Back to Journals » Clinical Interventions in Aging » Volume 18
An Exploratory Study of Sleep-Wake Differences of Autonomic Activity in Patients with Mild Cognitive Impairment: The Role of Melatonin as a Modulating Factor
Authors Abulafia C, Vidal MF, Olivar N, Odzak A, Brusco I, Guinjoan SM, Cardinali DP , Vigo DE
Received 5 November 2022
Accepted for publication 8 May 2023
Published 12 May 2023 Volume 2023:18 Pages 771—781
DOI https://doi.org/10.2147/CIA.S394749
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Carolina Abulafia,1,2 María F Vidal,3 Natividad Olivar,4 Andrea Odzak,5 Ignacio Brusco,4– 6 Salvador M Guinjoan,7 Daniel P Cardinali,8 Daniel E Vigo1,9
1Laboratory of Chronophysiology, Institute for Biomedical Research (BIOMED), Pontifical Catholic University of Argentina (UCA) and CONICET, Buenos Aires, Argentina; 2Facultad de Psicología, Universidad de Buenos Aires, Buenos Aires, Argentina; 3Servicio de Psiquiatría, Departamento de Neurología, Fleni, Buenos Aires, Argentina; 4Hospital de Clínicas “José de San Martín”, Universidad de Buenos Aires, Buenos Aires, Argentina; 5Servicio de Clínica Médica, Hospital Argerich, Buenos Aires, Argentina; 6CONICET, Buenos Aires, Argentina; 7Laureate Institute for Brain Research, Tulsa, OK, USA; 8Facultad de Ciencias Médicas, Universidad Católica Argentina, Buenos Aires, Argentina; 9Faculty of Psychology and Educational Sciences, Katholieke Universiteit Leuven, Leuven, Belgium
Correspondence: Daniel E Vigo, Instituto de Investigaciones Biomédicas, Pontificia Universidad Católica Argentina, Alicia Moreau de Justo 1500, 4° piso, Buenos Aires, C1107AAZ, Argentina, Tel +54 0810-2200-822 ext 1152, Email [email protected]; [email protected]
Purpose: The objective of the present study was to assess sleep-wake differences of autonomic activity in patients with mild cognitive impairment (MCI) compared to control subjects. As a post-hoc objective, we sought to evaluate the mediating effect of melatonin on this association.
Patients and Methods: A total of 22 MCI patients (13 under melatonin treatment) and 12 control subjects were included in this study. Sleep-wake periods were identified by actigraphy and 24hr-heart rate variability measures were obtained to study sleep-wake autonomic activity.
Results: MCI patients did not show any significant differences in sleep-wake autonomic activity when compared to control subjects. Post-hoc analyses revealed that MCI patients not taking melatonin displayed lower parasympathetic sleep-wake amplitude than controls not taking melatonin (RMSSD − 7 ± 1 vs 4 ± 4, p = 0.004). In addition, we observed that melatonin treatment was associated with greater parasympathetic activity during sleep (VLF 15.5 ± 0.1 vs 15.1 ± 0.1, p = 0.010) and in sleep-wake differences in MCI patients (VLF 0.5 ± 0.1 vs 0.2 ± 0.0, p = 0.004).
Conclusion: These preliminary findings hint at a possible sleep-related parasympathetic vulnerability in patients at prodromal stages of dementia as well as a potential protective effect of exogenous melatonin in this population.
