Back to Journals » Vascular Health and Risk Management » Volume 18
Angiotensin-Converting Enzyme Inhibitor Dose Optimization and Its Associated Factors at Felege Hiwot Comprehensive Specialized Hospital, Bahir Dar, Ethiopia
Authors Gelaye AT, Seid MA , Baffa LD
Received 18 February 2022
Accepted for publication 8 June 2022
Published 7 July 2022 Volume 2022:18 Pages 481—493
DOI https://doi.org/10.2147/VHRM.S363051
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Abebech Tewabe Gelaye,1 Mohammed Assen Seid,1 Lemlem Daniel Baffa2
1Department of Clinical Pharmacy, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Human Nutrition, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Abebech Tewabe Gelaye, Email [email protected]
Introduction: Angiotensin-converting enzyme inhibitors dose optimizations (ACEIs) are essential to boost the treatment outcome in heart failure patients (HF) with reduced ejection fraction. Therefore, the main purpose of this study was to evaluate dose optimization and associated factors of ACEIs among HF patients.
Method: An institutional-based retrospective study was conducted on 256 study participants from May 20 to August 30, 2020 in ambulatory care clinic at Felege Hiwot Comprehensive Specialized Hospital. A systematic random sampling method was carried out to select study participants. Data were collected from the patient interview and the review of medical records. Epidata and SPSS version 22 were used for data entry and analysis. A bivariate logistic regression analysis was done to determine the association of independent variables with a dose optimization of ACEIs.
Results: The mean age of the subjects in the study was 53.82 years with a standard deviation (SD) of 17.067 and more than half of (60.9%) the patients were unable to read and write. Among participants who were receiving ACEIs, only 30.6% were taking an optimal dose. Age ≥ 65 years (AOR 5.04 (2.81– 12.56)) and a dose of furosemide ≥ 40 mg (AOR, 2.62 (1.28– 16.74)) were significantly associated with the suboptimal dose of ACEIs.
Conclusion: Only one-third of patients received the optimum dose of ACEIs. Older age and dose of furosemide greater > 40 mg were significantly associated with suboptimal dosing of ACEIs. Therefore, more attention must be given to older patients with HF in order to optimize the dose of ACEIs administered.
Keywords: ACEIS, dose optimization, HF, Ethiopia
Introduction
Heart failure (HF) is a diverse clinical syndrome caused by any structural or functional impairment of ventricular filling or blood ejection.1,2 It is an overwhelming illness that is associated with a high burden of morbidity and mortality, reduced quality of life, and increased health care expenditure in most developed and some developing countries.3–5 Most HF patients have comorbidities.6 Comorbidity of HF patients increased with age and may worsen the disease condition and clinical severity of HF.7
Nowadays, therapeutic policies have been deliberated to counter the progression of heart failure and to increase ‘long term lives by using ACEIs for HF patients, which is measured currently as one of the important steps towards effective treatment of patients with HF.8 The available evidence recommends that in chronic HF patients, high doses of ACEIs are more effective than low doses. Clinical trials targeting of ACEIs dosing regimens recognized mortality and morbidity benefits when increased properly.9,10 Evidence-based HF guidelines recommend increasing ACEIs to a target dose unless there is a tolerability problem.1,2,11
Concerning the tolerability of ACEIs, renal function, serum potassium, signs and symptoms of cough and angioedema should be measured within 1–2 weeks from the start of treatment.1,11,12 ACEIs are generally well tolerated and high dose can be reached and maintained in the majority of patients with HF if increased properly, but some side effects such as hyperkalemia and hypotension might occur while using high dose of ACEIs.13,14 However, a study conducted in Sweden reported that 77% of patients practiced angioedema within the first 3 weeks of starting treatment.15 Several prospective observational studies have stated that patients with HF discharged from hospital and continued with “high” ACEIs doses have improved clinical outcomes compared to those receiving low-dose treatment. The benefit includes decreasing patients symptom status, low rates of death, and re-hospitalization, thus acquiring low cost.16,17 However, many studies reported that large numbers of patients receiving under the target dose of ACEIs.18–20 Mostly underdosing of ACEIs reduces morbidity and mortality rates of HF patients.21 The target daily doses of ACEIs are 20–40 mg enalapril, 10 mg Ramipril, 150 mg captopril, 20–40 mg Lisinopril, 40 mg fosinopril, 4 mg trandolapril, 40 mg quinapril, or 8–16 mg perindopril.1,2 In this hospital, the study of dose optimization of ACEIs has not been addressed. Therefore, this study aimed to evaluate dose optimization and its associated factors of ACEIs in the treatment of ambulatory heart failure patients.
