Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
An Autologous Topical Serum Derived from Platelet-Rich Plasma Therapy for the Management of Sensitive Skin Alterations: A Case Series Report
Authors García-Millan C, Pino A, Rodrigues R, Segurado-Miravalles G, Alegre-Sánchez A, Jaén P, Anitua E
Received 23 August 2022
Accepted for publication 20 September 2022
Published 29 September 2022 Volume 2022:15 Pages 2077—2086
DOI https://doi.org/10.2147/CCID.S379323
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Cristina García-Millan,1 Ander Pino,2 Rita Rodrigues,1 Gonzalo Segurado-Miravalles,1,3 Adrián Alegre-Sánchez,1 Pedro Jaén,1,3 Eduardo Anitua2
1Grupo de Dermatología Pedro Jaén, Madrid, Spain; 2BTI Biotechnology Institute, Vitoria, Spain; 3University Hospital Ramon y Cajal, Madrid, Spain
Correspondence: Eduardo Anitua, BTI Biotechnology Institute, Jacinto Quincoces 39, Vitoria, Spain, Email [email protected]
Background: Although the underlying pathophysiology of sensitive skin remains unknown, it presents clinical symptoms like erythema, burning and dryness associated with other inflammatory dermatoses such as dermatitis or rosacea.
Objective: The aim of the present report was to provide preliminary data about the efficacy of Endoret-Serum (ES) as an autologous therapy for the topical management of sensitive skin alterations.
Materials and Methods: Five patients underwent a daily topical ES treatment that was maintained for three months. Clinical assessment was carried out using validated dermatological surveys (DLQI, IGA, Likert, PGI-I). Additionally, skin hydration measurement and high-resolution topographic and reflectance confocal imaging analysis were carried out.
Results: No adverse events were observed during the treatment. At the end of the follow-up period, surveys highlighted a significant therapeutic effect compared to baseline. Skin hydration was also improved, and topographic images showed a decrease in patient’s underlying inflammatory and vascular condition.
Conclusion: This preliminary report suggests that Endoret-Serum may be useful in the management of clinical symptoms derived from sensitive skin alterations.
Keywords: platelet-rich plasma, growth factors, sensitive skin, topical formulation, case report
Introduction
The concept of sensitive skin is widely used by patients and is becoming increasingly recognized by dermatologists. Rather than a medical diagnosis, this term is generally used to describe altered skin conditions with clinical signs of inflammation, erythema, stinging, itching, burning, redness, dryness, scaling, peeling, bumps and hives.1 Although often transient, rarely serious and in many cases unaccompanied by visual alterations, sensitive skin affects severely patient’s quality of life.2 Initially, it was believed to be an unusual reaction to common products; however, recent epidemiological studies find a high prevalence of these alterations reaching up to 68% of the population.3 This syndrome is usually associated with a reduced topical medication tolerance and hyperreactivity after cutaneous application that becomes incompatible with the patient’s skin. Nevertheless, the underlying pathophysiology remains unknown and these alterations may also occur in individuals with normal skin or as a part of symptoms associated with other inflammatory dermatoses. In fact, eczema, atopic dermatitis, seborrheic dermatitis and rosacea are likely the most common causes of sensitive skin.4 Other conditions such as contact dermatitis and chronic urticaria have also been shown to elicit the clinical symptomatology.5
Usually, an impaired skin barrier function is associated with sensitive skin, together with an increase in transepidermal water loss (TEWL) and imbalance of intercellular stratum corneum lipids. These factors lead to an increase in cutaneous dryness that leaves the tissue exposed to irritants.6 Individuals show an increased incidence of atopy, higher pH and elevated erythema index which are also observed in other eczematous diseases.7 Additionally, the presence of vasodilation demonstrates the involvement of underlying inflammation that result from the release of neurotransmitters such as substance P and other peptides like interleukin-1/8 and TNF-alpha.8 It has also been reported that the innate immune system triggers an abnormal inflammatory response that mediates blushing erythema. Specific mediators may induce a pro-inflammatory cytokine cascade that trigger vasodilation and other downstream reactions including the activation of peripheral innervation, lymphatic microvasculature spreading and local mast cell degranulation.9
Owing to the multifactorial pathogenesis of sensitive skin, there is no current standardized or validated treatment. However, several actives can be used to control or improve various aspects of the disease related alterations. In particular, moisturizers and optimized lipid mixtures can be used to improve barrier function.10 Anti-inflammatory compounds like flavonoids, corticosteroids and antioxidants can minimize inflammatory reactions and modulate vascular reactivity. Specific inhibitors of vanilloid receptors have also been shown to be helpful in the management of the disease.11 Nevertheless, current dermatological treatments do not always reach the desired efficacy; hence, novel therapies are essential to reduce morbidity.
