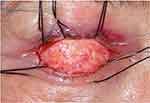 |
Figure 3 Bare DFG positioned in the socket with slight volume oversizing. (Image is the property of the authors). |
Some studies documented a greater fat atrophy after secondary procedures, maybe related to insufficient socket vascularization.46,54 Excessive grafts may lead to compression of the surrounding tissues, can compromise vascularization and cause central necrosis; on the other hand, small grafts may not completely restore an acceptable orbital volume for the possibility of fat atrophy.
DFG is useful in pediatric patients both as primary or secondary implant.56,57 It is a suitable option in children affected by congenital anophthalmia, and it can also be considered to address the volume deficit following explantation of exposed implants and in contracted sockets.56
The main drawback is that DFG is a time-consuming procedure and there is higher morbidity due to an additional surgical site. Graft necrosis may occur in 2.9% of patients after primary procedures and in 4.5% of patients after secondary implantation;46 cyst formation is caused by persistence of epithelial islands and occurs in up to 11% of patients;46,58,59 late shallowing of the fornices and superior sulcus deformity have been described in respectively 20% and 13% of patients.34 Other complications can be granulomas formation, infection, atrophy, keratinization of the socket, hair growth, fat prolapse, seroma and posterior hemorrhage.54,56,60
Subperiosteal Implants
Orbital floor implants are useful if there is the need to repair an orbital floor fracture. This is sometimes overlooked in anophthalmic patients, and may determine an orbital dystopia that should be addressed prior to other volume enhancing surgical procedures.
Sometimes superior sulcus deformity and enophthalmos persist after implantation if the implant is small or if there is an excess of orbital fat reabsorption. In these cases, a subperiosteal implant can enhance the orbital volume if increasing the size of the prosthesis does not compensate for the volume deficiency.61
Implants may be autogenous, such as bone or cartilage grafts, or non autogenous, such as silicon, porous polyethylene, synthetic porous composite of Teflon polymer and alumina,61,62 and can be positioned via a subciliary incision or a transconjunctival approach.
Infection, extrusion or anterior displacement are possible complications. In our experience, a moderate shallowing of the lower fornix postoperatively is not uncommon, and care must be taken in choosing an adequate size and positioning the implant. A skin-muscle approach should be preferred in case of shallow lower fornix. The mobility of the orbital contents and of the upper lid is usually not improved by this procedure.63
Fat Grafting – Dermal Fillers
Volume augmentation with fat grafting and dermal fillers has been proposed as an alternative procedure for rehabilitation of the volume-deficient anophthalmic socket, and can be combined with various surgical techniques.
The ideal filler should be safe, easy to administer, not expensive and have predictable outcomes.64 Orbital fat grafting can result in a gain of orbital fat volume for up to 5 years, and micro-fat grafting produces minimal donor site morbidity.65,66 Complications include a lumpy appearance when fat is injected into the orbicularis oculi muscle and embolism has been reported in case of intravascular injections.65
A DFG positioned at the level of the superior orbital rim periosteum helps to reduce a superior deep sulcus present after positioning of an adequate intraconal implant and a subperiosteal implant.67 The fat is placed to fill the pre-aponeurotic fat pad, the dermis is sutured to the periosteum of the superior orbital rim (Figure 4), and the levator muscle can be advanced at the same time to correct ptosis, if necessary.68 In our experience oversizing of 10% seems adequate in these cases; results are stable after a long follow up and outcomes are more satisfactory than with fat transfer.
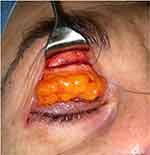 |
Figure 4 DFG to upper sulcus. (Image is the property of the authors). |
Calcium hydroxyapatite gel (Radiesse) is a biocompatible material with longstanding effect and it has been suggested for orbital volume augmentation, with relatively long‑term effects and low complications.69 Volume enhancement using this technique may be however limited to orbits that demonstrate significant fibrosis resulting from multiple surgeries, trauma or radiation.70 Hyaluronic acid fillers are relatively easy to administer, but may migrate and are temporary. However, hyaluronic acid gel can be considered for correction of deep upper sulcus, or enophthalmos related to trauma or phthisis bulbi.
Malhotra described a peculiar technique of injecting Restylane Sub-Q (Q-Med, Uppsala, Sweden) in anophthalmic patients.71 Filler administered in the intraconic and extraconic region through infratemporal transcutaneous injections obtained a 2mm reduction in enophthalmos and an improvement in upper eyelid sulcus with no significant complications.71
Restylane Sub-Q has been evaluated in another series of anophthalmic patients: the reduction of enophthalmos decreased over time and significant loss of volume occurred by 6 months in 2 patients.72
A retrospective case series evaluated the use of Juvederm Voluma (Allergan) in the anophthalmic syndrome. Volume loss was corrected in most cases with a single injection, and only 2 patients required an additional injection. Persistent edema and ptosis requiring surgical treatment were reported as possible complications.73
Another study reported the long-term clinical outcomes of filler injection in patients with a deep superior sulcus, comparing retrobulbar with direct superior sulcus injections. The duration of the deep superior sulcus correction was similar after both injections and both protocols were considered safe and effective.74
The rate of filler absorption in the orbit is faster than in the dermis, and this must be considered and included in patient counselling, as multiple injections are needed to maintain a satisfactory aesthetic appearance and repeated treatment could be a potential source of inflammation.
Self-inflating pellet hydrogel expanders have been originally proposed for orbital volume deficiency in congenital anophthalmia.75 In our experience migration is possible and this may lead to early removal.56 However, transcutaneous intraconal injection of a limited number of self-inflating hydrogel pellets could be considered in case of mild socket volume deficiency.
A system for socket volume enhancement procedures is proposed in Figure 5.
 |
Figure 5 System for socket volume enhancement. (Image is the property of the authors). |
Ptosis in Anophthalmic Patients
Upper eyelid ptosis occurs in 2 to 25% of patients with anophthalmic syndrome.76 Ptosis may be related to trauma, surgery, anatomical modifications of the socket, or to continuous mechanical stimuli for insertion and removal of the prosthesis.77
Adequate primary surgery can improve postoperative eyelid function by maximizing implant size, but patients should be counseled regarding the possibility of developing a ptosis after enucleation or evisceration.78
Mild ptosis can be compensated adjusting the size and the shape of the prosthesis, transposing the levator fulcrum forward and upward. Severe ptosis require surgery and levator muscle advancement through an anterior approach is usually the procedure of choice.6
Encouraging results have been reported with resection of conjunctiva and Müller’s muscle through a posterior approach. However, this procedure should be reserved to patients affected by ptosis and enlarged superior fornix, as it usually produces a satisfactory aesthetic improvement and a reduction of chronic discharge.79 In other anophthalmic patients, posterior approach should be carefully evaluated for the risk of shortening the posterior lamella and shallowing of the upper fornix.