Materials and Methods
Study Setting and Period
The study was carried out at Felege Hiwot Comprehensive and Specialized Hospital (FHCSH) from May 20, 2020, to August 30, 2020. FHCSH is located in Bahir Dar (the capital city of Amhara National Regional State), 562 km northwest of Addis Ababa. The hospital is expected to serve more than seven million people in its catchment area. It has 400 beds and 15 adult outpatient departments. It serves as a specialized and follow-up clinic for patients with chronic diseases. Chronic illness care is one of the services in the Hospital. The hospital provides for the population both within and outside of Bahir-Dar city. Delivery of treatments for chronic heart failure patients is one of the chronic illnesses included in chronic follow-up care.
Study Design
An institutional-based retrospective study was conducted to evaluate dose optimization and associated factors of ACEIs medications among heart failure patients at Felege Hiwot Comprehensive and Specialized Hospital, ambulatory clinic Northwest Ethiopia, 2020.
Source and Study Population
The source population was all adult ambulatory HF patients who were undergoing HF treatments.
The study population in this study was all adult ambulatory HF patients who were obtaining services at the cardiology unit of FHCSH and whose treatment regimen on ACEIs.
Eligibility Criteria
Ambulatory HF patients who had a regular follow-up at least 3 months, Patients ≥18 years old, and with Ejection fraction <40% confirmed by Echocardiogram were included in the study. In contrast, ambulatory HF patients who were using ACEIs for <3 months and with no baseline data or incomplete medical chart (whose ejection fraction was not recorded) were excluded from the study.
Sample Size Determination and Sampling Procedures
A sample of 388 was calculated by using 35.7% proportion of ACEIs dose optimization among patients with HF22, a confidence interval of 95%, the margin error 5%, and 10% of contingency for nonresponse rate. From a total of 388 study patients approached, 132 study participants were excluded from the study due to their follow-up period being less than three months, no baseline data (EF was not recorded), and involuntary participation in this study. The study participants were picked from heart failure patients who visited the ambulatory clinic during the data collection period. We have used a systematic random sampling technique to select a sample population, and the sampling interval k was calculated by dividing the projected number of HF patients on ACEIs attending the ambulatory cardiac clinic for three months by the total calculated sample size. In this study, the projected number of HF patients on ACEIs for three months was 850 and the calculated total sample size was 388 therefore k=850/388=2.19, then Every 2nd participant was selected using their medical record order.
Study Variables
The dose optimization of ACEIs was the primary outcome (dependent variable) of this study. Besides to this, the socio-demographic variables (sex and age), place of residence and clinical characteristics such as causes of heart failure (HTN), atrial fibrillation (AF), dilated cardiomyopathy (DCMP), chronic rheumatic heart disease (CRHD), anemia, chronic obstructive pulmonary disease, thyroid disorder, and renal disease), cardiac medications used for ambulatory management of chronic HF, New York Heart Association (NYHA) functional class and ACCF/AHA staging system were considered as independent variables.
Data Collection Instrument and Procedure
Data were collected by three qualified data collectors, two nurses and one pharmacist, by reviewing the patient’s medical record for four-month duration. A structured interviewer-administered questionnaire was used for socio-demographic characteristics, which was designed by reviewing relevant literature keeping physical distancing (due to COVID-19).19,22,23 The data collection tools had two parts. The first section composed questions about the participant’s socio-demographic characteristics, which were obtained via interviewer-administered questionnaire. The second section was about clinical and treatment-related characteristics of HF patients with reduced ejection fraction on ACEIs which were extracted from medical charts. The clinical and treatment-related characteristics consist of comorbidity such as hypertension, diabetes mellitus, hospitalization and combination of HF medications. Data were collected from their medical charts, and sociodemographic characteristics were gained from the patients.