In this sense, the progression in the field of biological treatments has led to the emergence of autologous products based on the patient’s own bioactive proteins known as growth factors. Human origin preparations like platelet-rich plasma (PRP) and derivatives have emerged as an intriguing modality designed to promote skin regeneration.12 In fact, several findings suggest that these preparations may elicit an important anti-inflammatory and immunomodulatory effect for the management of different dermatological conditions.13–15 Recently, a topical formulation based on plasma rich in growth factors technology (PRGF) has been developed.16 This product, known as Endoret-Serum (ES), is 100% autologous and can be in situ prepared after patient’s blood withdrawal. Additionally, it has been shown that the formulation maintains its physicochemical and biological properties after long-term storage.17 ES contains a pool of bioactive proteins that undergo a sustained percutaneous absorption and play a major role in cutaneous regeneration.18 Unlike other PRPs, ES has been designed for a topical and needle-free application with no need of physician intervention. Thus, ES provides a minimally invasive approach for maintaining a sustained dosage of growth factors in the management of chronic dermatological conditions. Here, preliminary results about ES in the management of sensitive skin alterations are reported.
Materials and Methods
The study was conducted following the principles established in the Declaration of Helsinki amended in 2013, and patients gave their informed consent. All described procedures were performed according to the common clinical practice of the centre. Patients gave consent for their case and photograph publication. The manuscript is exempt from ethical committee approval. The case series that are described in the manuscript are based on a regular clinical practice and no control group is included. The treatment described is obtained by using a commercially available kit with CE clearance that can be used under medical prescription. The Spanish Agency of Medicines and Medical Devices indicates that a case-by-case authorization by a local ethical committee is not required when using platelet-rich plasma as a regular clinical practice.
Endoret-Serum Preparation
Manufacturer’s instructions were followed for Endoret-Serum (ES) preparation (KMU10-TPC, BTI Biotechnology Institute, Vitoria, Spain). Peripheral blood was harvested into 9-mL collection tubes containing 0.4 mL of 3.8% (wt/v) trisodium citrate as anticoagulant. Afterwards, the blood was centrifuged (580G 8 minutes) and the whole plasma column was collected, avoiding the leukocyte-rich buffy coat. Part of the plasma was steadily gelated (76°C) and mixed with the remaining calcium chloride-activated plasma at a 2:1 ratio. Once obtained, the ES was dispensed into airless pump bottles that were specially designed to avoid external air intake.
The platelet enrichment before ES preparation was measured with a haematology analyser (Micros 60, Horiba ABX, Montpellier, France). The pH of the product was checked with a pH meter GLP 21+ (Sigma-Aldrich, St. Louis, MO). A rheometer AR 1000 (TA Instruments, New Castle, EEUU) was used to determine the viscosity of the formulation. One mL of sample was applied to the sample holder, and viscosity was determined at a shear rate of 1s−1 at 25°C. For growth factor content determination, extracts were subjected to enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN). Several key proteins for skin regeneration were quantified including epidermal growth factor (EGF), insulin-like growth factor-I (IGF-I), platelet-derived growth factor-AB (PDGF-AB) and transforming growth factor-β1 (TGF-β1).