Keywords: heart rate variability, actigraphy, circadian rhythms, wavelets
Introduction
Mild cognitive impairment (MCI) has been defined as an intermediate state between healthy ageing and dementia, where patients exhibit clinically significant mild cognitive decline from expected performance for subjects’ age group, yet not severe enough to impact functional independence in activities of daily living.1 MCI is present in 15–25% of older adults over 65 years old and 32% of MCI subjects convert to dementia due to Alzheimer’s disease (AD) (the main cause for dementia) over a five-year period.2 For this reason, MCI is a critical stage to assess subjects at risk of developing dementia and a promising target population to implement potential prevention and treatment interventions before neurodegeneration is more advanced.1
As a transitional phase to dementia, MCI due to AD already manifests some pathophysiological changes in the nervous system1,3,4 such as accumulation of phosphorylated tau in the locus coeruleus and allocortical and diencephalic limbic structures involved in the autonomic nervous system regulation, along with a disrupted diurnal variation of multiple physiological processes.5 Several cross-sectional and prospective studies have described associations between sleep disturbances and increased risk of cognitive decline.6–9 Indeed, there is a high prevalence of sleep-aid medication use, such as zolpidem, benzodiazepines, and melatonin in this population.10–14
Sleep-wake cycle disruption is associated with abnormalities in the circadian regulation of the autonomic nervous system. Furthermore, cognitive function and sympathovagal cardiac modulation seem to influence each other in MCI and dementia patients, who exhibit reduced parasympathetic modulation.15 It is believed that such anomalies in vagal tone are most likely caused to neurofibrillary degeneration and neuronal death in the insular cortex and brainstem.16
Heart rate variability (HRV) is considered an index of autonomic control of the heart17 and represents a non-invasive, cost-effective method to assess cardiac autonomic function. HRV measures interval variations between successive heartbeats, reflecting the sympathetic and parasympathetic balance on the sinoatrial node.18 Changes in HRV as a marker of autonomic activity have been associated with neuropsychiatric disorders and cognitive impairment, where high HRV is associated with better cognitive performance in healthy adults, older adults and patients with dementia.19–21 However, the literature exploring this relationship specifically in MCI is surprisingly limited, and it reports mixed results.15,16,22–25 Analysis of autonomic activity throughout the whole sleep-day cycle has been proposed as the better approach to study sleep-related changes in HRV,26 yet only two studies explored autonomic function in MCI with 24hr HRV. One study did not assess the sleep-wake cycle.27 The other publication explored HRV limited to time-domain analysis, which may explain why the authors found no significant results for the MCI group.28 Therefore, the purpose of the present study was to assess sleep-wake differences of autonomic activity in patients with MCI through 24hr time- and frequency-domain HRV measures. Based upon previous literature,5,16 we hypothesized that incipient neurodegeneration of limbic structures would induce changes in the circadian variation of the autonomic input to the heart. We specifically predicted that MCI would be associated with decreased vagal function, as defined by lower HRV measurements in the high-frequency domain. Finally, a post-hoc objective was to evaluate the mediating effect of melatonin on the relationship between MCI and vagal function.
Materials and Methods
Subjects and Design
A total of 39 outpatients with probable amnestic MCI and 12 control subjects comparable in gender, age and education level were examined at the facilities of the Centro de Neuropsiquiatría y Neurología de la Conducta (CENECON), Hospital de Clínicas “José de San Martín” and at the Service of Psychiatry of Fleni Foundation.
For this study, MCI was diagnosed by trained neuropsychiatrists and neurologists following Petersen’s diagnostic criteria:29 1) cognitive complaint reported by the patient or by an informant, 2) objective cognitive impairment, 3) preserved independence in functional abilities, 4) no dementia. Clinical diagnosis was complemented with standardized neuropsychological assessment when available. Neuropsychological assessment included the following tests (only techniques common to all assessments available were included in the present study): Rey Auditory Verbal Learning Test (RAVLT) to assess verbal episodic memory and learning;30 Semantic Fluency (SF, “animals”) and Phonemic Fluency (PS, “P”) to assess verbal fluency;31 Trail Making Test A (TMT A) to assess sustained attention and Trail Making Test B (TMT B) to assess cognitive flexibility.32
Inclusion criteria for MCI subjects were as follows: 1) probable amnestic MCI diagnosis according to standard diagnostic criteria,29 2) >7 years of formal education. Exclusion criteria were as follows: 1) a diagnosis of dementia, neurological or other medical conditions known to affect cognition; 2) current alcohol or substance abuse; 3) diagnosis of a major psychiatric disorder (eg, psychosis); 4) cardiovascular conditions including stroke, transient ischemic attack, severe ischemic heart disease, unstable tachycardia, severe valvular heart disease, non-sinus rhythm including atrial fibrillation and other arrhythmias, paced rhythms; 5) regular use of beta-blockers.
From the total of 39 patients with probable MCI, nine subjects (17%) were excluded due to diagnosis of depression with memory complaint, and did not meet MCI diagnostic criteria, and eight (16%) due to undefined diagnosis. Thus, the final MCI sample consisted of 22 subjects.
Sleep-aiding medication information was collected. Of the MCI group (n = 22), ten subjects were under melatonin treatment (3 mg), three participants were under both melatonin (3 mg) and benzodiazepine treatment (0.5–2 mg), one subject was taking both a benzodiazepine (1 mg) and zolpidem (10 mg), and one subject was taking zolpidem (10 mg). Among control subjects (n = 12), six participants were under benzodiazepine treatment (0.25–1 mg), and two participants were taking melatonin (3–50 mg). All individuals under melatonin treatment took it approximately 30 minutes before the expected sleep time. All participants completed the Pittsburgh Sleep Quality Index (PSQI),33 which is a sleep quality self-report questionnaire that assesses sleep problems in the last month to screen for sleep problems. MCI subjects reported a mean score of 8.4 ± 3.4, consistent with mild-to-moderate sleep problems, while control subjects reported a mean score of 5.8 ± 4.3, which reflects none to mild sleep problems. However, comparison between groups did not show significant differences.