Data Quality Control
Training was given to data collectors for two days. The training was concerning on data handling, participants’ approach and also on ethical aspects of data collection. Those trained data collectors were conquered to supervision by a single supervisor. The questionnaire was pre-tested in 10% of sample size in the University of Gondar comprehensive and specialized hospital to assess the understandability and availability of the data. Daily close supervision was done during the period of data collection. The questionnaire was revised and checked for completeness, accuracy, and consistency by the supervisor.
Data Processing and Analysis
The data was coded and entered into Epidata version 4.4 software and then transferred to SPSS version 22 software for analysis. The descriptive statistics were described using frequency distribution and measures of central tendency. The association between the dependent variable and independent variables was identified using a binary logistic regression model. The independent variable with p-value less than 0.2 in univariable analysis was involved in the multivariable binary logistic regression model to identify factors of dose optimization of ACEIs.
The strength of association between the dependent variables and associated factors was identified by using an adjusted odds ratio (AOR) with a p-value <0.05 and 95% CI. Model fitness was checked, and multi-collinearity was tested by running a false linear regression restating the independent variables as a dependent variable, and the result showed all the variance inflation factor (VIF) values to be less than three and tolerance less than ten which established the absence of multicollinearity.24 The data were also checked for outliers by box plot and no beating outlier effect was detected. The models’ fit of goodness was also checked using omnibus tests of model coefficients for the overall (global) fitness of the model and Hosmer and Lemeshow test for goodness fit of the model. Therefore, the omnibus test result was significant with a p-value = 0.001, and the Hosmer and Lemeshow test shows good model fit with a p-value=1.00, which indicates the goodness fit of the model.25
Ethics Approval and Consent to Participate
Ethical clearance was obtained from the University of Gondar, College of Medicine and Health Sciences, School of Pharmacy, Research and Ethical Review Committee before the data was collected. Necessary permission was gained from the Felege Hiwot Comprehensive and Specialized Referral Hospital. The respondents were informed about the importance of the study, and their consent to participate in the study was obtained. The data were gathered after receiving written informed consent and maintaining the confidentiality of the information. The study was conducted using the criteria established by the Declaration of Helsinki.
Operational Definitions
The doses of ACEIs were said to be optimal if it was given based on the guideline-recommended target dose or a maximum tolerable dose was given to the patients, whereas the dose was said to be suboptimal if the patient was receiving any dose of ACEIs below the target dose in the absence of contraindications to reach the maximum dose. ACEIs were considered to be “tolerated” if the blood pressure was greater than 90/60 mmHg, serum creatinine was less than 3 mg/dL, and serum potassium was <5.5 mEq/L, and there was no history or current complaint of cough or angioedema. Otherwise, the ACEIs were considered to be ‘non-tolerated.
Results
Sociodemographic Characteristics
Socio-demographic and clinical issues of the study participants are summarized in Table 1.
 |
Table 1 Sociodemographic Related Characteristics of HF Patients Taking ACEIs at the Cardiac Clinic of FHCSH, 2020. (n = 256) |
388 study subjects were approached in this study from May 20 to August 30, 2020. Finally, 256 HF patients were involved in this study. More than half (63.7%) of the patients were females. The majority of the study subjects were dwelling in urban areas (53.3%). In addition to this, the mean age of study subjects was 53.82 years with a standard deviation (SD) of ± 17.067, and more than half of 156 (60.9%) patients were unable to read and write (Table 1).