Patients
Patients between 30 and 60 years old from “Grupo Dermatología Pedro Jaén Clinic” (Madrid, Spain) were treated. A total of five patients with clinically diagnosed symptoms of sensitive skin alteration within the facial area were treated.
Endoret-Serum Treatment
Subjects did not use their usual facial formulations during ES treatment. A single extraction of peripheral blood and personalized ES preparation was carried out for each participant as described above. The ES was used every 12 hours. Prior to application patients cleansed their face and washed their skin with water. Afterwards, the product was applied over the face using slight rubbing movements in order to optimize surface area covered and was left to air dry. Patients underwent an uninterrupted treatment of ES for three months. The product was cold stored at 4°C during treatment.
Clinical Assessment
Participants were clinically assessed at baseline and at one and three months after the beginning of the treatment. Any undesired side effects or adverse reactions were recorded. The clinical assessment was performed by trained technicians in a controlled environment. Skin hydration was determined by triplicate using a Moisture-checker MY-808S based on the electrical capacitance of the skin (Scalar corporation, Tokyo, Japan). Reflectance confocal microscopic images (RCM) of the affected areas were obtained as a non-invasive technique for in vivo skin inflammation assessment (VivaScope 1500, Caliber Imaging and Diagnostics, Rochester, NY, USA). The Reveal System (Canfield Imaging Systems, Fairfield, NJ) was employed as a topographic imaging technology to obtain high resolution photographs with the aim of assessing the skin underlying condition only. During the follow-up period, subjects were asked to complete a self-assessment questionnaire and rated their overall satisfaction using a Likert’s scale. Additionally, patients fulfilled the “Dermatology Life Quality Index (DLQI)” as a measurement of the impact of their dermatological condition on their life quality at baseline and during the study. Participants also completed the “Patient Global Impression of Improvement scale (PGI-I)” by comparing their post-treatment condition to baseline status. Finally, two trained clinicians were asked to fulfil the “Investigator’s Global Assessment survey (IGA)” at the end of treatment. Self-assessment and clinical improvement questionnaires fulfilled by patients and healthcare specialists are summarized in Table 1.
 |
Table 1 Self-Assessment and Clinical Improvement Questionnaires Fulfilled by Patients and Healthcare Specialists During the Follow-Up Period |
Results
Four women and one man that met the inclusion criteria were selected. The mean age of the patients was 43 ± 10 years old. No adverse events were observed during the treatment and follow-up period. At baseline, patients presented sensitive skin alterations that were also related to other dermatoses such as dermatitis/eczema, rosacea, melasma and seborrheic dermatitis. Before ES therapy, medicines that were prescribed for the treatment of patients’ condition included moisturizers, topical lotions, cleansing agents, anti-itching creams, steroid creams, topical antihistamines and pain relievers. However, these medicaments had achieved moderate efficacy on participants’ life quality. For this reason, ES was prescribed as an alternative topical therapy.
The hematologic analysis revealed that the formulation was obtained from a leukocyte-free platelet enriched plasma. ES presented a neutral pH value and showed a shear thinning viscosity behaviour which is common in pseudoplastic topical lotions with optimal rheologic profile (Table 2). The growth factor content within ES was quantified using enzyme-linked immunosorbent assays. As it is summarized in Table 2, high levels of key proteins involved in skin regeneration such as EGF, IGF-I, PDGF-AB and TGF-β1 were detected.
 |
Table 2 Biological and Physicochemical Characterization of Endoret-Serum |
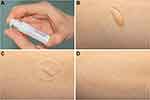
Subjects referred a pleasant fresh and relieving feeling immediately after the hydro-lotion administration (Figure 1A). The viscous nature of the product and the storage device allowed an easy handling of the formulation which was translated into a soft superficial application over the skin that rapidly got integrated within the tissue (Figure 1B–D). At baseline DLQI-index showed that the dermatological condition affected the life quality of patients in a moderate to very large way (10.4 ± 5.3). However, after one and three months of ES treatment subjects referred a significant improvement as 1.4 ± 1.3 and 0.8 ± 1.1 DLQI-index were achieved, respectively (no effect on patient’s life) (Figure 2A).