The study was approved by the institutional review board at the School of Medicine, University of Buenos Aires and Fleni Foundation and was conducted according to the Declaration of Helsinki. All participants signed an informed consent to participate in the study.
Sleep Assessment
Wrist accelerometers (MicroMini Motionlogger Actigraphs, Ambulatory Monitoring Inc., Ardsley, NY) were used to assess the sleep-wake cycle. Subjects were asked to wear the actigraphs for seven days on their nondominant wrist and to complete a sleep diary. The following variables were derived using the software provided by the manufacturer (Action-W User’s Guide, Version 2.4; Ambulatory Monitoring, Inc., Ardsley, NY): sleep onset (starting time of the first sleep episode after bedtime, as recorded by actigraphy), sleep offset (ending time of the last sleep episode before waking-up time, as recorded by actigraphy), sleep duration (elapsed time between sleep onset and sleep offset), sleep efficiency [(100 X (wake time during sleep / sleep duration)], and wake after sleep onset (WASO, wake minutes during sleep episode).34
Heart Rate Variability
A digital Holter device was used for the recording of the electrocardiogram signal for 24 hours. R waves (ventricular depolarizations) were detected through the device software. RR intervals (time elapsed between R waves) were then computed. Heart rate variability (HRV) indexes were calculated in 30 min bins. An automated filter was used to identify lost or ectopic beats, being replaced by RR intervals resulting from linear interpolation. Segments with more than 20% of missing intervals were excluded from further analysis.30
Time domain analyses were performed by assessing indexes of variation over time. Among these, RRm (mean duration of RR intervals in milliseconds) quantifies the mean heart rate, SDNN (standard deviation of RR intervals in milliseconds) represents a coarse quantification of global variability, and RMSSD (square root of the mean squared differences of successive normal RR) measures short-term heart rate variations. Frequency domain variables provide a measure of the amplitude of the frequencies contributing to the HRV signal. Its high-frequency (HF) component (0.15–0.4 Hz) is mediated by parasympathetic activity and related to respiratory sinus arrhythmia and its low-frequency (LF) component (0.04–0.15 Hz) relies on sympathetic and parasympathetic mechanisms and is related to baroreflex control. Its very low frequency (VLF) component (0.003–0.04 Hz) stems from the outflow of parasympathetic nervous system and its origin has been attributed to humoral factors such as the renin–angiotensin system and to thermoregulatory fluctuations in vasomotor tone.17 Sympathetic predominance is usually reflected by a relative increase in the LF component, while parasympathetic predominance is reflected by an increase in the HF component (or RMSSD), either isolated or accompanied by increases in LF and VLF components as well.35
The Discrete Wavelet Transform (DWT) was chosen instead of the traditional Fast Fourier Transform (FFT) to analyze the frequency components of HRV because it is less sensitive to the presence of nonstationarities or discontinuities. The mean value and the linear trend were subtracted from the signal before applying the DWT. In addition, the signal was evenly sampled with a frequency of 2.4 Hz by means of a spline interpolation algorithm and zero padded to the next higher power of two. A Daubechies four-wavelet function and a nine-level wavelet decomposition were employed to analyze the signal. Using this decomposition, wavelet levels D2-D3 approximately correspond to the high-frequency band (HF, 0.15–0.6 Hz), wavelet levels D4-D5 to the low-frequency band (LF, 0.0375–0.15 Hz), wavelet levels D6-D9 to the very low-frequency band (VLF, 0.0023–0.0375 Hz), and wavelet levels D2-D9 and A9 represent the total power (TP, 0–0.6 Hz). The spectral power of a level is concordant with the square of the standard deviation of wavelet coefficients at each level.36,37
Reported values are expressed as the natural logarithm of TP, HF, LF and VLF wavelet power coefficients (wpc); normalized units (nu) of HF [HFnu= 100XHF/(TP – VLF)]; and the ratio between LF and HF. HRV bins were averaged along wake and sleep periods. Sleep periods were defined based on actigraphy data. HRV differences between sleep and wake averages were also calculated.36
Statistical Analysis
Values are presented as mean ± SEM for numerical variables or frequency and % for categorical variables. Several comparisons between groups and subgroups were conducted. First, demographics, clinical data, sleep-wake characteristics and autonomic activity were compared between MCI patients and controls. Comparisons between these groups were evaluated by means of a t-test for independent samples. For categorical variables “biological sex” and “higher education” a Fisher's exact test was used.