Clinical and Medication-Related Characteristics
The majority of (63.7%) the study participants were hospitalized two or more times during their treatment period. Almost all of the study participants 251 (96.9%) had one or more comorbidities. The most commonly known comorbidity was hypertension 113 (44.1%) followed by diabetes mellitus 78 (30.5%). The known cause of HF was dilated cardiomyopathy 81 (31.3%) followed by anemia 62 (23.9). The median duration of diagnosis of HF for the patients was 4 years (interquartile range was 2 to 6 years) (Table 2).
 |
Table 2 Clinical and Medication Related Characteristics of HF Patients Taking ACEIs at the Cardiac Clinic of FHCSH, 2020. (n = 256) |
Combination of HF Medication
The most commonly used combination of HF medications were ACEIs and loop diuretics 107 (29.1%) followed by a combination of ACEIs with calcium channel blocker and statin 40 (10.7) (Table 3).
 |
Table 3 Common Combination of HF Medications at FHCSH (n = 256) |
The ACCF/AHA Staging System and NYHA Class of HF Patients
According to the ACCF/AHA staging system, 185 (71.7%) of the patients had stage “C” HF. In addition to staging, the most predominant category of NYHA class was class IV, which accounted for 178 (69.0%) followed by class III 44 (17.1%) (Figures 1 and 2) and the mean EF of heart failure patients were 28.47± 6.374.
 |
Figure 1 Proportion of ACCF/AHA staging system among heart failure patients taking ACEIs at the cardiac clinic of FHCSH. |
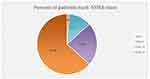 |
Figure 2 Proportion of NYHA classification system among heart failure patients taking ACEIs at the cardiac clinic of FHCSH. |
Dose Optimization and Overall Tolerability of ACEIs
Among participants who were receiving ACEIs, 30.6% [95% CI (24.6–36.3)] were taking optimal dose. Regarding the toleration using blood pressure, potassium level, serum creatinine, cough, and angioedema as a monitoring parameter, 89.1% [95% CI (85.0–92.2)] of the study participants were tolerating to the ACEIs (Table 4).
 |
Table 4 Tolerability, and Dose Optimization ACEIs Treatment at FHCSH (n = 256) |
Among patients who were receiving ACEIs 15 (5.9%) discontinued the treatment and 11 (4.3%) of patients were reducing the dose. From discontinued patients of the medication 12 (4.7%) of the patient had a known reason and 3(1.2%) patients did not have any known reason (Table 5).
 |
Table 5 Presence of Cough, Angioedema and Tolerability of ACEIs During Different Dose Titration Periods, of the Study Participants at the Cardiac Clinic of FHCSH, 2020 (n = 256) |
Factors Associated with Dose Optimization of ACEIs
In bivariate logistic regression analysis, it was shown that age, the total number of medications at diagnosis and current dose of furosemide at diagnosis, ischemic heart disease, hypertension, and diabetes mellitus were significantly associated with the dose optimization of ACEIs. However, in the multivariable regression model, only dose of furosemide at diagnosis and age of the patient were significantly associated with the dose optimization of ACEIs. Those participants aged ≥65 years old AOR (5.01 95%, CI (2.89–10.95)) was more than five times sub-optimized than those aged <65 years. And those participants who were taking furosemide dose at diagnosis ≥40 mg AOR (2.12, 95% CI (1.43–15.74)) was more than twice as sub-optimized as those who were taking <40 mg (see Table 6).
 |
Table 6 Factor Associated with the Dose Optimization of ACEIs Among HF Patients at FHCSH 2020 (n = 256) |
Discussion
This study assessed to explain the dose optimization and associated factors of ACEIs among ambulatory HF patients following at FHCSH. Assessment of dose optimization of ACEI is vital to provide important information for clinicians working in the management of chronic HF patients.26 Studies have shown a dose-related clinical benefit of ACEIs therapy in HF patients,27 and a higher dose was related to a better treatment outcome.28 Therefore, evidence-based guidelines recommend up-titration (increasing) of ACEIs to a target dose unless there is a tolerability problem.1,2,29 Most of the study participants in our study were tolerating ACEIs (89.1%) [95% CI (85.0–92.2)] considering potassium level, serum creatinine, cough, angioedema, and blood pressure were a monitoring parameter. A possible investigation of this study indicated that most of the patients were continued at the starting dose without increasing for a long period of time and also patients could not state the potential side effects of ACEIs. This percentage of tolerability (89.1%) is in line with the studies done in 13 European countries which showed that a tolerability rate (90.2%) of patients were tolerated to ACEIs.30 This is correlated due to the similarity of socio-demographic and clinical characteristics.17 Another reason might be that most patients were continued on a similar dose to the first dose without reaching the maximum dose.