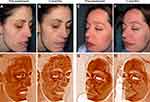
Before the beginning of the treatment, the skin showed a hydration of 27.4% ± 3.9%. During ES treatment, cutaneous moisture increased to 29.4% ± 4.6% and 35% ± 4.8% after one and three months, respectively (Figure 2B). In some patients, cutaneous RCM evaluation showed a decrease in dermal inflammatory infiltrate when comparing baseline and post-treatment status (Figure 3A). Self-assessment questionnaire showed that patients referred to be very satisfied with ES treatment as 8.4 ± 1.5 and 8.4 ± 0.9 in Likert’s score was achieved after one and three months, respectively (Figure 3B). Participants also referred a “much better” condition when compared to their baseline dermatological status (2.2 ± 0.8 and 2.4 ± 0.5 PGI-I score at one and three months, respectively) (Figure 3B). Healthcare specialists concluded that the symptoms at the end of the study were resolved or minimal (1.0 ± 0.0 and 0.8 ± 0.5 IGA score after one and three months, respectively) (Figure 3B).
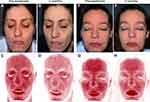
Reveal software and RBX images highlighted a noticeable improvement in the underlying vascular condition of patients after ES therapy. Erythema and overall skin inflammation decreased along with reduction of intermittent flushing and blushing. The excess of haemoglobin within the vascular structure at the papillary dermis, which is responsible for red coloration of skin tone, is shown to diminish (Figure 4). The abnormal melanin concentration at the epidermal layer, which is associated with hyperpigmented spots, was also shown to be reduced at the end of the study (Figure 5).
Discussion
In the last few years, the concept of endogenous regenerative medicine is gaining the attention of dermatologists. Plasma rich in growth factors (Endoret-PRGF) is a specific technology based on platelet-rich plasma therapy. A small blood sample is obtained and is briefly centrifuged at low rate in order to obtain a leucocyte-free platelet-rich volume at an optimal concentration.19 The alpha granules within platelets behave as a natural reservoir of growth factors and bioactive proteins that are released to the local milieu with the aim of promoting tissue repair and regeneration. These morphogens include EGF, IGF-I, PDGF-AB and TGF-β1. The biologic potential of PRGF has been widely evaluated in different medical fields such as oral/maxillofacial surgery, sports medicine, traumatology and ophthalmology.20–24 Moreover, several studies have demonstrated its regenerative effect when facing different dermatological disorders.25–29 As a versatile technology, further advances in the field have enabled the development of different therapeutic formulations that can be easily prepared and used in the clinical setting.