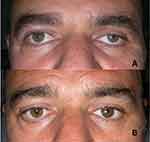
It is mandatory to correct the volume deficit before any eyelid surgery. Although lower lid malposition is usually corrected prior to ptosis correction, the elevation of the lower lid at the time of ptosis correction can be performed in selected patients.6 (Figure 6).
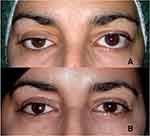 |
Figure 6 (A) Right ptosis and lower lid laxity following secondary implantation. (B) Ptosis corrected simultaneously with right and left lower lid malpositions. (Image is the property of the authors. |
A vertical alignment of the pupils neglecting the position of the upper eyelid with a custom-made temporary prosthesis before surgery is essential, and it helps to reduce the risk of overcorrection.80
Ipsilateral frontalis muscle activation in anophthalmic patients with ptosis is an interesting phenomenon that demonstrates the presence of non-visual stimuli in frontalis recruitment, showing that a sensory trigger - and not a visual stimulus - might be responsible for frontalis recruitment in these patients.81,82 Histologic studies have confirmed the presence of Golgi tendon organs, muscle spindles, and myotendinous cylinders within the levator muscle,83 and neural structures have been demonstrated in Müller’s muscle fibers.84 These nerve terminals may be implicated in proprioception, and the pathway for frontalis contraction might originate in proprioceptive fibers in the Müller’s muscle through the trigeminal ganglion.82 The eyelid, in close contact with the prosthesis, may provide an alternative pathway to stimulate ipsilateral frontalis recruitment.83–85
Contracted Socket
Socket contraction may be due to poor previous surgical technique, early removal of the conformer positioned at surgery, incorrect wearing of the prosthesis, recurrent socket inflammation, previous external beam radiotherapy, immunologic disease such as Stevens Johnson syndrome, chemical or thermal trauma.86,87
An important role in the pathogenesis of socket contraction is due to the activity of the myofibroblasts. Tawfik et al have demonstrated histologically in an animal model that a single injection of mitomycin-C, triamcinolone, 5-Fluorouracil, or bevacizumab could reduce the number of myofibroblasts in an actively healing socket, reducing the tendency to socket contraction.88
Contracted socket can be classified into four categories:86
- Grade I: Minimal contraction due to horizontal lower lid laxity with retraction of the inferior fornix; anteriorly displaced implants.
- Grade II: Mild contracture of superior and/or inferior fornix with upper and/or lower lid entropion.
- Grade III: Advanced scarring with impossibility to wear a prosthesis.
- Grade IV: Phimosis of palpebral fissure; recurrent contraction; irradiated sockets.
In our experience, in grades I and II contraction the use of specially designed progressively larger conformers positioned with a firm pad – socket moulders – may help to allow the socket to achieve enough space to fit a custom prosthesis, avoiding or delaying socket surgery. Upper and lower eyelid entropion can be corrected with standard techniques (Figure 7).
 |
Figure 7 System for contracted socket. (Image is the property of the authors). |
In Grades II and III contraction, fornices may be deepened by positioning autologous spacers: MMG is useful for moist sockets and skin graft for dry sockets.68
Amniotic membrane has been suggested as an alternative in case of mild grade of socket contracture, but previous radiation therapy is a contraindication because of the absence of healthy conjunctival cells that could proliferate and differentiate over it.89,90
Repair of the contracted socket using oral MMG can instead allow the resumption of prosthesis wear also in patients previously submitted to high-dose radiotherapy with severe socket contracture.91 Porous orbital implants together with MMG can be proposed in severely contracted sockets to reconstruct the fornices and stabilize the retention of the implant.92
Auricular cartilage, dermis and hard palate can be used as posterior lamella spacers to correct fornix contraction in patients with moderate or severe retraction, and dermis is more effective in our experience.
DFG can be a viable option up to grade III contraction in cases with no previous radiotherapy, as insufficient socket vascularity acts against composite grafts.68
According to our experience, it may be attempted in moderately trophic sockets following radiotherapy, warning patients of the possibility of failure. In these cases, a large dermis graft with reduced amount of fat can help to keep the graft adequately vascularized. Further lining enhancement with MMG, or volume enhancement with secondary implantation can be delayed to a later stage. Other procedures have been suggested. Composite hard palate and DFG together with adjunctive use of 5-Fluorouracil injections to delay cicatrization may restore volume, lengthen the posterior lamella, and expand the fornices to allow prosthesis retention.93
Mu et al used a temporal superficial artery pedicled skin flap to restore orbital volume and repair severely contracted fornices.94 Sterker and Frerich proposed the use of microvascular radial forearm free flaps to correct severe socket contraction with successful functional outcomes.95
Recently, an innovative technique to reconstruct deep-set sockets with shallow fornices has been proposed by the transplant of an autogenic dermal sphere connected to epidermidis.96
Recurrent contracted sockets are complex situations: when previous surgeries have failed, temporalis muscle transfer or combined surgery together with long-term fixation using three-dimensional custom-made printed conformers could be a feasible option. This method would enable the retention of a prosthesis in previously failed socket surgeries, and staged operations may be avoided.97
Chronic Anophthalmic Socket Pain
Persistent socket pain is related to different pathological entities and to psychological elements, and its incidence rate is difficult to assess for its subjective nature and the heterogeneity of the studies.98
An inadequate prosthesis may determine mechanical damage and irritation by continually rubbing against the socket walls.99 A dry socket, caused by a reduced secretion of tears due to the absence of corneal reflex, can lead to socket pain.100 The eyelids of anophthalmic sockets have a reduced density of meibomian glands acinar units and a more inhomogeneous appearance of the periglandular interstices and the acinar unit walls; in these cases, patients benefit from the regular use of lubricants and anti-inflammatory drops.101 Pain may be due to trochleitis, to supraorbital nerve entrapment or to sensory infraorbital nerve compression.102–105
Trochleitis is relatively rare and is caused by supratrochlear nerve compression. It should be suspected in case of pain evoked by palpation of trochlear region or by movement of the prosthesis. Treatment is based on the use of corticosteroids, prostaglandin synthetase inhibitors and triamcinolone injections,99,103 or implant removal and DFG if due to implant migration.