Concerning dose optimization of ACEIs, the majority of the study participants (69.4%) [95% CI (63.3–74.7)] were sub-optimized. This study was consistent with other similar studies done in England that reported 69%31 and also in line with the study done in Ethiopia Jimma (64.7%)22 and Mekele (72.7%).23 Possible reasons for the low dose of ACEIs were associated with fear of adverse effects and patients intolerant to higher doses. Another explanation might be that some studies suggested that clinicians often prefer the use of low doses of ACEIs and had the knowledge that low doses are as effective as high doses.32 In contrast, this finding was relatively different from another study in Germen33 in which 62% of the ACEIs doses were at the guideline-recommended target dose. This might be due to the difference in medical doctor experience, which was general practitioners tend to prescribe higher doses than specialists and in the previous study, those patients had good adherence to the ACEIs.34,35
As observed in our study, there might be less understanding of the benefits of maximum dose of ACEIs. As a result, prescribing suboptimal dose was practiced. In our current study, the age of the study participants and the dose of furosemide at diagnosis were significantly associated with the dose optimization of ACEIs. Even though studies have shown that the maximum dose of ACEIs had long-term benefits in older individuals with systolic HF,36 older patients (age ≥65) were more likely to receive a suboptimal dose of ACEIs compared to those of younger ages (age < 65) in this study. Despite optimal doses were being practicable in the majority of older patients with HF37, (61.4%) of the older patients were receiving a suboptimal dose of ACEIs in the present study. This could be due to the reason that medical doctors may forget to prescribe a high dose of ACEIs for older patients due to fear of the side effect of ACEIs which includes hyperkalemia, hypotension, the elevation of creatinine and cough which are more common in older patients than younger patients.38–40
The dose of furosemide was significantly associated with the suboptimal dose of ACEIs, which is in line with other similar studies.22,41 This can be justified following previous studies. First, higher doses of loop diuretics at baseline are associated with more severe heart failure, more congestion, worsening renal function, and worse outcomes.41 Second, this could be explained by the reason that a high dose of diuretics can increase the risk of hypotension and renal insufficiency with ACEIs causing volume depletion.10 This result can also be explained by a significant association between higher loop diuretic dosage and less successful up-titration of guideline-recommended doses of ACEIs.42 Even though there is an improvement in patients’ symptoms of congestion, the dose of loop diuretics is not reduced which causes poor up-titration of ACEIs.11,41 Mainly, the dose of furosemide needs to be optimized to allow titration of ACEIs to the target dose. So, clinicians should prescribe a dose of diuretics with careful attention taken for their negative effect of furosemide on the up-titration of ACEIs.
Limitations of the Study
This study had the following limitations. First, its retrospective nature might limit the cause-and-effect relationship between dependent and independent variables and also did not allow follow-up of the study participants. Second, the apparent dose optimization of ACEIs may in some cases reflect undocumented contraindications. This may be the cause for incremental suboptimal doses of ACEIs. Third, due to secondary data being used since important variables might have been missed and the diagnosis only considered the base-line information, this is difficult to clarify the correct NYHA class of HF patients. It would be better if a prospective cohort study could be done. In addition, non-included confounding factors like adherence may have a potential contribution to bias for the optimal dose of ACEIs.
Conclusion
This study suggests a baseline for dose optimization practice of ACEIs in the treatment of chronic heart failure patients, which remains the determined priority. However, old age (≥65) and dose of furosemide ≥40 were significantly associated with suboptimal dosing of ACEIs.