In this study, Endoret-Serum (ES) has been tested as a topical formulation for the management of sensitive skin alterations. There are several recognised medical causes of sensitive skin, such as irritant or allergic dermatitis, contact urticaria, rosacea, melasma, aquagenic pruritus, eczema or cutaneous mastocytoses.30 Dry skin and epidermal barrier disturbance are the main symptoms associated with these dermatoses.6 Results presented herein showed that a daily topical application of ES resulted in an increase of surface moisture. These results are consistent with previous studies were PRGF treatment increased skin hydration as measured by a corneometer in aged skin.31 This could be related to the previously reported ability of ES to induce glycosaminoglycan and fibrillar structure production and their ability to retain water.16
In the present study, participants described ES as an opaque yellowish and odourless serum, with smooth texture and homogeneous oil-in-water emulsion appearance. During the follow-up period, ES modulated cutaneous inflammation and ameliorated some of the main symptoms of sensitive skin such as local erythema, burning and redness. In line with these outcomes, recent studies have suggested that PRGF is able to mitigate inflammation and pain due to interconnected acute-phase proteins, linked to the nuclear-factor-κβ (NF-κβ) pathway and peripheral endocannabinoid metabolism.32,33 In fact, it has been also demonstrated that PRGF reduces the expression of pro-inflammatory cytokines such as IL-1, IL-8 and TNF-alpha in hostile microenvironments.34 These molecules are important mediators in several cutaneous dermatoses that trigger abnormal vasodilation and excessive inflammatory cell recruitment.8 The biologic mechanism of PRP may also be associated with its ability to reduce pro-inflammatory M1 macrophage phenotype and foster the differentiation of monocytes to anti-inflammatory cell populations.35,36 Other findings reveal that key inflammatory activators such as TLR4 and p-p65 can be inhibited by use of platelet-rich plasma.35
In this study, the presence of hyperpigmented melanin spots showed a slight reduction after ES treatment. Melanin deposition usually occurs during photo-oxidative stress after solar damage. In this sense PRGF has previously been shown to reduce cutaneous photo-damage including collagen deterioration, interstitial oedema and actinic elastosis.37,38 Additional findings revealed that ES downregulated the expression of reactive oxygen species (ROS) and free radicals over UV irradiated skin models. In addition, ES prevented DNA cleavage that occurs after solar exposure which is directly related to a decrease in tissue necrosis and cell apoptosis.39 Moreover, it has been suggested that PRGF mediated photo-protection may be directly related to the activation of several antioxidant enzymes and the reduction of apoptotic caspases that ultimately maintain tissue viability.40,41 Thus, hyperpigmented melanin spot reduction observed herein could be an additional effect of ES when counteracting solar photo-damage symptoms.
During the follow-up period, patients included in this study fulfilled specific surveys and dermatological life quality tests. Taken together, anti-inflammatory and photo-protective outcomes are usually related to a good prognosis of several skin conditions such as acne, rosacea, dermatitis, melasma, photoaging and telangiectasia.42 Therefore, participants and physicians referred a noticeable improvement of their symptoms with no signs of adverse events. Accordingly, researchers are beginning to envisage injectable platelet-rich plasma as an alternative for the treatment of eczema, psoriatic plaques, radiodermatitis, chronic urticaria, vitiligo, lichen sclerosus or leprosy.43
At the European level, there are no real common rules for platelet-rich plasma therapies that are applicable to all member countries. The European Medicines Agency (EMA) has certain regulations, but each state has the authority to independently regulate PRP therapy.44,45 As this study has been conducted in Spain, this work has been conducted in accordance with Spanish guidelines. The Spanish Agency of Medicines and Medical Devices (AEMPS) stands a comprehensive report and subsequent resolution regarding the medicinal classification of PRP.44,45 AEMPS has clarified that, although it is a medicinal product, obtaining PRP does not involve an industrial process and therefore PRP cannot be considered an industrially produced medicinal product. AEMPS states that the use of PRP must be prescribed by doctors with appropriate qualification and that it must be obtained using commercially available disposable kits. These kits must always be medical device and have the Conformite Europeenne (CE) clearance indicating that they comply with European directives and should be used following the manufacturer’s instructions.44,45 ES preparation is attached to the use of a disposable kit which is a medical device with CE clearance.
Conclusion
To the author’s knowledge this is the first study where a platelet based 100% autologous topical product is used for the management of sensitive skin-related alterations. However, the present study presents important limitations in its design such as the low sample size, the short follow-up period and the absence of a control group. Hence, additional randomized and controlled clinical trials that consider additional outcomes such as TEWL are needed to clearly assess the efficacy and safety of ES. Nevertheless, this pilot study provides valuable preliminary data that would support further research involving the therapeutic use of Endoret-Serum over inflammatory dermatological conditions.