In case of infraorbital nerve compression, a surgical nerve decompression can be proposed.105 Implant exposure, an infected orbital implant and persistent inflammation can also determine socket pain and may require implant replacement.99,106 Previous surgery may also be the cause of pain, if conjunctival cysts are present or if residues of a silicon band due to previous retinal detachment surgery had not been removed at the time of evisceration.102
Socket pain could also be related to the presence of neuromas in the tissue surrounding the optic nerve and the implant junction. Neuromas can cause pain for mechanical irritation of the surrounding scar tissue or due to pressure derived from adjacent cysts; removal of both the neuroma and the orbital implant may resolve the pain.107,108
Pain can also be caused by malignant tumors of the socket; treatment is specifically related to the type and the extension of the tumor.99,109 Retrobulbar injections of neurolytic agents such as alcohol and chlorpromazine have been used to treat painful blind eyes,110 and can be an effective technique for the resolution of socket pain.111
Many psychological factors play an important role in the maintenance of pain; most patients experience intermittent attacks of pain that last seconds or hours, with intervals ranging from days to weeks.112 If the cause of pain fails to be identified and an implant is present, it should be removed and a DFG can be suggested.107
Phantom Eye Syndrome
The Phantom Eye Syndrome can be described as a perception of the amputated eye area, and pain can be associated.98,113 It may be caused by an alteration of the somatosensory system or by a damage of central or peripheral neurons.113–116 Although the eye has an important somatosensory innervation and a large cortical representation, phantom perceptions are not frequent,114 and a recent report stated that it is present in approximately one third of anophthalmic patients.117 Visual hallucinations are usually elementary and are related to past life. Rasmussen et al reported this phenomenon in 36% of anophthalmic patients studied, but only 1% of these patients experiencing complex visual hallucinations.115
Phantom sensations can be described as paresthesia, dysesthesia or hyperpathia; patients may feel periorbital itching, perceive the presence of eyelids and imagine that they can see with both eyes.98
Various factors could act as trigger, such as fatigue, stress or different lighting.115 Different studies showed the similarity between the triggers and relievers of phantom pain of amputated limb and those of amputated eye, and the duration of pain before eye removal and the risk of developing subsequent phantom pain appear to be related.114,115
Several drugs such can be used for treatment, such as antidepressant, anticonvulsants, sodium channel blockers, N-methyl-D aspartate receptor antagonist and opioids. Psychological support is essential, as all these patients need to reduce anxiety to improve their quality of life.112
Congenital Anophthalmia and Microphthalmia
Congenital anophthalmia is an anomaly in which there is the absence of the eye, determined by a deficiency in the development of the primary optic vesicle early during embryonic development, approximately at 6–10 weeks of gestation, or due to a failure in the formation or closure of the fetal fissure.118–120
Microphthalmia indicates the presence of a hypoplastic eye with corneal diameter less than 11 mm, or axial length less than 21 mm. Both anophthalmia and microphthalmia may occur isolated or as part of complex syndromes.118
Anophthalmia and microphthalmia have an estimated incidence of 3 and 14 per 100,000 births respectively.118 According to a recent study from China, mothers with disease during pregnancy - mainly influenza or common cold infection - and fathers with systemic disease are risk factors specific for congenital anophthalmia, but not for microphthalmia.121
In some cases of clinical anophthalmos imaging may show eye remnants within the orbit, outlining a condition more properly defined as clinical congenital anophthalmia (CCA), that identifies a phenotypic range between anophthalmia and microphthalmia.120
The treatment should aim at simultaneous expansion of the eyelids, socket and orbital bones, and it should begin after birth as soon as possible to achieve a good prosthetic rehabilitation and an adequate facial development.120,122 There is still no consensus about the most appropriate treatment strategy. Although orbital and socket expansion with self-inflating hydrogel expanders appears useful in selected patients, in mild to moderate cases preferred treatment is conservative and is managed by progressively enlarging customized prostheses.120,123,124 Expansion can begin early and it is usually acceptable to parents; satisfactory increase in the horizontal eyelid length can be achieved by means of an outpatient procedure, with no need for hospitalization and repeated general anaesthesia.123,124
Severe cases need instead strategies of orbital volume replacement and lid and socket reconstruction.120,122 Several types of implants or expanders have been proposed. Early dermis-fat graft is considered a feasible option, following adequate lid and socket expansion, as it exerts adequate orbital pressure and continues to expand the eyelids and the soft tissues of the socket.56
The implant may grow at puberty. In our experience, some patients with CCA submitted to early dermis-fat graft needed further surgery up to 15 years following primary surgery, to debulk excessive volume and reconstruct the lateral fornix by transposition of the conjunctivalized dermis.
Patients with microphthalmos need adequate support to detect the possibility of visual development. Cysts associated with microphthalmos can be retained in most cases, as they provide useful orbital volume and allow a proper development of the orbit.125,126 Reasons for cyst excision may be impossibility to fit an adequate conformer, or distortion of the fornices, that usually happens when cysts are too anterior; in all these cases imaging is mandatory. If removal of the eye is necessary, DFG is a viable option.
In case of CCA, early management is crucial for the outcome. A multidisciplinary approach is essential and should include imaging and genetic assessment. Inadequate soft tissues and bony orbital growth is possible in very severe cases, resulting in facial asymmetry, and maldeveloped orbits may need relatively complex craniofacial surgery at a later stage.120
Conclusions
Current clinical evidence does not support a specific orbital implant and acrylic implants, inexpensive and easily available, are still widely used. Understanding the pathophysiology of implant exposure is crucial to preoperative planning. Late exposure of porous implants may be due to pegging,34 a procedure that at present is rather infrequent. History of smoking and immunomodulatory therapy appears associated with higher rates of implant exposure.127
Socket contraction results in significant functional and psychological disability: cure is challenging and there are few studies on the activity of conjunctival myofibroblasts, which are responsible for socket contraction.88
Anophthalmic socket pain and Phantom Eye Syndrome may be misunderstood and have a great impact on affected patients, who need specific counseling.99,117
A standardized protocol adopted by different clinical centers would be auspicable for children with congenital anophthalmia and microphthalmia.120
Adequate clinical care is essential to improve the quality of life of anophthalmic patients.5
Disclosure
The authors report no conflicts of interest for this work.