It is better to widespread ACEIs dose optimization in heart failure patients with LVSD and highlights the ongoing need for efforts to optimize the dose of ACEIs. We recommended the involvement of clinical pharmacists in the medication review and patient monitoring process at ambulatory care clinic for optimization of ACEIs and reaching definite outcomes in patients with HF. In addition, more efforts need to be made to minimize potentially modifiable risk factors for suboptimal doses of ACEIs in HF patients. It is better to conduct prospective studies with a larger sample size to fully understand the effect of those factors on dose optimization and associated factors of ACEIs among HF patients.
Abbreviations
ACCF/AHA, American College of Cardiology Foundation/American Heart Association; ACEIs, angiotensin-converting enzyme inhibitors; AF, atrial fibrillation; AOR, adjusted odds ratio; BB, Beta-blockers; CKD, chronic kidney disease; COR, crude odds ratio; CI, confidence interval; DM, diabetes mellitus; FHCSRH, Felege Hiwot Comprehensive and Specialized Referral Hospital; HF, heart failure; HFrEF, heart failure reduced ejection fraction; HTN, hypertension; IHD, ischemic heart disease; NYHA, New York Heart Association; RVHD, rheumatic valvular heart disease; SD, standard deviation; VHD, valvular heart disease; LVSD, left ventricular systolic dysfunction.
Data Sharing Statement
The data sets generated and/or analyzed during the present study are not available in public due to the obligation of confidentiality upon which the study was accepted by the Ethical Review Committee and consent was protected from the officially authorized representatives but it is obtainable from the corresponding author on reasonable request.
Ethical Approval
Ethical clearance was obtained from the University of Gondar, College of Medicine and Health Sciences, School of Pharmacy, Research and Ethical Review Committee before the data was collected. Necessary permission was gained from the Felege Hiwot Comprehensive and Specialized Referral Hospital. The study was conducted using the criteria established by the Declaration of Helsinki.
Consent to Publish
Participants’ consent was taken to publish this work.
Acknowledgments
We would like to acknowledge the University of Gondar for funding this research project, supporting and facilitating the study. We are also thankful to the study participants for their collaboration and participation in the study and to the data collectors and supervisors for their contribution to this research.
Author Contributions
All authors made extensive contributions to the conception, design, and acquisition of data; analysis and interpretation of data; took part in drafting the article and revising it extremely for important intellectual content; agreed to submit the current journal; gave final approval for the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This research was done with the financial support of the University of Gondar. The funding agencies had no direct or indirect participation in the study write-up and analysis.
Disclosure
The authors reported no potential conflicts of interest with respect to this work, the research, authorship, and publication of this article.
References
1. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. doi:10.1016/j.jacc.2013.05.019
2. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi:10.1093/eurheartj/ehw128
3. McMurray JJJV. Improving outcomes in heart failure: a personal perspective†. Eur Heart J. 2015;36(48):3467–3470. doi:10.1093/eurheartj/ehv565
4. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7–11. doi:10.15420/cfr.2016:25:2
5. Agbor VN, Essouma M, Ntusi NAB, Nyaga UF, Bigna JJ, Noubiap JJ. Heart failure in sub-Saharan Africa: A contemporaneous systematic review and meta-analysis. Int J Cardiol. 2018;257:207–215. doi:10.1016/j.ijcard.2017.12.048
6. Widmer F. [Comorbidity in heart failure]. Ther Umsch. 2011;68(2):103–106. German. doi:10.1024/0040-5930/a000127
7. Lawson CA, Solis-Trapala I, Dahlstrom U, et al. Comorbidity health pathways in heart failure patients: a sequences-of-regressions analysis using cross-sectional data from 10,575 patients in the Swedish heart failure registry. PLoS Med. 2018;15:e1002540. doi:10.1371/journal.pmed.1002540
8. Sleiman O, Ghanem W, Murin J. Angiotensin-converting enzyme inhibitors: do we utilize our knowledge in heart failure patients? J Clin Basic Cardiol. 2001;4(4):279–283.
