Back to Journals » Clinical Ophthalmology » Volume 16
Analysis of Systemic Risk Factors and Post-Insult Visual Development in a Danish Cohort of Patients with Nonarteritic Anterior Ischemic Optic Neuropathy
Authors Citirak G, Malmqvist L, Hamann S
Received 5 August 2022
Accepted for publication 9 September 2022
Published 14 October 2022 Volume 2022:16 Pages 3415—3424
DOI https://doi.org/10.2147/OPTH.S384740
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Gülsenay Citirak, Lasse Malmqvist, Steffen Hamann
Department of Ophthalmology, Rigshospitalet, University of Copenhagen, Glostrup, Denmark
Correspondence: Steffen Hamann, Department of Ophthalmology, Rigshospitalet, University of Copenhagen, Valdemar Hansens Vej 13, Glostrup, 2600, Denmark, Tel +45 3863 4653, Email [email protected]
Purpose: Nonarteritic anterior ischemic optic neuropathy (NAION) is associated with vascular as well as anatomical risk factors. Following the insult, the visual development varies from minor to severe deterioration. The aim of this study was to examine possible prognostic systemic risk factors and their eventual impact on post-insult visual development in NAION patients.
Methods: A retrospective chart review of all NAION patients (18– 79 years at time of diagnosis) seen a minimum of two times in a tertiary eye department during a 10-year period in regard to systemic diseases, medication, lifestyle factors and ophthalmic examination was performed. Visual outcome was assessed according to the development of best corrected visual acuity (BCVA) and visual field from initial to final visit.
Results: There were 163 eligible patients. A greater proportion of the patients in the total cohort were over 50 years of age (79.8%) and men (66.3%). In total, 59.5% of the patients had a stable BCVA, while 25.8% experienced improvement, and 14.7% had deterioration. Seventy-two percent of the patients had a stable visual field, while 14% had improvement, and 14% had deterioration. No association between visual outcome and clinical characteristics, medication or systemic risk factors were identified.
Conclusion: We did not find any association between patient characteristics and systemic risk factors at time of diagnosis and visual development post-insult. This could suggest that the optic nerve head anatomy plays a larger role for visual outcome than previously estimated.
Keywords: NAION, nonarteritic anterior ischemic optic neuropathy, risk factors, visual outcome, optic neuropathy, NAION
Introduction
Nonarteritic anterior ischemic optic neuropathy (NAION) is characterized by a sudden ischemic insult to the anterior, prelaminar portion of the optic nerve head. It is the most common acute optic neuropathy among patients over 50 years of age and the disease is the second most common cause of permanent optic nerve-related vision loss in adults after glaucoma. NAION typically presents unilaterally with sudden, painless visual loss. No specific sex predilection has been found, with both men and women affected equally.1,2
The exact pathogenesis still remains unknown, however, NAION is assumed to be due to a transient disruption in the circulation of the optic nerve head resulting in hypoperfusion and ischemia of the short posterior ciliary arteries.1 NAION is associated with several vascular risk factors such as hypertension,3,4 diabetes mellitus,3 hypercholesterolemia,5,6 smoking,7 obstructive sleep apnea,8,9 anemia,10,11 and hypercoagulability.4,5 Use of vasoactive medication may also be a risk factor for NAION. Phosphodiesterase type-5 inhibitors (PDE-5i) have been suggested to increase the risk of NAION,12 however the role of PDE-5i in NAION pathogenesis is still not clarified.13 NAION has also been reported in patients under the age of 40 years with and without the above-mentioned risk factors.14 More than 50% of NAION patients under the age of 50 years have underlying optic disc drusen, pointing at these structures constituting an important anatomic risk factor.15,16 Neither medical or surgical treatment options have proven effect on the disease.2 However, 40% of the patients experience spontaneous improvement in visual acuity for unexplained reasons.2,17 The visual field defects (VFD) are less likely to improve. There is less than a 5% risk of recurrence in the same eye,1 while the incidence of fellow eye NAION has been reported to be 15% at 5 years after the first eye was affected.18 There is no proven prophylactic treatment to prevent second-eye involvement.2
Patients diagnosed with NAION are naturally concerned about their visual prognosis. However, the number of studies that have examined the visual development in NAION patients following the initial insult is limited. A minority may suffer a progressive form, where the vision deteriorates further following the ischemic event.19,20 In this study, we investigated the clinical profile and characteristics of Danish patients who were diagnosed with NAION over a period of 10 years. The aim of this study was to investigate possible prognostic systemic risk factors and post-insult visual development in patients with NAION.