References
1. Wang KJ, Li SS, Wang HY. Psychological symptoms in anophthalmic patients wearing ocular prosthesis and related factors. Medicine. 2020;99(29):e21338. doi:10.1097/MD.0000000000021338
2. Hatamleh MM, Alnazzawi AA, Abbariki M, Alqudah N, Cook AE. Survey of ocular prosthetics rehabilitation in the United Kingdom, Part 2: anophthalmic patients’ satisfaction and acceptance. J Craniofac Surg. 2017;28(5):1297–1301. doi:10.1097/SCS.0000000000003656
3. McBain HB, Ezra DG, Rose GE, Newman SP. The psychosocial impact of living with an ocular prosthesis. Orbit. 2014;33:39–44. doi:10.3109/01676830.2013.851251
4. Ahn JM, Lee SY, Yoon JS. Health-related quality of life and emotional status of anophthalmic patients in Korea. Am J Ophthalmol. 2010;149:1005–1011. doi:10.1016/j.ajo.2009.12.036
5. Ruiters S, Mombaerts I. The prevalence of anophthalmic socket syndrome and its relation to patient quality of life. Eye (Lond). 2020;35(7):1909–1914. doi:10.1038/s41433-020-01178-2
6. Quaranta-Leoni FM. Treatment of the anophthalmic socket. Curr Opin Ophthalmol. 2008;19(5):422–427. doi:10.1097/ICU.0b013e32830b1551
7. Rokohl AC, Adler W, Koch KR, et al. Cryolite glass prosthetic eyes-the response of the anophthalmic socket. Graefes Arch Clin Exp Ophthalmol. 2019;257(9):2015–2023. doi:10.1007/s00417-019-04395-y
8. Rokohl AC, Trester M, Pine KR, Heindl LM. Risk of breakage of cryolite glass prosthetic eyes. Graefes Arch Clin Exp Ophthalmol. 2019;257(2):437–438. doi:10.1007/s00417-018-4155-x
9. Rokohl AC, Koch KR, Adler W, et al. Concerns of anophthalmic patients-a comparison between cryolite glass and polymethyl methacrylate prosthetic eye wearers. Graefes Arch Clin Exp Ophthalmol. 2018;256(6):1203–1208. doi:10.1007/s00417-018-3942-8
10. Rokohl AC, Trester M, Mor JM, Loreck N, Koch KR, Heindl LM. Customizing a cryolite glass prosthetic eye. J Vis Exp. 2019;(152). doi:10.3791/60016.PMID:31736496.
11. Chiu SJ, Tan JHY, Currie ZI. To implant or not to implant: emergency orbital eviscerations with primary orbital implants. Eye (Lond). 2021. Epub ahead of print. doi:10.1038/s41433-020-01382-0
12. Dresner SC, Karesh JW. Primary implant placement with evisceration in patients with endophthalmitis. Ophthalmology. 2000;107:1661–1664. doi:10.1016/S0161-6420(00)00262-1
13. Tawfik HA, Budin H. Evisceration with primary implant placement in patients with endophthalmitis. Ophthalmology. 2007;114:1100–1103. doi:10.1016/j.ophtha.2006.09.027
14. Ye J, Lou L, Jin K, et al. Vision-related quality of life and appearance concerns are associated with anxiety and depression after eye enucleation: a cross-sectional study. PLoS One. 2015;10:e0136460. doi:10.1371/journal.pone.0136460
15. Wang J, Zhang H, Chen W, Li G. The psychosocial benefits of secondary hydroxyapatite orbital implant insertion and prosthesis wearing for patients with anophthalmia. Ophthalmic Plast Reconstr Surg. 2012;28(5):324–327. doi:10.1097/IOP.0b013e31825238c9
16. Pine NS, De Terte I, Pine KR. The impact of eye loss and prosthetic eye wear on recreational, occupational and social areas of functioning. J Ophthalmol Vis Sci. 2017;2(1):1016.
17. Pine NS, Pine KR. Depression, anxiety and stress indicators for prosthetic eye wearers. Clin Ophthalmol. 2020;14:1715–1723. doi:10.2147/OPTH.S254910
18. Heindl LM, Trester M, Guo Y, et al. Anxiety and depression in patients wearing prosthetic eyes. Graefes Arch Clin Exp Ophthalmol. 2021;259(2):495–503. doi:10.1007/s00417-020-04908-0
19. Pine NS, De Terte I, Pine KR. Time heals: an investigation into how anophthalmic patients feel about eye loss and wearing a prosthetic eye. J Ophthalmol Vis Sci. 2017;2(2):1018.
20. Rokohl AC, Trester M, Pine KR, Heindl LM. Prevention of socket complications in anophthalmic patients. Curr Eye Res. 2020;45(12):1625–1626. doi:10.1080/02713683.2020.1770294
21. Baino F, Potestio I. Orbital implants: state-of-the-art review with emphasis on biomaterials and recent advances. Mater Sci Eng C Mater Biol Appl. 2016;69:1410–1428. doi:10.1016/j.msec.2016.08.003
22. Baino F. How can bioactive glasses be useful in ocular surgery?. J Biomed Mater Res A. 2015;103(3):1259–1275. doi:10.1002/jbm.a.35260
23. Schellini S, El Dib R, Silva LR, Farat JG, Zhang Y, Jorge EC. Integrated versus non-integrated orbital implants for treating anophthalmic sockets. Cochrane Database Syst Rev. 2016;11(11):CD010293.
24. Custer PL, Kennedy RH, Woog JJ, Kaltreider SA, Meyer DR. Orbital implants in enucleation surgery: a report by the American Academy of Ophthalmology. Ophthalmology. 2003;110(10):2054–2061. doi:10.1016/S0161-6420(03)00857-1
25. Viswanathan P, Sagoo MS, Olver JM. UK national survey of enucleation, evisceration and orbital implants trends. Br J Ophthalmol. 2007;91:616–619. doi:10.1136/bjo.2006.103937
26. Quaranta-Leoni FM. Reply re: “Management of porous orbital implants requiring explantation: a clinical and histopathological study”. Ophthalmic Plast Reconstr Surg. 2014;30(6):528–529. doi:10.1097/IOP.0000000000000262
27. Piest KL, Welsh MG. Pediatric enucleation, evisceration, and exenteration techniques. In: Katowitz JA, editor. Pediatric Oculoplastic Surgery. NewYork: Springer; 2002:617–627.