9. Konstam MA, Neaton JD, Dickstein K, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374(9704):1840–1848. doi:10.1016/S0140-6736(09)61913-9
10. Cowie MR, Schöpe J, Wagenpfeil S, et al. Patient factors associated with titration of medical therapy in patients with heart failure with reduced ejection fraction: data from the QUALIFY international registry. ESC Heart Fail. 2021;8(2):861–871. doi:10.1002/ehf2.13237
11. Wang -C-C, Wu C-K, Tsai M-L, et al. 2019 Focused update of the guidelines of the Taiwan society of cardiology for the diagnosis and treatment of heart failure. Acta Cardiol Sin. 2019;35(3):244–283. doi:10.6515/ACS.201905_35(3).20190422A
12. Massie BM, Armstrong PW, Cleland JG, et al. Toleration of high doses of angiotensin-converting enzyme inhibitors in patients with chronic heart failure: results from the ATLAS trial. Arch Intern Med. 2001;161(2):165–171. doi:10.1001/archinte.161.2.165
13. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93(9):1137–1146. doi:10.1136/hrt.2003.025270
14. Simpson CR, Lewsey JD, Stewart S, et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 millionpeople.Circulation. 2009;119:515–523. doi:10.1161/CIRCULATIONAHA.108.812172
15. Gibbs C, Lip G, Beevers D. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol. 1999;48(6):861. doi:10.1046/j.1365-2125.1999.00093.x
16. Chen Y-T, Wang Y, Radford MJ, Krumholz HM. Angiotensin-converting enzyme inhibitor dosages in elderly patients with heart failure. Am Heart J. 2001;141(3):410–417. doi:10.1067/mhj.2001.113227
17. Luzier AB, Forrest A, Feuerstein SG, Schentag JJ, Izzo JL
18. Barywani SB, Ergatoudes C, Schaufelberger M, Petzold M, Fu ML. Does the target dose of neurohormonal blockade matter for outcome in systolic heart failure in octogenarians? Int J Cardiol. 2015;187:666–672. doi:10.1016/j.ijcard.2015.03.428
19. Sweileh WM, Sawalha AF, Rinno TM, Zyoud S, Al-Jabi SW. Optimal dosing of angiotensin-converting enzyme inhibitors in patients with chronic heart failure: a cross-sectional study in Palestine. Ann Saudi Med. 2009;29(2):119–122. doi:10.4103/0256-4947.51794
20. Solal AC, Leurs I, Assyag P, et al. Optimization of heart FailUre medical Treatment after hospital discharge according to left ventricUlaR Ejection fraction: the FUTURE survey. Arch Cardiovasc Dis. 2012;105(6–7):355–365. doi:10.1016/j.acvd.2012.04.003
21. Schmidt S, Hürlimann D, Starck CT, et al. Treatment with higher dosages of heart failure medication is associated with improved outcome following cardiac resynchronization therapy. Eur Heart J. 2014;35(16):1051–1060. doi:10.1093/eurheartj/eht514
22. Niriayo YL, Kumela K, Gidey K, Angamo MT. Utilization and dose optimization of angiotensin-converting enzyme inhibitors among heart failure patients in Southwest Ethiopia. Biomed Res Int. 2019;2019:9463872. doi:10.1155/2019/9463872
23. Atey TM, Teklay T, Asgedom SW, Mezgebe HB, Teklay G, Kahssay M. Treatment optimization of angiotensin converting enzyme inhibitors and associated factors in ayder comprehensive specialized hospital: a cross-sectional study. BMC Res Notes. 2018;11(1):209. doi:10.1186/s13104-017-2820-5
24. O’brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41(5):673–690. doi:10.1007/s11135-006-9018-6
25. Hosmer DW
26. Komajda M, Boehm M, Borer JS, et al. Incremental benefit of drug therapies for chronic heart failure with reduced ejection fraction: a network meta‐analysis. Eur J Heart Fail. 2018;20(9):1315–1322. doi:10.1002/ejhf.1234
27. Rochon PA, Sykora K, Bronskill SE, et al. Use of angiotensin-converting enzyme inhibitor therapy and dose-related outcomes in older adults with new heart failure in the community. J Gen Intern Med. 2004;19(6):676–683. doi:10.1111/j.1525-1497.2004.30328.x
28. Kojima N, Williams JM, Slaughter TN, et al. Renoprotective effects of combined SGLT 2 and ACE inhibitor therapy in diabetic D ahl S rats. Physiol Rep. 2015;3(7):e12436. doi:10.14814/phy2.12436
29. Chang HY, Wang CC, Wei J, et al. Gap between guidelines and clinical practice in heart failure with reduced ejection fraction: results from TSOC-HFrEF registry. J Chin Med Assoc. 2017;80(12):750–757. doi:10.1016/j.jcma.2017.04.011
30. Komajda M, Lutiger B, Madeira H, et al. Tolerability of carvedilol and ACE‐Inhibition in mild heart failure. Results of CARMEN (Carvedilol ACE‐Inhibitor Remodelling Mild CHF EvaluatioN). Eur J Heart Fail. 2004;6(4):467–475. doi:10.1016/j.ejheart.2003.12.019
31. Gotsman I, Rubonivich S, Azaz-Livshits T. Use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients with congestive heart failure: an observational study of treatment rates and clinical outcome. Isr Med Assoc J. 2008;10(3):214–218.