Materials and Methods
This regional, retrospective cohort study adhered to the tenets of the Declaration of Helsinki. The protocol was approved by the Danish Patient Safety Authority and the Danish Data Management Authority (approval # VD-2019-146). This retrospective study did not require ethics review board approval under the laws of the Kingdom of Denmark.
A retrospective chart review was performed on all patients seen in our department between 2009 and 2019 with a diagnosis of “ischemic optic neuropathy” based on International Classification of Diseases (ICD) codes (ICD-10 code DH470C). Patients were included if the NAION diagnosis was verified by at least two ophthalmologists, if their age was between 18–79 years at time of diagnosis and they attended a minimum of two eye examinations in our department. The first eye examination was usually performed by a supervised ophthalmology resident on call, the second (and subsequent, if more than two visits) was always with a neuro-ophthalmologist. The last follow-up visit was defined as the last visit and examination of the patient before the patient was discharged from the outpatient clinic. Exclusion criteria were: (1) the presence of other eye disorders that would impair central vision or cause VFDs (mild cataract was accepted); (2) eye surgery (uncomplicated cataract surgery more than 1 month prior to onset of NAION and external eye surgery were accepted), newly initiated treatment for NAION; and (3) clinical features that suggested disorders other than NAION.
Following data from patients’ medical records were collected: age, sex, time of follow up (the time from initial visit and given diagnosis to final follow-up visit), type of onset of symptoms, use of alcohol and smoking, family history of NAION, history of previous NAION in contralateral eye, medication and presence of systemic comorbidities including diabetes, hypertension, hypercholesterolemia, ischemic heart disease, arrhythmia, anemia, cerebrovascular disease including history of stroke and transient ischemic attack, obstructive sleep apnea and autoimmune disorders. As data on body mass index and blood pressure are not systematically recorded in the medical records at our department, these data were not registered. Medication list for all medications except hormonal contraceptives, medicines for pain relief and topical treatments, and presence of systemic disease were recorded according to the time of initial visit and diagnosis of NAION. The following parameters were recorded from the ophthalmic examinations: Best corrected visual acuity (BCVA) using the Snellen visual acuity chart, visual fields with a computerized perimetry (various models of Octopus perimeters), presence of relative afferent pupillary defect (RAPD), slit lamp examination of the anterior segment, lens and vitreous, intraocular pressure, indirect ophthalmoscopy, blood pressure at time of diagnosis of NAION, VFDs and the morphology of the optic disc were recorded separately both at initial visit and at the final follow-up visit. However, results of VFD were only recorded if the reliability factor (RF) was below 20%.
BVCA was graded in 6 levels: (0) mild or no visual impairment (BCVA better than 0.7), (1) moderate visual impairment (BCVA 0.7 to better than 0.3), (2) marked visual impairment (BCVA 0.3 to better than 0.1), (3) severe visual impairment (BCVA 0.1 to better than 0.01), (4) blind (BCVA 0.01 to no perception of light (PL)) and (5) totally blind (no PL). Change in visual acuity of ≥2 lines in Snellen VA was considered a significant change in either direction (improvement or deterioration). Similarly, we divided VFD according to a perimetrical mean deviation (MD) grading scale from Hatem et al:21 (0) Normal visual field, (1) MD less than −4.0 with a VFD, (2) MD −4.0 to −11.9 with VFD, (3) MD −12.0 to −19.9 and (4) MD ≥-20. Change in MD of ≥3 MD was defined significant in either direction (improvement or deterioration). In patients with simultaneous, bilateral NAION, only the result of the eye with most impaired vision was used in the comparative statistical analysis.
Due to NAION being more prevalent among patients over 50 years, we did a separate analysis of patients in two groups according to age: 18–50 years and 51–79 years. In selected cases, we compared clinical and medical characteristics between the groups.
Demographic factors and clinical data were analyzed using the Mann–Whitney U-test, Fischer exact test or chi-squared exact test with Monte Carlo estimate. To analyze for the association of systemic diseases and medical treatments on visual outcome, exact chi-squared analysis and in relevant cases a post hoc analysis of the chi-squared test was performed with Bonferroni correction. Finally, Bonferroni-Holm method was used to counteract the problem of multiple comparisons and thereby control the family-wise error rate. A p value ≤ 0.05 was treated as statistically significant. All the analyzes were carried out in SAS Studio 3.8 (SAS Institute, Cary NC).