28. Ho VWM, Hussain RN, Czanner G, Sen J, Heimann H, Damato BE. Porous versus nonporous orbital implants after enucleation for uveal melanoma: a randomized study. Ophthalmic Plast Reconstr Surg. 2017;33(6):452–458. doi:10.1097/IOP.0000000000000824
29. Paik JS, Park HY, Cho WK, Yang SW. Effects of secondary porous orbital implantation in anophthalmic sockets. J Craniofac Surg. 2012;23(6):1677–1682. doi:10.1097/SCS.0b013e318266fb7c
30. Yoon JS, Lew H, Kim SJ, Lee SY. Exposure rate of hydroxyapatite orbital implants a 15-year experience of 802 cases. Ophthalmology. 2008;115(3):566–572.e2. doi:10.1016/j.ophtha.2007.06.014
31. Jordan DR, Klapper SR. Wrapping hydroxyapatite implants. Ophthalmic Surg Lasers. 1999;30(5):403–407. doi:10.3928/1542-8877-19990501-14
32. Sobti MM, Shams F, Jawaheer L, Cauchi P, Chadha V. Unwrapped hydroxyapatite orbital implants: our experience in 347 cases. Eye. 2020;34(4):675–682. doi:10.1038/s41433-019-0571-3
33. Schellini S, Jorge E, Sousa R, Burroughs J, El-Dib R. Porous and nonporous orbital implants for treating the anophthalmic socket: a meta-analysis of case series studies. Orbit. 2016;35(2):78–86. doi:10.3109/01676830.2016.1139591
34. Lin CW, Liao SL. Long-term complications of different porous orbital implants: a 21-year review. Br J Ophthalmol. 2017;101(5):681–685. doi:10.1136/bjophthalmol-2016-308932
35. Ye J, Gao Q, He JJ, Gao T, Ning QY, Xie JJ. Exposure rate of unwrapped hydroxyapatite orbital implants in enucleation surgery. Br J Ophthalmol. 2016;100(6):860–865. doi:10.1136/bjophthalmol-2015-307412
36. Custer PL, Trinkaus KM. Porous implant exposure: incidence, management, and morbidity. Ophthal Plast Reconstr Surg. 2007;23:1–7. doi:10.1097/01.iop.0000249432.18688.ee
37. Verhoekx JSN, Rengifo Coolman A, Tse WHW, Paridaens D. A single- versus double-layered closure technique in anophthalmic surgery. Ophthalmic Plast Reconstr Surg. 2017;33(5):329–333. doi:10.1097/IOP.0000000000000784
38. McGrath LA, McNab AA. Temporary suture tarsorrhaphy at the time of orbital ball implantation. Graefes Arch Clin Exp Ophthalmol. 2018;256(12):2437–2441. doi:10.1007/s00417-018-4090-x
39. Quaranta-Leoni FM, Moretti C, Sposato S, Nardoni S, Lambiase A, Bonini S. Management of porous orbital implants requiring explantation: a clinical and histopathological study. Ophthalmic Plast Reconstr Surg. 2014;30(2):132–136. doi:10.1097/IOP.0000000000000028
40. Liao SL, Kao SC, Tseng JH, Lin LL. Surgical coverage of exposed hydroxyapatite implant with retroauricular myoperiosteal graft. Br J Ophthalmol. 2005;89(1):92–95. doi:10.1136/bjo.2003.038778
41. Medel R, Cicinelli MV, Arboleda Hurtado JC, Sánchez España JC, Bahamondes AT, Vasquez LM. Retroauricular myoperiosteal autograft for orbital implant exposure: 11 years of experience. Orbit. 2020;39(5):342–349. doi:10.1080/01676830.2019.1692039
42. Klapper SR, Jordan DR, Ells A, et al. Hydroxyapatite orbital implant vascularization assessed by magnetic resonance imaging. Ophthal Plast Reconstr Surg. 2003;19:46–52. doi:10.1097/00002341-200301000-00006
43. Curragh DS, Kamalarajah S, Lacey B, et al. Primary replacement for the management of exposed orbital implant. Orbit. 2019;38(6):461–467. doi:10.1080/01676830.2019.1573262
44. Jung SK, Cho WK, Paik JS, Yang SW. Long-term surgical outcomes of porous polyethylene orbital implants: a review of 314 cases. Br J Ophthalmol. 2012;96(4):494–498. doi:10.1136/bjophthalmol-2011-300132
45. Nentwich MM, Schebitz-Walter K, Hirneiss C, Hintschich C. Dermis fat grafts as primary and secondary orbital implants. Orbit. 2014;33:33–38. doi:10.3109/01676830.2013.844172
46. Dave TV, Ezeanosike E, Basu S, Ali MJ, Kaliki S, Naik MN. Effect of optic nerve disinsertion during evisceration on nonporous implant migration: a comparative case series and a review of literature. Ophthalmic Plast Reconstr Surg. 2018;34(4):336–341. doi:10.1097/IOP.0000000000000987
47. Dave TV, Tiple S, Vempati S, et al. Low-cost three-dimensional printed orbital template-assisted patient-specific implants for the correction of spherical orbital implant migration. Indian J Ophthalmol. 2018;66(11):1600–1607. doi:10.4103/ijo.IJO_472_18
48. Quaranta-Leoni FM, Sposato S, Lorenzano D. Secondary orbital ball implants after enucleation and evisceration: surgical management, morbidity, and long-term outcome. Ophthalmic Plast Reconstr Surg. 2015;31(2):115–118. doi:10.1097/IOP.0000000000000212
49. Axmann S, Paridaens D. Anterior surface breakdown and implant extrusion following secondary alloplastic orbital implantation surgery. Acta Ophthalmol. 2018;96(3):310–313. doi:10.1111/aos.13611
50. Sundelin KC, Dafgård Kopp EM. Complications associated with secondary orbital implantations. Acta Ophthalmol. 2015;93:679–683. doi:10.1111/aos.12818