Post-hoc analyses were performed to assess a possible modulating effect of melatonin in autonomic activity when comparing MCI patients and controls. In order to remove the potential effect of melatonin, a further comparison was conducted excluding subjects under melatonin treatment. Additionally, we assessed MCI patients with or without melatonin treatment. Lastly, we assessed the independent effect of MCI and melatonin over sleep-wake characteristics and autonomic activity conducting two-way ANOVA models.
Exploratory studies in early stages such as the present work aim to discover new hypothesis,38 so minimizing Type II errors is highly recommended.39,40 Therefore, false discovery rate (FDR) correction41 at a moderate q = 0.2 was applied to multiple intergroup comparisons and the significance level of two-tailed tests was established at α = 0.05. The value q = 0.2 is a sensitive statistical threshold applied in various exploratory studies on different subjects with promising results.42–45
Results
Demographic and Clinical Data
Demographic and clinical characteristics are depicted in Table 1. Groups were comparable in terms of age, gender and education level. Statistically significant differences in favor of control subjects were observed in verbal episodic memory and learning (RAVLT total learning, RAVLT delayed recall, RAVLT recognition), verbal fluency (semantic fluency), and cognitive flexibility (Trail Making Test B), confirming MCI diagnosis.
 |
Table 1 Demographic Data |
Actigraphy
Table 2 shows the sleep-wake characteristics of the sample. The original sample was reduced to 29 subjects due to technical issues related to actigraphic signal. MCI patients exhibited greater sleep fragmentation (wake after sleep onset) than control subjects. However, this result did not survive correction for multiple comparisons (FDR).
 |
Table 2 Actigraphy Analysis |
Heart Rate Variability
Table 3 summarizes HRV analyses. The original sample was reduced to 28 subjects due to technical issues related to HRV signal quality. No significant differences were displayed between groups in wake, sleep or sleep-wake differences of HRV.
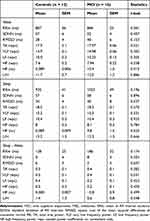 |
Table 3 HRV Analysis |
Post-Hoc Analyses: Melatonin Effect Assessment
Supplementary Table 1 shows demographic and clinical characteristics of controls and MCI patients without melatonin treatment. Removing subjects taking melatonin treatment did not affect the results: both groups remained comparable in terms of age, gender and education level, and controls not taking melatonin still exhibited better performance than MCI patients not taking melatonin in verbal episodic memory and learning (RAVLT delayed recall, RAVLT recognition), verbal fluency (semantic fluency, phonemic fluency) and cognitive flexibility (Trail Making Test B).
Supplementary Table 2 portrays demographic data of MCI patients with and without melatonin treatment. The MCI group under melatonin treatment showed lower education level. No significant differences were observed in cognitive performance.
Supplementary Table 3 shows sleep-wake characteristics in patients with MCI and controls without melatonin treatment. No significant differences were observed. Supplementary Table 4 shows sleep-wake characteristics in MCI patients with and without melatonin treatment. No significant differences were observed.
Table 4 shows the results of the independent effect of MCI and melatonin in sleep characteristics as evidenced by a two-way ANOVA model. No significant effect was observed relating to the presence of MCI or melatonin treatment in sleep characteristics.
 |
Table 4 Actigraphy Multivariate Model |
Supplementary Table 5 displays HRV differences between MCI patients and controls without melatonin treatment. Sleep-wake differences of HRV (RMSSD and absolute values of LF) were decreased in MCI patients, denoting a diminished parasympathetic sleep-wake amplitude in this group.
Supplementary Table 6 shows HRV differences in patients with MCI with and without melatonin treatment. Results show an HRV pattern of greater parasympathetic activity during the night in patients that were taking melatonin as evidenced by the increased values of RRM, SDNN, TA, VLF and LF. Accordingly, sleep-wake differences of HRV were also significantly increased in this group of patients.
Table 5 shows the results of the independent effect of MCI and melatonin in HRV as evidenced by the two-way ANOVA model. The effect of melatonin was confirmed for all variables exhibiting significant differences between MCI patients with and without melatonin treatment (Supplementary Table 6) during sleep and in sleep-wake differences. The effect of MCI was confirmed only for sleep-wake differences of LF.
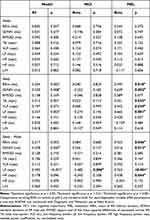 |
Table 5 HRV Multivariate Model |
Discussion
The present results add new information to the limited literature on sleep-wake differences of autonomic activity in MCI patients and the mediating effects of melatonin. The main findings of the present study are 1) MCI patients not taking melatonin displayed a reduced parasympathetic sleep-wake amplitude when compared to controls not taking melatonin during sleep and in sleep-wake differences, 2) the independent effect of MCI was confirmed for sleep-wake differences of LF HRV, and 3) the independent effect of melatonin was confirmed for all variables during sleep and in sleep-wake differences.