Results
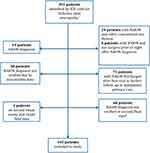
Three hundred ninety-one patients were identified by ICD code for “ischemic optic neuropathy” in the 10-year period. Of these, 163 patients were included in the study. The remaining 228 patients were excluded according to the flowchart in Figure 1.
Demographics
Patient demographics are shown in Table 1. Overall, a greater proportion of the patients in the total cohort were over 50 years of age (79.8%) and predominantly men (66.3%). Active smokers made up 22.7% of the included patients, 16.6% reported regular alcohol consumption, and 71.2% had comorbidity. We identified a higher prevalence of comorbidity (62%) in the age group over 50 years (p<0.001). In the same age group, 53.4% of the patients used medication vs. 7.4% in the age group 18–50 years (p = 0.0037). Follow-up time between the two groups varied overall with 12.6 months in the age group 18–50 years vs. 4.7 months in the group of patients among 50–79 years (p = 0.0016). No statistically significant difference was seen between the groups regarding smoking.
 |
Table 1 Patient Demographics and Statistical Difference Between Age Groups (p-value) |
More than half (50.9%) of the patients reported impaired vision as the initial onset symptom that made them seek medical care, while 45.4% of the patients noticed a VFD as the onset symptom. A few patients (3.7%) had not noticed any change in their vision, but a routine control at their primary ophthalmologist was with findings compatible with NAION (decreased BCVA, VFDs or/and optic disc edema or atrophy). Some patients (17.8%) had a history of previous NAION in the fellow eye. Four patients in the cohort had bilateral simultaneous NAION. Only 2 patients in the total cohort could report a family history of NAION.
Clinical Characteristics
The clinical characteristics of the included patients are shown in Table 2. Nearly all patients had their initial visit in our eye emergency department and were examined by supervised ophthalmology residents. At initial eye examination 85.3% were noted to have optic disc edema, while at the final examination 75.5% of the patients were noted to have optic atrophy based on the ophthalmoscopic appearance of a pale optic disc. On their first visit to our department, 27.6% of the patients were noted to have RAPD. It was not possible to record data about RAPD in 52.8% of the patients, in most cases because they were medically dilated from primary eyecare before they were seen in our eye emergency department. However, 63.2% had RAPD confirmed at least at their final follow-up visit.
 |
Table 2 Clinical Characteristics and Statistical Difference Between Age Groups (p-value) |
Baseline BCVA was minimally to markedly affected (grade 0–2) in 81% and severely impaired to blindness (grade 3–5) in 19% of the study group. Visual outcome, both BCVA (59.5%) and VF (72%) was overall stable from time of initial visit to final follow-up visit. No significant statistical difference was found for BCVA or VF grade at presentation and last available follow-up visit. Similarly, no statistically significant differences in BCVA or VF outcome were found between the age groups.
Systemic Comorbidities and Medication
Divided in age groups, 55% of patients in the 18–50 years category did not have any systemic comorbidities at all. This number decreased to 22% in the category of patients from 51–79 years. In the patients that did have systemic comorbidities, a subanalysis of the percentage distribution of five selected systemic comorbidities, with relevance to NAION, was performed (see Figure 2, top panel). No significant difference was found between the two age groups for any of these selected comorbidities. In the enrolled patients in the study, 31% did not have any of the selected comorbidities, 26% had one of these, and 43% had two or more of these.
Divided in age groups, 63% of patients in the 18–50 years category did not receive any medication at all and this number decreased to 32% in the category from 51–79 years. In the patients that did receive medication, a subanalysis of the percentage distribution of seven selected medications, with relevance to NAION, was performed (see Figure 2, lower panel). No significant difference was found between the two age groups for any of these selected medications. In the enrolled patients in the study, 18% did not receive any of the selected medications, 38% received one, and 44% received two or more of these.