51. Sagoo MS, Rose GE. Mechanisms and treatment of extruding intraconal implants: socket aging and tissue restitution (the “Cactus Syndrome”). Arch Ophthalmol. 2007;125:1616–1620. doi:10.1001/archopht.125.12.1616
52. Gawdat TI, Diab MM. Transcutaneous approach for orbital augmentation with alloplastic implants in acquired anophthalmia. Orbit. 2021;40(3):233–238. doi:10.1080/01676830.2020.1767156
53. Migliori ME, Putterman AM. The domed dermis-fat graft orbital implant. Ophthalmic Plast Reconstr Surg. 1991;7:23–30. doi:10.1097/00002341-199103000-00003
54. Jovanovic N, Carniciu AL, Russell WW, Jarocki A, Kahana A. Reconstruction of the orbit and anophthalmic socket using the dermis fat graft: a major review. Ophthalmic Plast Reconstr Surg. 2020;36(6):529–539. doi:10.1097/IOP.0000000000001610
55. Aryasit O, Preechawai P. Indications and results in anophthalmic socket reconstruction using dermis-fat graft. Clin Ophthalmol. 2015;9:795–799. doi:10.2147/OPTH.S77948
56. Quaranta-Leoni FM, Sposato S, Raglione P, Mastromarino A. Dermis-fat graft in children as primary and secondary orbital implant. Ophthalmic Plast Reconstr Surg. 2016;32(3):214–219. doi:10.1097/IOP.0000000000000471
57. Tarantini A, Hintschich C. Primary dermis fat grafting in children. Orbit. 2008;27:363–369. doi:10.1080/01676830802345125
58. Vagefi MR, McMullan TF, Burroughs JR, et al. Autologous dermis graft at the time of evisceration or enucleation. Br J Ophthalmol. 2007;91:1528–1531. doi:10.1136/bjo.2007.115543
59. Baum SH, Schmeling C, Pförtner R, Mohr C. Autologous dermis - fat grafts as primary and secondary orbital transplants before rehabilitation with artificial eyes. J Craniomaxillofac Surg. 2018;46(1):90–97. doi:10.1016/j.jcms.2017.10.016
60. Betharia SM, Patil ND. Dermis fat grafting in contracted socket. Indian J Ophthalmol. 1988;36:110–112.
61. Choi BH, Lee SH, Chung WS. Correction of superior sulcus deformity and enophthalmos with porous high-density polyethylene sheet in anophthalmic patients. Korean J Ophthalmol. 2005;19(3):168–173. doi:10.3341/kjo.2005.19.3.168
62. Jordan DR. Microplate fixation of prefabricated subperiosteal orbital floor implants. Ophthalmic Surg. 1995;26(1):78–79.
63. Rose GE, Sigurdsson H, Collin R. The volume-deficient orbit: clinical characteristics, surgical management, and results after extraperiorbital implantation of Silastic block. Br J Ophthalmol. 1990;74(9):545–550. doi:10.1136/bjo.74.9.545
64. Tan P, Kwong TQ, Malhotra R. Non-aesthetic indications for periocular hyaluronic acid filler treatment: a review. Br J Ophthalmol. 2018;102(6):725–735. doi:10.1136/bjophthalmol-2017-310525
65. Hardy TG, Joshi N, Kelly MH. Orbital volume augmentation with autologous micro-fat grafts. Ophthal Plast Reconstr Surg. 2007;23:445–449. doi:10.1097/IOP.0b013e31815928f8
66. Fox DM. Orbital fat injection: technique and 5-year follow-up. Aesthetic Plast Surg. 2019;43(1):123–132. doi:10.1007/s00266-018-1205-z
67. Rose GE, Collin R. Dermofat grafts to the extraconal orbital space. Br J Ophthalmol. 1992;76:408–411. doi:10.1136/bjo.76.7.408
68. Collin JRO. Enucleation, Evisceration and Socket Surgery. A Manual of Systematic Eyelid Surgery.
69. Aletaha M, Salour H, Yadegary S, Fekri Y, Tavakoli M. Orbital volume augmentation with calcium hydroxyapatite filler in anophthalmic enophthalmos. J Ophthalmic Vis Res. 2017;12(4):397–401. doi:10.4103/jovr.jovr_201_16
70. Vagefi MR, McMullan TF, Burroughs JR, Georgescu D, McCann JD, Anderson RL. Orbital augmentation with injectable calcium hydroxylapatite for correction of postenucleation/ evisceration socket syndrome. Ophthalmic Plast Reconstr Surg. 2011;27(2):90–94. doi:10.1097/IOP.0b013e3181cff9fd
71. Malhotra R. Deep orbital Sub-Q restylane (nonanimal stabilized hyaluronic acid) for orbital volume enhancement in sighted and anophthalmic orbits. Arch Ophthalmol. 2007;125(12):1623–1629. doi:10.1001/archopht.125.12.1623
72. Zamani M, Thyagarajan S, Olver JM. Adjunctive use of hyaluronic acid gel (Restylane Sub-Q) in anophthalmic volume deficient sockets and phthisical eyes. Ophthal Plast Reconstr Surg. 2010;26(4):250–253. doi:10.1097/IOP.0b013e3181ba76f5
73. Crochelet O, Maalouf T, Duron S, Froussart F, Rigal-Sastourné J, George J. Efficacité et tolérance des injections d’acide hyaluronique hautement réticulé dans la prise en charge des énophtalmies. J Fr Ophtalmol. 2012;35:441. doi:10.1016/j.jfo.2012.04.005
74. Sung Y, Goldberg RA, Lew H. Periorbital injection of hyaluronic acid gel in patients with deep superior sulcus. J Craniofac Surg. 2020;31(1):271–273. doi:10.1097/SCS.0000000000006060
75. Schittkowski MD, Guthoff RF. Injectable self-inflating hydrogel pellet expanders for the treatment of orbital volume deficiency in congenital microphthalmos: preliminary results with a new therapeutic approach. Br J Ophthalmol. 2006;90:1173–1177. doi:10.1136/bjo.2006.092478