No significant findings related to sleep parameters were observed. This could be explained by multiple factors, many relating to the heterogeneous nature of MCI, either associated with severity and staging of the neurodegenerative process or regarding the underlying etiology of the MCI syndrome.46 Therefore, additional sleep abnormalities might manifest at different stages or might not emerge at all in MCI.47,48 MCI is considered a possible transitional stage to dementia in that not all subjects will develop dementia, and those who do, might have different underlying etiologies:1 biomarker studies showed that about 25% of MCI subjects exhibit non-AD pathology.49 Therefore, the various underlying pathologies of the MCI syndrome may not affect sleep equally or at all. Another possibility is that the limited sample size lacks statistical power to exhibit differences related to sleep parameters.
Initial analyses showed no differences in HRV measures, which is not entirely surprising, given the mixed and limited results in this field.15,16,22–25,27,28 When comparing MCI and control subjects without melatonin treatment, a reduced parasympathetic amplitude (reduced RRm, SDNN and absolute values of TA, LF and VLF) was observed in the MCI group during sleep and in sleep-wake differences. Indeed, when performing multivariate analysis controlling for melatonin intake, a moderate negative association between MCI and LF sleep-wake differences was identified.
Our findings align with Kong et al’s, who recently reported reduced parasympathetic function in MCI subjects during sleep.25 It is not surprising that the only significant results observed in the current study were related to the sleep interval, given that MCI and dementia usually exhibit sleep disturbances very early on, even prior to cognitive symptoms50–55 and sleep disorders are mostly related to decreased parasympathetic function during sleep.56,57 Autonomic nervous system activity varies greatly between wakefulness and sleep, exhibiting parasympathetic predominance during sleep. Parasympathetic dysfunction at nighttime and during sleep has already been reported in AD patients,28 which supports our results in MCI patients, arguing for a potential sleep-related parasympathetic vulnerability in this clinical population.25
In light of these observations, we were curious to explore whether melatonin might be mediating sleep-wake effects on the clinical sample, given that over half of the subjects were under melatonin treatment. Actigraphy analyses between MCI patients with versus without melatonin intake did not display significant differences, although actigraphic measures did show significant differences between MCI and control subjects related to sleep fragmentation. A possible explanation could be that patients under melatonin treatment had reported greater sleep difficulties, which is why they were prescribed melatonin in the first place. However, comparison of HRV measures showed MCI patients under melatonin treatment presented greater parasympathetic activity (RRm, SDNN, TA, VLF, LF, HF) during sleep and in sleep-wake differences than patients not taking melatonin. Additionally, two-way ANOVA analysis confirmed this effect of melatonin on HRV parasympathetic variables during sleep and sleep-wake differences, showing a protective effect of melatonin on parasympathetic function during sleep. These findings agree with the literature on the effect of exogenous melatonin on HRV, which suggests that melatonin administration increases parasympathetic activity and reduces sympathetic function,58 and more specifically, that melatonin promotes parasympathetic activity during nighttime sleep.59,60
Limitations for this exploratory study include a small sample size, which may have lacked statistical power to detect additional differences in sleep parameters. Significant results observed in this work have been corrected for multiple comparisons with FDR method, with a moderate threshold of q=0.2 which is considered appropriate for preliminary studies.39 However, subsequent studies with larger sample size should be developed to allow for multiple comparison correction with conservative methods like Bonferroni. Finally, another limitation is related to the potential heterogeneity within the MCI group due to lack of biomarkers to determine underlying pathology behind the MCI syndrome.61
Conclusion
Overall, the present study demonstrated parasympathetic reduction during sleep in MCI patients not taking melatonin. These findings hint at a possible sleep-related parasympathetic vulnerability in patients at prodromal stages of dementia as well as a potential protective effect of exogenous melatonin on such vulnerability. These results complement our previous observation of therapeutic effects of melatonin on cognitive function and depressive symptoms in a different MCI sample62 and contribute to the burgeoning literature on melatonin as an adjunctive treatment in MCI, aiding sleep quality, sleep-wake cycle regulation, and cognition before brain function is too compromised by the underlying neurodegenerative processes of dementia.63 To our knowledge, this is the first study reporting a relationship between melatonin and HRV in MCI patients. Additional, more extensive, longitudinal studies should be performed to confirm the observed results in larger, culturally diverse populations.