To shed light on possible prognostic factors for visual acuity and visual field, we assessed suspected risk factors for their potential effect on visual outcome (Table 3). Overall, no statistically significant correlation was identified between selected risk factors and visual outcome for NAION patients after adjusting with Holm-Bonferroni method. Similarly, no significant correlation was found between risk factors and outcome in VA or VF in neither of the two age groups. And finally, we did not find any correlation between greater number of risk factors and visual outcome.
 |
Table 3 Comorbidity and Medication in Relation to Visual Outcome |
Discussion
In this retrospective cohort study, we describe the demographic and clinical profile of 163 Danish patients diagnosed with NAION. We did not identify any correlation between suspected vascular risk factors at time of diagnosis and visual development following the ischemic insult.
NAION is a disease of the small vessels supplying the anterior optic nerve head. The exact cause remains unknown. However, an anatomical pre-disposition, the narrow scleral canal and the so-called disc at risk, is considered essential for the development of NAION.22–24 Other anatomic abnormalities, that result in axonal crowding, such as optic disc drusen15,16 or papilledema24 may predispose to NAION. In addition to anatomical risk factors, past studies have demonstrated that vascular risk factors, such as cardiovascular disease, diabetes, and hypercholesterolemia are associated with NAION.3–5,9,25–28 It is unclear whether vascular risk factors are as important as the anatomical ones for acquiring NAION, and it is unclear whether pre-existing vascular or anatomic risk factors are important for the development of visual acuity and fields following the initial ischemic event. Our primary aim was therefore to examine potential prognostic vascular risk factors for visual outcome.
Mean age at diagnosis of NAION in this cohort was 60 years. This finding corresponds well with previous studies, where the mean age ranged from 57 to 65 years.4,25,29,30 Overall, there was an overweight of men diagnosed with NAION in our study. However, we did not find a statistically significant sex predisposition for NAION, which also correlates well with previous studies.4,25,29,30
We assessed the prevalence of comorbidities in two age groups: Below and above 50 years of age. As expected, we identified a slightly higher proportion of comorbidities in the age group over 50 years. However, we did not find a statistically significant difference between the age groups when analyzing the risk factors independently.
Previous studies have tried to assess whether some of the suspected risk factors examined in this study do have an effect on visual outcome.20,31,32 Sharma et al found that ischemic heart disease and increasing age were associated with a declined final visual outcome. Furthermore, they did not find that diabetes or obstructive sleep apnea were independent risk factors affecting visual outcome.31 Hayreh et al did not find any association with age at diagnosis, sex, smoking, diabetes mellitus, hypertension, ischemic heart disease, hypercholesterolemia and visual outcome one year after time of diagnosis.20 Bialer et al neither found any association between clinical characteristics and visual outcome. Our study supports a hypothesis of a non-existing or minor association between visual outcome and proposed systemic risk factors. Bialer et al suggested that the detection of deterioration is predominantly related with the timing of medical evaluation and not with the patient’s characteristics or clinical presentation.32
When looking at risk factors in relation to post-insult visual development, we found that most of the risk factors did not play a role for visual development after the patients were diagnosed with NAION. Most patients experienced stable and unchanged visual acuity and visual fields regardless of the type of risk factor and the number of risk factors they had. We cannot explain why the greater proportion of those who experienced improved visual development were smokers. However, it is important to note that the sample groups were small regarding the risk factors and therefore it is necessary to do further studies with a bigger sample size to confirm the observations more accurately.
Several limitations of the present study should be addressed; most of them are primarily related to the study being a retrospective analysis. A limitation of this study was first the need to exclude the majority (228/391) of patients from the initial data because of primarily inadequate follow-up in the department. Secondly, the lack of eye examination at a standardized follow-up time for all patients is also a limitation in the retrospective format. Third, the lack of an RAPD in 42% of patients examined for RAPD at first visit, could question the NAION diagnosis, as one would expect this number to be lower. It is possible that some RAPDs may have been too subtle to detect in a busy emergency setting, especially if the NAION only affected the optic nerve head segmentally, and the lack of an RAPD was only noted in 26% of the examined patients at follow-up, where the diagnosis was confirmed by trained neuro-ophthalmologists. Fourth, optic atrophy was only noted in about 75% at final examination, where one would have expected some degree of optic atrophy in almost all cases. The observed optic atrophy was based on the fundoscopic appearance of a pale disc, not on peripapillary retinal nerve fiber layer atrophy, which probably would have been detected on optical coherence tomography scans. Fifth, in this study BCVA (59.5%) and VF (72%) were found overall stable from initial visit to final follow-up. These percentages are high in respect to what has been previously reported with spontaneous partial improvement usually observed in about 40% of patients within the first few weeks from onset.2,17 In some cases we could have missed an initial improvement in BCVA as time between initial and final visit in our study in many cases was longer. Finally, this study did not include a control group matched for age and sex. As systemic comorbidities are common in older patients without NAION, an analysis of a control group could potentially have yielded important results.