32. Lonn E. Dose response of ACE inhibitors: implications of the SECURE trial. Trials. 2001;2(4):155. doi:10.1186/CVM-2-4-155
33. Komajda M, Anker SD, Cowie MR, et al. Physicians’ adherence to guideline‐recommended medications in heart failure with reduced ejection fraction: data from the QUALIFY global survey. Eur J Heart Fail. 2016;18(5):514–522. doi:10.1002/ejhf.510
34. Hirt MN, Muttardi A, Helms TM, van den Bussche H, Eschenhagen T. General practitioners’ adherence to chronic heart failure guidelines regarding medication: the GP-HF study. Clin Res Cardiol. 2016;105(5):441–450. doi:10.1007/s00392-015-0939-8
35. Cowie MR, Komajda M. Quality of physician adherence to guideline recommendations for life-saving treatment in heart failure: an international survey. Card Fail Rev. 2017;3(2):130. doi:10.15420/cfr.2017:13:1
36. Sargento L, Simões AV, Longo S, Lousada N, Dos Reis RP. Treatment with optimal dose angiotensin-converting enzyme inhibitors/angiotensin receptor blockers has a positive effect on long-term survival in older individuals (aged> 70 years) and octogenarians with systolic heart failure. Drugs Aging. 2016;33(9):675–683. doi:10.1007/s40266-016-0393-y
37. Gianni M, Bosch J, Pogue J, et al. Effect of long-term ACE-inhibitor therapy in elderly vascular disease patients. Eur Heart J. 2007;28(11):1382–1388. doi:10.1093/eurheartj/ehm017
38. Bangalore S, Kumar S, Messerli FH. Angiotensin-converting enzyme inhibitor associated cough: deceptive information from the Physicians’ Desk Reference. Am J Med. 2010;123(11):1016–1030. doi:10.1016/j.amjmed.2010.06.014
39. Raebel M. Hyperkalemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cardiovasc Ther. 2011;30:e156–166. doi:10.1111/j.1755-5922.2010.00258.x
40. Caldeira D, David C, Sampaio C. Tolerability of angiotensin-receptor blockers in patients with intolerance to angiotensin-converting enzyme inhibitors. Am J Cardiovasc Drugs. 2012;12(4):263–277. doi:10.1007/BF03261835
41. Ter Maaten JM, Martens P, Damman K, et al. Higher doses of loop diuretics limit uptitration of angiotensin-converting enzyme inhibitors in patients with heart failure and reduced ejection fraction. Clin Res Cardiol. 2020;109(8):1048–1059. doi:10.1007/s00392-020-01598-w
42. Jessup M, Abraham WT, Casey DE, et al; 2009 Writing Group to Review New Evidence and Update the 2005 Guideline for the Management of Patients with Chronic Heart Failure Writing on Behalf of the 2005 Heart Failure Writing Committee. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):1977–2016. doi:10.1161/CIRCULATIONAHA.109.192064
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