Acknowledgments
Carolina Abulafia and María F. Vidal should be considered joint first authors. Carolina Abulafia is postdoctoral fellow from CONICET. Supported by a PICT-2014–0633 Grant from the Agencia de Promoción, Argentine Government.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275(3):214–228. doi:10.1111/joim.12190
2. Ward A, Tardiff S, Dye C, Arrighi HM. Rate of conversion from prodromal Alzheimer’s disease to Alzheimer’s dementia: a systematic review of the literature. Dement Geriatr Cogn Dis Extra. 2013;3(1):320–332. doi:10.1159/000354370
3. Stephan BC, Hunter S, Harris D, et al. The neuropathological profile of mild cognitive impairment (MCI): a systematic review. Mol Psychiatry. 2012;17(11):1056–1076. doi:10.1038/mp.2011.147
4. Jack CR, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–562. doi:10.1016/j.jalz.2018.02.018
5. Buijs FN, Leon-Mercado L, Guzman-Ruiz M, Guerrero-Vargas NN, Romo-Nava F, Buijs RM. The circadian system: a regulatory feedback network of periphery and brain. Physiology. 2016;31(3):170–181. doi:10.1152/physiol.00037.2015
6. Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006;20(1):41–48. doi:10.1097/01.wad.0000201850.52707.80
7. Lim AS, Yu L, Costa MD, et al. Increased fragmentation of rest-activity patterns is associated with a characteristic pattern of cognitive impairment in older individuals. Sleep. 2012;35(5):633–40B. doi:10.5665/sleep.1820
8. Ju YE, McLeland JS, Toedebusch CD, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70(5):587–593. doi:10.1001/jamaneurol.2013.2334
9. Faubel R, Lopez-Garcia E, Guallar-Castillon P, Graciani A, Banegas JR, Rodriguez-Artalejo F. Usual sleep duration and cognitive function in older adults in Spain. J Sleep Res. 2009;18(4):427–435. doi:10.1111/j.1365-2869.2009.00759.x
10. Linden M, Bar T, Helmchen H. Prevalence and appropriateness of psychotropic drug use in old age: results from the Berlin Aging Study (BASE). Int Psychogeriatr. 2004;16(4):461–480. doi:10.1017/s1041610204000420
11. Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349:g5205. doi:10.1136/bmj.g5205
12. Kaufmann CN, Spira AP, Alexander GC, Rutkow L, Mojtabai R. Trends in prescribing of sedative-hypnotic medications in the USA: 1993–2010. Pharmacoepidemiol Drug Saf. 2016;25(6):637–645. doi:10.1002/pds.3951
13. Voyer P, Verreault R, Mengue PN, Morin CM. Prevalence of insomnia and its associated factors in elderly long-term care residents. Arch Gerontol Geriatr. 2006;42(1):1–20. doi:10.1016/j.archger.2005.06.008
14. Ancoli-Israel S. Sleep and aging: prevalence of disturbed sleep and treatment considerations in older adults. J Clin Psychiatry. 2005;66(Suppl 9):
15. Toledo MA, Junqueira LF. Cardiac autonomic modulation and cognitive status in Alzheimer’s disease. Clin Auton Res. 2010;20(1):11–17. doi:10.1007/s10286-009-0035-0
16. Collins O, Dillon S, Finucane C, Lawlor B, Kenny RA. Parasympathetic autonomic dysfunction is common in mild cognitive impairment. Neurobiol Aging. 2012;33(10):2324–2333. doi:10.1016/j.neurobiolaging.2011.11.017
17. Malik M. Heart rate variability: standards of measurement, physiological interpretation, and clinical use: task force of the European Society of Cardiology and the North American Society for Pacing and Electrophysiology. Ann Noninvasive Electrocardiol. 1996;1(2):151–181.