Ethical Statement
The study was conducted following the principles established in the Declaration of Helsinki amended in 2013, and patients gave their informed consent. All described procedures were performed according to the common clinical practice of the centre. Patients gave consent for their case and photograph publication. The manuscript is exempt from ethical committee approval. The case series that are described in the manuscript are based on a regular clinical practice and no control group is included. The treatment described is obtained by using a commercially available kit with CE clearance that can be used under medical prescription. The Spanish Agency of Medicines and Medical Devices indicates that a case-by-case authorization by a local ethical committee is not required when using platelet-rich plasma as a regular clinical practice.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Eduardo Anitua is the scientific director and Ander Pino is researcher at BTI Biotechnology Institute, the company that has developed the Endoret®PRGF® technology. The rest of the authors declare no conflict of interest. No funding was received for this study.
References
1. Misery L. Sensitive skin. Expert Rev Dermatol. 2013;8(6):631–637. doi:10.1586/17469872.2013.856688
2. Farage MA, Maibach HI. Sensitive skin: closing in on a physiological cause. Contact Dermatitis. 2010;62(3):137–149. doi:10.1111/j.1600-0536.2009.01697.x
3. Farage M. How do perceptions of sensitive skin differ at different anatomical sites? An epidemiological study. Clin Exp Dermatol. 2009;34(8):e521–e30. doi:10.1111/j.1365-2230.2009.03487.x
4. Lev-Tov H, Maibach HI. The sensitive skin syndrome. Indian J Dermatol. 2012;57(6):419. doi:10.4103/0019-5154.103059
5. Misery L, Boussetta S, Nocera T, Perez‐Cullell N, Taieb C. Sensitive skin in Europe. J Eur Acad Dermatol Venereol. 2009;23(4):376–381. doi:10.1111/j.1468-3083.2008.03037.x
6. Berardesca E, Farage M, Maibach H. Sensitive skin: an overview. Int J Cosmet Sci. 2013;35(1):2–8. doi:10.1111/j.1468-2494.2012.00754.x
7. Seidenari S, Francomano M, Mantovani L. Baseline biophysical parameters in subjects with sensitive skin. Contact Dermatitis. 1998;38(6):311–315. doi:10.1111/j.1600-0536.1998.tb05764.x
8. Reilly D, Parslew R, Sharpe G, Powell S, Green M. Inflammatory mediators in normal, sensitive and diseased skin types. Acta Dermato-Venereologica. 2000;80(3):171–174.
9. Quatresooz P, Piérard‐Franchimont C, Piérard GE. Vulnerability of reactive skin to electric current perception–a pilot study implicating mast cells and the lymphatic microvasculature. J Cosmet Dermatol. 2009;8(3):186–189. doi:10.1111/j.1473-2165.2009.00445.x
10. Honari G, Andersen R, Maibach HL. Sensitive Skin Syndrome. CRC Press; 2017.
11. Pauly G, Moussou P, Contet‐Audonneau JL, et al. New peptidic active ingredient to reduce discomfort and painful sensations in sensitive skin. Int J Cosmet Sci. 2009;31(6):480. doi:10.1111/j.1468-2494.2009.00532_4.x
12. Anitua E, Pino A, Orive G. Opening new horizons in regenerative dermatology using platelet‐based autologous therapies. Int J Dermatol. 2017;56(3):247–251. doi:10.1111/ijd.13510
13. Choi SY, Lee YJ, Kim JM, Kang HJ, Cho SH, Chang SE. Epidermal growth factor relieves inflammatory signals in staphylococcus aureus-treated human epidermal keratinocytes and atopic dermatitis-like skin lesions in Nc/Nga mice. Biomed Res Int. 2018;2018(7):1–9.
14. Kumaravel S, Manjula J, Balamurugan L, Sindhuja S, Anandan H. Chronic autoimmune urticaria and efficacy of autologous serum therapy. Int J Sci Study. 2017;4(11):163–166.
15. Ghani R. Platelet rich plasma use in the treatment of eczema (atopic dermatitis): case report. Glob Sci J. 2018;6(12):22–31.