This study highlights the clinical profile of a Danish population diagnosed with NAION. A comprehensive list of factors, including comorbidities, medications and lifestyle factors, were examined in this large study with 163 patients to see if there were any associations with visual outcome after the ischemic event took place. However, no association was found between clinical characteristics, systemic vascular risk factors and visual outcome. The lack of this association could indicate a role for visual outcome of the pre-existing optic nerve head anatomy, which should be further explored in future studies.
Acknowledgments
This was not an industry supported study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Berry S, Lin WV, Sadaka A, Lee AG. Nonarteritic anterior ischemic optic neuropathy: cause, effect, and management. Eye Brain. 2017;9:23–28. doi:10.2147/EB.S125311
2. Miller NR, Arnold AC. Current concepts in the diagnosis, pathogenesis and management of nonarteritic anterior ischemic optic neuropathy. Eye. 2015;29(1):65–79. doi:10.1038/eye.2014.144
3. Hayreh SS, Joos KM, Podhajsky PA, Long CR. Systemic diseases associated with nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1994;118(6):766–780. doi:10.1016/S0002-9394(14)72557-7
4. Cestari DM, Gaier ED, Bouzika P, et al. Demographic, systemic, and ocular factors associated with nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 2016;123(12):2446–2455. doi:10.1016/j.ophtha.2016.08.017
5. Giambene B, Sodi A, Sofi F, et al. Evaluation of traditional and emerging cardiovascular risk factors in patients with non-arteritic anterior ischemic optic neuropathy: a case-control study. Graefes Arch Clin Exp Ophthalmol. 2009;247(5):693–697. doi:10.1007/s00417-008-0981-6
6. Deramo VA, Sergott RC, Augsburger JJ, Foroozan R, Savino PJ, Leone A. Ischemic optic neuropathy as the first manifestation of elevated cholesterol levels in young patients. Ophthalmology. 2003;110(5):1041–1046. doi:10.1016/S0161-6420(03)00079-4
7. Chung S, Gay C, McCrary III J. Nonarteritic ischemic optic neuropathy. The impact of tobacco use. J Neuro Ophthalmol. 1994;101(4):779–782.
8. Bilgin G, Koban Y, Arnold AC. Nonarteritic anterior ischemic optic neuropathy and obstructive sleep apnea. J Neuro Ophthalmol. 2013;33(3):232–234. doi:10.1097/WNO.0b013e31828eecbd
9. Palombi K, Renard E, Levy P, et al. Non-arteritic anterior ischemic optic neuropathy is nearly systematically associated with obstructive sleep apneea. Br J Ophthalmol. 2006;90(7):879–882. doi:10.1136/bjo.2005.087452
10. Brouzas D, Charakidas A, Ladas I, Apostolopoulos M. Nonarteritic anterior ischemic optic neuropathy associated with chronic anemia: a case series of myelodysplastic syndrome patients. Clin Ophthalmol. 2009;3:133–137.