18. Stauss HM. Heart rate variability. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R927–31. doi:10.1152/ajpregu.00452.2003
19. Forte G, Favieri F, Casagrande M. Heart rate variability and cognitive function: a systematic review. Front Neurosci. 2019;13:710. doi:10.3389/fnins.2019.00710
20. Cheng YC, Huang YC, Huang WL. Heart rate variability in patients with dementia or neurocognitive disorders: a systematic review and meta-analysis. Aust N Z J Psychiatry. 2020;4867420976853. doi:10.1177/0004867420976853
21. Liu KY, Elliott T, Knowles M, Howard R. Heart rate variability in relation to cognition and behavior in neurodegenerative diseases: a systematic review and meta-analysis. Ageing Res Rev. 2022;73:101539. doi:10.1016/j.arr.2021.101539
22. Kim MS, Yoon JH, Hong JM. Early differentiation of dementia with Lewy bodies and Alzheimer’s disease: heart rate variability at mild cognitive impairment stage. Clin Neurophysiol. 2018;129(8):1570–1578. doi:10.1016/j.clinph.2018.05.004
23. Nicolini P, Ciulla MM, Malfatto G, et al. Autonomic dysfunction in mild cognitive impairment: evidence from power spectral analysis of heart rate variability in a cross-sectional case-control study. PLoS One. 2014;9(5):e96656. doi:10.1371/journal.pone.0096656
24. Terroba-Chambi C, Abulafia C, Vigo DE, Merello M. Heart rate variability and mild cognitive impairment in Parkinson’s disease. Mov Disord. 2020;35(12):2354–2355. doi:10.1002/mds.28234
25. Kong SDX, Hoyos CM, Phillips CL, et al. Altered heart rate variability during sleep in mild cognitive impairment. Sleep. 2020. doi:10.1093/sleep/zsaa232
26. Grassler B, Dordevic M, Herold F, et al. Relationship between resting state heart rate variability and sleep quality in older adults with mild cognitive impairment. Int J Environ Res Public Health. 2021;18(24):13321. doi:10.3390/ijerph182413321
27. Zulli R, Nicosia F, Borroni B, et al. QT dispersion and heart rate variability abnormalities in Alzheimer’s disease and in mild cognitive impairment. J Am Geriatr Soc. 2005;53(12):2135–2139. doi:10.1111/j.1532-5415.2005.00508.x
28. Kim J-S, Park H-E, Oh Y-S, et al. Blood pressure and heart rate variability in Alzheimer’s disease. Dement Neurocogn Disord. 2015;14(3):128–134.
29. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi:10.1111/j.1365-2796.2004.01388.x
30. Rey A. Rey auditory verbal learning test (RAVLT). In: L’Examen Clinique En Psychologie. PUF; 1964:5–6.
31. Spreen O, Benton A. Neurosensory Center Comprehensive Examination for Aphasia. Victoria (BC): University of Victoria; 1977.
32. Reitan RM, Wolfson D. The Halstead–Reitan neuropsychological test battery for adults—theoretical, methodological, and validational bases. Neuropsychol Assess Neuropsychiatr Neuromed Disord. 2009;1:3–24.
33. Escobar-Córdoba F, Eslava-Schmalbach J. Validación colombiana del índice de calidad de sueño de Pittsburgh. Revista de Neurologia. 2005;40(3):150–155.
34. Diez JJ, Vigo DE, Lloret SP, et al. Sleep habits, alertness, cortisol levels, and cardiac autonomic activity in short-distance bus drivers: differences between morning and afternoon shifts. J Occup Environ Med. 2011;53(7):806–811. doi:10.1097/JOM.0b013e318221c6de
35. Seely AJ, Macklem PT. Complex systems and the technology of variability analysis. Crit Care. 2004;8(6):R367–84. doi:10.1186/cc2948
36. Vigo DE, Tuerlinckx F, Ogrinz B, et al. Circadian rhythm of autonomic cardiovascular control during Mars500 simulated mission to Mars. Aviat Space Environ Med. 2013;84(10):1023–1028. doi:10.3357/asem.3612.2013
37. Pichot V, Gaspoz JM, Molliex S, et al. Wavelet transform to quantify heart rate variability and to assess its instantaneous changes. J Appl Physiol. 1999;86(3):1081–1091. doi:10.1152/jappl.1999.86.3.1081
38. Jaeger RG, Halliday TR. On confirmatory versus exploratory research. Herpetologica. 1998;1998:S64–S66.
39. McDonald JJB. Handbook of Biological Statistics.
40. Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003;19(3):368–375. doi:10.1093/bioinformatics/btf877
41. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statist Soc. 1995;57(1):289–300.
42. Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. Journal of Clinical Epidemiology. 2014;67(8):850–857. doi:10.1016/j.jclinepi.2014.03.012
43. Terroba-Chambi C, Bruno V, Vigo DE, Merello M. Heart rate variability and falls in Huntington’s disease. Clin Autonomic Res. 2021;31(2):281–292. doi:10.1007/s10286-020-00669-2
44. Feng W, Yokoyama JS, Yu S, et al. APOE genotype affects cognitive training response in healthy shanghai community-dwelling elderly individuals. J Alzheimers Dis. 2015;47(4):1035–1046. doi:10.3233/JAD-150039
45. Rhein C, Muhle C, Richter-Schmidinger T, Alexopoulos P, Doerfler A, Kornhuber J. Neuroanatomical correlates of intelligence in healthy young adults: the role of basal ganglia volume. PLoS One. 2014;9(4):e93623. doi:10.1371/journal.pone.0093623
46. Pereira T, Lemos L, Cardoso S, et al. Predicting progression of mild cognitive impairment to dementia using neuropsychological data: a supervised learning approach using time windows. BMC Med Inform Decis Mak. 2017;17(1):110. doi:10.1186/s12911-017-0497-2
47. Wams EJ, Wilcock GK, Foster RG, Wulff K. Sleep-wake patterns and cognition of older adults with amnestic mild cognitive impairment (aMCI): a comparison with cognitively healthy adults and moderate Alzheimer’s disease patients. Curr Alzheimer Res. 2017;14(10):1030–1041. doi:10.2174/1567205014666170523095634
48. Reda F, Gorgoni M, Lauri G, et al. In search of sleep biomarkers of Alzheimer’s disease: k-complexes do not discriminate between patients with mild cognitive impairment and healthy controls. Brain Sci. 2017;7(12):51. doi:10.3390/brainsci7050051
49. Nelson PT, Trojanowski JQ, Abner EL, et al. “New Old Pathologies”: AD, PART, and cerebral age-related TDP-43 with sclerosis (CARTS). J Neuropathol Exp Neurol. 2016;75(6):482–498. doi:10.1093/jnen/nlw033
50. Westerberg CE, Mander BA, Florczak SM, et al. Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J Int Neuropsychol Soc. 2012;18(3):490–500. doi:10.1017/S135561771200001X
51. Jeong J. EEG dynamics in patients with Alzheimer’s disease. Clin Neurophysiol. 2004;115(7):1490–1505. doi:10.1016/j.clinph.2004.01.001
52. D’Rozario AL, Chapman JL, Phillips CL, et al. Objective measurement of sleep in mild cognitive impairment: a systematic review and meta-analysis. Sleep Med Rev. 2020;52:101308. doi:10.1016/j.smrv.2020.101308
53. Naismith SL, Rogers NL, Hickie IB, Mackenzie J, Norrie LM, Lewis SJ. Sleep well, think well: sleep-wake disturbance in mild cognitive impairment. J Geriatr Psychiatry Neurol. 2010;23(2):123–130. doi:10.1177/0891988710363710
54. Cavuoto MG, Kinsella GJ, Ong B, Pike KE, Nicholas CL. Naturalistic measurement of sleep in older adults with amnestic mild cognitive impairment: anxiety symptoms do not explain sleep disturbance. Curr Alzheimer Res. 2019;16(3):233–242. doi:10.2174/1567205016666190301104645
55. Holth J, Patel T, Holtzman DM. Sleep in Alzheimer’s disease - beyond amyloid. Neurobiol Sleep Circadian Rhythms. 2017;2:4–14. doi:10.1016/j.nbscr.2016.08.002
56. Tobaldini E, Nobili L, Strada S, Casali KR, Braghiroli A, Montano N. Heart rate variability in normal and pathological sleep. Front Physiol. 2013;4:294. doi:10.3389/fphys.2013.00294
57. Fink AM, Bronas UG, Calik MW. Autonomic regulation during sleep and wakefulness: a review with implications for defining the pathophysiology of neurological disorders. Clin Auton Res. 2018;28(6):509–518. doi:10.1007/s10286-018-0560-9
58. Nishiyama K, Yasue H, Moriyama Y, et al. Acute effects of melatonin administration on cardiovascular autonomic regulation in healthy men. Am Heart J. 2001;141(5):E9. doi:10.1067/mhj.2001.114368
59. Rechcinski T, Trzos E, Wierzbowska-Drabik K, Krzeminska-Pakula M, Kurpesa M. Melatonin for nondippers with coronary artery disease: assessment of blood pressure profile and heart rate variability. Hypertens Res. 2010;33(1):56–61. doi:10.1038/hr.2009.174
60. Campos LA, Bueno C, Barcelos IP, et al. Melatonin therapy improves cardiac autonomic modulation in pinealectomized patients. Front Endocrinol (Lausanne). 2020;11:239. doi:10.3389/fendo.2020.00239
61. Jacquemont T, De Vico Fallani F, Bertrand A, et al. Amyloidosis and neurodegeneration result in distinct structural connectivity patterns in mild cognitive impairment. Neurobiol Aging. 2017;55:177–189. doi:10.1016/j.neurobiolaging.2017.03.023
62. Cardinali DP, Vigo DE, Olivar N, Vidal MF, Furio AM, Brusco LI. Therapeutic application of melatonin in mild cognitive impairment. Am J Neurodegener Dis. 2012;1(3):280–291.
63. Vecchierini MF, Kilic-Huck U, Quera-Salva MA; Members of the MELcgotS. Melatonin (MEL) and its use in neurological diseases and insomnia: recommendations of the French Medical and Research Sleep Society (SFRMS). Rev Neurol (Paris). 2021;177(3):245–259. doi:10.1016/j.neurol.2020.06.009
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