76. Shams PN, Selva D. Upper eyelid retraction in the anophthalmic socket: review and survey of the Australian and New Zealand Society of Ophthalmic Plastic Surgeons (ANZSOPS). Ophthalmic Plast Reconstr Surg. 2014;30(4):309–312. doi:10.1097/IOP.0000000000000098
77. Custer PL, Maamari RN, Huecker JB, Gordon MO. Anophthalmic ptosis and the effects of enucleation on upper eyelid function. Ophthalmic Plast Reconstr Surg. 2021;37(3S):S80–S84. doi:10.1097/IOP.0000000000001823
78. Shah CT, Hughes MO, Kirzhner M. Anophthalmic syndrome: a review of management. Ophthalmic Plast Reconstr Surg. 2014;30(5):361–365. doi:10.1097/IOP.0000000000000217
79. Jones DF, Lyle CE, Fleming JC. Superior conjunctivoplasty-mullerectomy for correction of chronic discharge and concurrent ptosis in the anophthalmic socket with enlarged superior fornix. Ophthal Plast Reconstr Surg. 2010;26:172–175. doi:10.1097/IOP.0b013e3181b8c49a
80. Mombaerts I, Groet E. Upper eyelid ptosis surgery using a preparatory ocular prosthesis. Ophthal Plast Reconstr Surg. 2009;25:90–93. doi:10.1097/IOP.0b013e3181994d5a
81. Beaulieu R, Andre K, Mancini R. Frontalis muscle contraction and the role of visual deprivation and eyelid proprioception. Ophthalmic Plast Reconstr Surg. 2018;34(6):552–556. doi:10.1097/IOP.0000000000001096
82. Segal KL, Lelli GJ, Djougarian A, Rosenberg CR, McCullough AJ, Lisman RD. Proprioceptive phenomenon with involutional ptosis: evidential findings in anophthalmic ptosis. Ophthalmic Plast Reconstr Surg. 2016;32(2):113–115. doi:10.1097/IOP.0000000000000438
83. Vrcek I, Blumer R, Blandford A, et al. Histologic evaluation of nonvisual afferent sensory upper eyelid proprioception. Ophthalmic Plast Reconstr Surg. 2020;36(1):7–12. doi:10.1097/IOP.0000000000001424
84. Yuzuriha S, Matsuo K, Ishigaki Y, Kikuchi N, Kawagishi K, Moriizumi T. Efferent and afferent innervations of Mueller’s muscle related to involuntary contraction of the levator muscle: important for avoiding injury during eyelid surgery. Br J Plast Surg. 2005;58:42–52. doi:10.1016/j.bjps.2004.06.006
85. Karacalar A, Korkmaz A, Kale A, Kopuz C. Compensatory brow asymmetry: anatomic study and clinical experience. Aesthetic Plast Surg. 2005;29:119–123. doi:10.1007/s00266-004-0086-5
86. Tawfik HA, Raslan AO, Talib N. Surgical management of acquired socket contracture. Curr Opin Ophthalmol. 2009;20(5):406–411. doi:10.1097/ICU.0b013e32832ed85b
87. AlHassan S, Galindo-Ferreiro A, Khandekar R, AlShaikh O, Schellini SA. Deepening fornix technique using central split-medium thickness skin graft to treat contracted anophthalmic sockets. J Craniofac Surg. 2018;29(6):1607–1611. doi:10.1097/SCS.0000000000004601
88. Tawfik HA, Abdulhafez MH, Fouad YA, Rashed HO, Osman WM. Revisiting the role of the myofibroblast in socket surgery: an immunohistochemical study. Ophthalmic Plast Reconstr Surg. 2016;32(4):292–295. doi:10.1097/IOP.0000000000000510
89. Bajaj MS, Pushker N, Singh KK, Chandra M, Ghose S. Evaluation of amniotic membrane grafting in the reconstruction of contracted socket. Ophthal Plast Reconstr Surg. 2006;22:116–120. doi:10.1097/01.iop.0000200887.26015.d4
90. Kumar S, Sugandhi P, Arora R, Pandey PK. Amniotic membrane transplantation versus mucous membrane grafting in anophthalmic contracted socket. Orbit. 2006;25:195–203. doi:10.1080/01676830600575527
91. Nasser QJ, Gombos DS, Williams MD, et al. Management of radiation-induced severe anophthalmic socket contracture in patients with uveal melanoma. Ophthalmic Plast Reconstr Surg. 2012;28(3):208–212. doi:10.1097/IOP.0b013e31824dd9b8
92. Bhattacharjee K, Bhattacharjee H, Kuri G, Das JK, Dey D. Comparative analysis of use of porous orbital implant with mucus membrane graft and dermis fat graft as a primary procedure in reconstruction of severely contracted socket. Indian J Ophthalmol. 2014;62(2):145–153. doi:10.4103/0301-4738.128593
93. Choi CJ, Tran AQ, Tse DT. Hard palate-dermis fat composite graft for reconstruction of contracted anophthalmic socket. Orbit. 2019;38(3):199–204. doi:10.1080/01676830.2018.1505920
94. Mu X, Dong J, Chang T. Correction of the contracted eye socket and orbitozygomatic hypoplasia using postauricular skin flap and temporal fascial flap. J Craniofac Surg. 1999;1:11–17. doi:10.1097/00001665-199901000-00004
95. Sterker I, Frerich B. Secondary reconstruction of the eye socket with a free radial forearm flap. Ophthalmologe. 2007;104:978–982. doi:10.1007/s00347-007-1501-5
96. Tian R, Xu H, Huang X, Wang X, Zhang J, Du Y. Reconstruction of contracted eye socket with autogenic dermal sphere connected to epidermis. J Craniofac Surg. 2018;29(6):1591–1595. doi:10.1097/SCS.0000000000004306
97. Groot AL, Remmers JS, Kloos RJ, Saeed P, Hartong DT. Recurrent contracted sockets treated with personalized, three-dimensionally printed conformers and buccal grafts. Eur J Ophthalmol. 2021;11:11206721211000013.