11. Humbertjean-Selton L, Selton J, Riou-Comte N, Lacour JC, Mione G, Richard S. Bilateral optic neuropathy related to severe anemia in a patient with alcoholic cirrhosis: a case report and review of the literature. Clin Mol Hepatol. 2018;24(4):417–423. doi:10.3350/cmh.2017.0021
12. Campbell UB, Walker AM, Gaffney M, et al. Acute nonarteritic anterior ischemic optic neuropathy and exposure to phosphodiesterase type 5 inhibitors. J Sex Med. 2015;12(1):139–151. doi:10.1111/jsm.12726
13. Nathoo NA, Etminan M, Mikelberg FS. Association between phosphodiesterase-5 inhibitors and nonarteritic anterior ischemic optic neuropathy. J Neuro Ophthalmol. 2015;35(1):12–15. doi:10.1097/WNO.0000000000000186
14. Mathews MK. Nonarteritic anterior ischemic optic neuropathy. Curr Opin Ophthalmol. 2005;16(6):341–345. doi:10.1097/01.icu.0000188361.52166.93
15. Fraser JA, Rueløkke LL, Malmqvist L, Hamann S. Prevalence of optic disc drusen in young patients with nonarteritic anterior ischemic optic neuropathy: a 10-year retrospective study. J Neuro Ophthalmol. 2021;41(2):200–205. doi:10.1097/WNO.0000000000000974
16. Hamann S, Malmqvist L, Wegener M, et al. Young adults with anterior ischemic optic neuropathy: a multicenter optic disc drusen study. Am J Ophthalmol. 2020;217:174–181. doi:10.1016/j.ajo.2020.03.052
17. Dickersin K, Everett D, Feldon S, et al. Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. JAMA J Am Med Assoc. 1995;273(8):625–632. doi:10.1001/jama.1995.03520320035038
18. Newman NJ, Scherer R, Langenberg P, et al. The fellow eye in NAION: report from the ischemic optic neuropathy decompression trial follow-up study. Am J Ophthalmol. 2002;134(3):317–328. doi:10.1016/S0002-9394(02)01639-2
19. Newman NJ. The ischemic optic neuropathy decompression trial. Arch Ophthalmol. 2007;125(11):1568–1570. doi:10.1001/archopht.125.11.1568
20. Hayreh SS, Zimmerman MB. Nonarteritic anterior ischemic optic neuropathy. natural history of visual outcome. Ophthalmology. 2008;115(2):298–305. doi:10.1016/j.ophtha.2007.05.027
21. Hatem CF, Yri HM, Sørensen AL, Wegener M, Jensen RH, Hamann S. Long-term visual outcome in a Danish population of patients with idiopathic intracranial hypertension. Acta Ophthalmol. 2018;96(7):719–723. doi:10.1111/aos.13664
22. Doro S, Lessell S. Cup-disc ratio and ischemic optic neuropathy. Arch Ophthalmol. 1985;103(8):1143–1144. doi:10.1001/archopht.1985.01050080055019
23. Beck RW, Servais GE, Hayreh SS. Anterior ischemic optic neuropathy IX Cup-to-disc ratio and its role in pathogenesis. Ophthalmology. 1987;94(11):1503–1508.
24. Burde RM. Optic disk risk factors for nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1993;116(6):759–764. doi:10.1016/S0002-9394(14)73478-6
25. Repka MX, Savino PJ, Schatz NJ, Sergott RC. Clinical profile and long-term implications of anterior ischemic optic neuropathy. Am J Ophthalmol. 1983;96(4):478–483. doi:10.1016/S0002-9394(14)77911-5
26. Salomon O, Huna-Baron R, Kurtz S, et al. Analysis of prothrombotic and vascular risk factors in patients with nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 1999;106(4):739–742. doi:10.1016/S0161-6420(99)90159-8
27. Jacobson DM, Vierkant RA, Belongia EA. Nonarteritic anterior ischemic optic neuropathy: a case-control study of potential risk factors. Arch Ophthalmol. 1997;115(11):1403–1407. doi:10.1001/archopht.1997.01100160573008
28. Lee MS, Grossman D, Arnold AC, Sloan FA. Incidence of nonarteritic anterior ischemic optic neuropathy: increased risk among diabetic patients. Ophthalmology. 2011;118(5):959–963. doi:10.1016/j.ophtha.2011.01.054
29. Johnson LN, Arnold AC. Incidence of nonarteritic and arteritic anterior ischemic optic neuropathy: population-based study in the State of Missouri and Los Angeles County, California. J Neuro Ophthalmol. 1994;14(1):38–44. doi:10.1097/00041327-199403000-00011
30. Newman NJ, Dickersin K, Kaufman D, et al. Characteristics of patients with nonarteritic anterior ischemic optic neuropathy eligible for the ischemic optic neuropathy decompression trial. Arch Ophthalmol. 1996;134(3):317–328.
31. Sharma S, Kwan S, Fallano KA, Wang J, Miller NR, Subramanian PS. Comparison of visual outcomes of nonarteritic anterior ischemic optic neuropathy in patients with and without diabetes mellitus. Ophthalmology. 2017;124(4):450–455. doi:10.1016/j.ophtha.2016.11.029
32. Bialer OY, Stiebel-Kalish H. Clinical characteristics of progressive nonarteritic anterior ischemic optic neuropathy. Int J Ophthalmol. 2021;14(4):517–522. doi:10.18240/ijo.2021.04.06
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.