16. Anitua E, Troya M, Pino A. A novel protein‐based autologous topical serum for skin regeneration. J Cosmet Dermatol. 2020;19(3):705–713. doi:10.1111/jocd.13075
17. Anitua E, Pino A, Troya M. Biological stability of plasma rich in growth factors-derived autologous topical serum after three-months storage. J Drugs Dermatol. 2018;17(10):1115–1121.
18. Anitua E, Pino A. The management of postsurgical wound complications with plasma rich in growth factors: a preliminary series. Adv Skin Wound Care. 2020;33(4):202–208. doi:10.1097/01.ASW.0000604168.62330.c7
19. Anitua E, Muruzabal F, de la Fuente M, Riestra A, Merayo-Lloves J, Orive G. PRGF exerts more potent proliferative and anti-inflammatory effects than autologous serum on a cell culture inflammatory model. Exp Eye Res. 2016;151(1):115–121. doi:10.1016/j.exer.2016.08.012
20. Sánchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012;28(8):1070–1078. doi:10.1016/j.arthro.2012.05.011
21. Anitua E, Sanchez M, Nurden AT, et al. Autologous fibrin matrices: a potential source of biological mediators that modulate tendon cell activities. J Biomed Mater Res A. 2006;77(2):285–293. doi:10.1002/jbm.a.30585
22. Anitua E, Begoña L, Orive G. Treatment of hemimandibular paresthesia in a patient with bisphosphonate-related osteonecrosis of the jaw (BRONJ) by combining surgical resection and PRGF-Endoret. Br J Oral Maxillofacial Surg. 2013;51(8):e272–e274. doi:10.1016/j.bjoms.2012.08.018
23. Anitua E, Muruzabal F, Tayebba A, et al. Autologous serum and plasma rich in growth factors in ophthalmology: preclinical and clinical studies. Acta Ophthalmol. 2015;93(8):e605–e614. doi:10.1111/aos.12710
24. Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofacial Implants. 1999;14(4):529–535.
25. Anitua E, Aguirre JJ, Algorta J, et al. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J Biomed Mater Res Part B. 2008;84(2):415–421. doi:10.1002/jbm.b.30886
26. Ramos-Torrecillas J, García-Martínez O, De Luna-Bertos E, Ocaña-Peinado FM, Ruiz C. Effectiveness of platelet-rich plasma and hyaluronic acid for the treatment and care of pressure ulcers. Biol Res Nurs. 2015;17(2):152–158. doi:10.1177/1099800414535840
27. Aguirre JJ, Anitua E, Francisco S, Cabezas AI, Orive G, Algorta J. Efficacy and safety of plasma rich in growth factors in the treatment of venous ulcers: a randomized clinical trial controlled with conventional treatment. Clin Dermatol. 2015;3(1):13–20.
28. Cardenosa ME, Domínguez-Maldonado G, Córdoba-Fernández A. Efficacy and safety of the use of platelet-rich plasma to manage venous ulcers. J Tissue Viability. 2017;26(2):138–143. doi:10.1016/j.jtv.2016.11.003
29. de la Portilla F, Segura-Sampedro J, Reyes-Díaz M, et al. Treatment of transsphincteric fistula-in-ano with growth factors from autologous platelets: results of a Phase II clinical trial. Int J Colorectal Dis. 2017;32(11):1545–1550. doi:10.1007/s00384-017-2866-9
30. Buhé V, Vié K, Guéré C, et al. Pathophysiological study of sensitive skin. Acta Dermato-Venereologica. 2016;96(3):314–319.
31. Anitua E, Sánchez M, Sarabia R, Sanz J, Aguirre J, Orive G. Efficacy and safety of PRGF in facial skin regeneration. Randomized clinical trial controlled with hyaluronic acid. J Spanish Assoc Aesthetic Plastic Surg. 2011;2011(1):23–33.