98. Rasmussen ML. The eye amputated – consequences of eye amputation with emphasis on clinical aspects, phantom eye syndrome and quality of life. Acta Ophthalmol. 2010;88:912. doi:10.1111/j.1755-3768.2010.02068.x
99. Hogeboom CSE, Mourits DL, Ket JCF, Tan HS, Hartong DT, Moll AC. Persistent socket pain postenucleation and post evisceration: a systematic review. Acta Ophthalmol. 2018;96(7):661–672. doi:10.1111/aos.13688
100. Rokohl AC, Trester M, Guo Y, et al. Dry anophthalmic socket syndrome - standardized clinical evaluation of symptoms and signs. Ocul Surf. 2020;18(3):453–459. doi:10.1016/j.jtos.2020.05.001
101. Rokohl AC, Trester M, Naderi P, et al. Dry anophthalmic socket syndrome - morphological alterations in meibomian glands. Eye (Lond). 2021. doi:10.1038/s41433-021-01426-z
102. Shah-Desai SD, Tyers AG, Manners RM. Painful blind eye: efficacy of enucleation and evisceration in resolving ocular pain. Br J Ophthalmol. 2000;84:437–438. doi:10.1136/bjo.84.4.437
103. Jordan DR, Bawazeer A. Experience with 120 synthetic hydroxyapatite implants (FCI3). Ophthalmic Plast Reconstr Surg. 2001;17:184–190. doi:10.1097/00002341-200105000-00007
104. Glatt HJ, Googe PB, Powers T, Apple DJ. Anophthalmic socket pain. Am J Ophthalmol. 1993;116:357–362. doi:10.1016/S0002-9394(14)71354-6
105. Bailey K, Ng JD, Hwang PH, Saulny SM, Holck DE, Rubin PA. Infraorbital nerve surgical decompression for chronic infraorbital nerve hyperesthesia. Ophthalmic Plast Reconstr Surg. 2007;23:49–51. doi:10.1097/IOP.0b013e31802dd3fc
106. Shams PN, Bohman E, Baker MS, Maltry AC, Kopp ED, Allen RC. Chronic anophthalmic socket pain treated by implant removal and dermis fat graft. Br J Ophthalmol. 2015;99(12):1692–1696. doi:10.1136/bjophthalmol-2014-306585
107. Messmer EP, Camara J, Boniuk M, Font RL. Amputation neuroma of the orbit. Report of two cases and review of the literature. Ophthalmology. 1984;91:1420–1423. doi:10.1016/S0161-6420(84)34134-3
108. Sutula FC, Weiter JJ. Orbital socket pain after injury. Am J Ophthalmol. 1980;90(5):692–696. doi:10.1016/S0002-9394(14)75139-6
109. Nguyen J, Ivan D, Esmaeli B. Conjunctival squamous cell carcinoma in the anophthalmic socket. Ophthalmic Plast Reconstr Surg. 2008;24:98–101. doi:10.1097/IOP.0b013e31816381fa
110. Al-faran MF, Al-omar OM. Retrobulbar alcohol injection in blind painful eyes. Ann Ophthalmol. 1990;22:460–462.
111. Schittkowski MP. Alcohol injection in a patient with chronic orbital pain after enucleation - a case report and review of the literature. Klin Monbl Augenheilkd. 2017;234:20–25.
112. Andreotti AM, Goiato MC, Pellizzer EP, et al. Phantom eye syndrome: a review of the literature. ScientificWorldJournal. 2014;2014:1–6. doi:10.1155/2014/686493
113. Rasmussen ML, Prause JU, Toft PB. Phantom pain after eye amputation. Acta Ophthalmol. 2011;89:10–16. doi:10.1111/j.1755-3768.2010.02058.x
114. Soroos P, Vo O, Husstedt IW, Evers S, Gerding H. Phantom eye syndrome – its prevalence, phenomenology, and putative mechanisms. Neurology. 2003;60:1542–1543. doi:10.1212/01.WNL.0000059547.68899.F5
115. Roed Rasmussen ML, Prause JU, Johnson M, Toft PB. Phantom eye syndrome: types of visual hallucinations and related phenomena. Ophthalmic Plast Reconstr Surg. 2009;25:390–393. doi:10.1097/IOP.0b013e3181b54b06
116. Hope-Stone L, Brown SL, Heimann H, Damato B, Salmon P. Phantom eye syndrome: patient experiences after enucleation for uveal melanoma. Ophthalmology. 2015;122:1585–1590. doi:10.1016/j.ophtha.2015.04.005
117. Martel A, Baillif S, Thomas P, et al. Phantom vision after eye removal: prevalence, features and related risk factors. Br J Ophthalmol. 2021. Epub ahead of print. doi:10.1136/bjophthalmol-2021-319091
118. Verma AS, FitzPatrick DR. Anophthalmia and microphthalmia. Orphanet J Rare Dis. 2007;2:47. doi:10.1186/1750-1172-2-47
119. Fuhrmann S. Eye morphogenesis and patterning of the optic vesicle. Curr Top Dev Biol. 2010;93:61–84.
120. Quaranta-Leoni FM. Congenital anophthalmia: current concepts in management. Curr Opin Ophthalmol. 2011;22(5):380–384. doi:10.1097/ICU.0b013e328349948a
121. Li Y, Hou Z, Ding J, Cui Y, Qin B, Li D. Distinguishing risk factors between congenital anophthalmia and microphthalmia using multivariable logistic regression. Ann Transl Med. 2020;8(11):704. doi:10.21037/atm.2019.12.103
122. Ragge NK, Subak-Sharpe ID, Collin JRO. A practical guide to the management of anophthalmia and microphthalmia. Eye. 2007;21:1290–1300. doi:10.1038/sj.eye.6702858
123. El Essawy RA, Abdelbaky SH. Successful conjunctival socket expansion in anophthalmic patients until the age of 2 years: an outpatient procedure. Clin Ophthalmol. 2016;10:1743–1748. doi:10.2147/OPTH.S109486
124. Changal N, Khandekar RB. Eye conformers as socket expanders in children: experience at a Tertiary Eye Hospital in Central Saudi Arabia. Cureus. 2021;13(2):e13465.
125. McLean CJ, Ragge NK, Jones RB, Collin JRO. The management of orbital cysts associated with congenital microphthalmos and anophthalmos. Br J Ophthalmol. 2003;87:860–863. doi:10.1136/bjo.87.7.860
126. Hamal D, Kafle PA, Poudyal P, Saiju R, Kc H, Kafle S. Congenital microphthalmia with orbital cyst: a case series. JNMA J Nepal Med Assoc. 2019;57(217):193–197.
127. Habib LA, North VS, Freitag SK, Yoon MK, Lefebvre DR, Grace Lee N. Medical comorbidities and orbital implant exposure. Acta Ophthalmol. 2021. Epub ahead of print. doi:10.1111/aos.14973
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.