32. Anitua E, Prado R, Azkargorta M, et al. High‐throughput proteomic characterization of plasma rich in growth factors (PRGF–Endoret)‐derived fibrin clot interactome. J Tissue Eng Regen Med. 2015;9(11):E1–E12. doi:10.1002/term.1721
33. Descalzi F, Ulivi V, Cancedda R, et al. Platelet-rich plasma exerts antinociceptive activity by a peripheral endocannabinoid-related mechanism. Tissue Eng Part A. 2013;19(19–20):2120–2129. doi:10.1089/ten.tea.2012.0557
34. Anitua E, Zalduendo M, Troya M, Padilla S, Orive G. Leukocyte inclusion within a platelet rich plasma-derived fibrin scaffold stimulates a more pro-inflammatory environment and alters fibrin properties. PLoS One. 2015;10(3). doi:10.1371/journal.pone.0121713
35. Zhang J, Yin C, Zhao Q, et al. Anti‐inflammation effects of injectable platelet‐rich fibrin via macrophages and dendritic cells. J Biomed Mater Res A. 2020;108(1):61–68. doi:10.1002/jbm.a.36792
36. Papait A, Cancedda R, Mastrogiacomo M, Poggi A. Allogeneic platelet‐rich plasma affects monocyte differentiation to dendritic cells causing an anti‐inflammatory microenvironment, putatively fostering wound healing. J Tissue Eng Regen Med. 2018;12(1):30–43. doi:10.1002/term.2361
37. Díaz‐Ley B, Cuevast J, Alonso‐Castro L, et al. Benefits of plasma rich in growth factors (PRGF) in skin photodamage: clinical response and histological assessment. Dermatol Ther. 2015;28(4):258–263. doi:10.1111/dth.12228
38. Anitua E, Pino A, Jaen P, Orive G. Plasma rich in growth factors enhances wound healing and protects from photo-oxidative stress in dermal fibroblasts and 3D skin models. Curr Pharm Biotechnol. 2016;17(6):556–570. doi:10.2174/1389201017666160301104139
39. Anitua E, Goñi F, Gomez P, Tierno R, Pino A, Pino A. A novel autologous topical serum based on plasma rich in growth factors technology counteracts ultraviolet light derived photo-oxidative stress. Skin Pharmacol Physiol. 2020;33(3):1–15. doi:10.1159/000507716
40. Anitua E, de la Fuente M, Del Olmo‐Aguado S, Suárez‐Barrio C, Merayo‐Lloves J, Muruzábal F. Plasma rich in growth factors reduces blue light‐induced oxidative damage on retinal pigment epithelial cells and restores their homeostasis by modulating vascular endothelial growth factor and pigment epithelium‐derived factor expression. Clin Experiment Ophthalmol. 2020;48(6):830–838. doi:10.1111/ceo.13767
41. Anitua E, Pino A, Orive G. Plasma rich in growth factors inhibits ultraviolet B induced photoageing of the skin in human dermal fibroblast culture. Curr Pharm Biotechnol. 2016;17(12):1068–1078. doi:10.2174/1389201017666160709200920
42. Primavera G, Berardesca E. Sensitive skin: mechanisms and diagnosis. Int J Cosmet Sci. 2005;27(1):1–10. doi:10.1111/j.1467-2494.2004.00243.x
43. Andia I, Maffulli N. A contemporary view of platelet-rich plasma therapies: moving toward refined clinical protocols and precise indications. Regen Med. 2018;13(6):717–728. doi:10.2217/rme-2018-0042
44. Anitua E, Prado R, Orive G. Closing regulatory gaps: new ground rules for platelet-rich plasma. Trends Biotechnol. 2015;33(9):492–495. doi:10.1016/j.tibtech.2015.07.002
45. Anitua E, Prado R, Orive G. A new regulatory framework for platelet-rich plasma in Spain. J Knee Surg. 2015;28(04):355–356. doi:10.1055/s-0035-1549025
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.