Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Altered Function of Superior Parietal Lobule Associated with Perceptive Awareness in First-Episode Drug-Naïve Panic Disorders: A Preliminary fMRI Study
Authors Jin H, Zhang B , Cui H, Li W, Li H, Hu Q, Wang J, Li C
Received 5 February 2020
Accepted for publication 15 June 2020
Published 3 July 2020 Volume 2020:16 Pages 1653—1659
DOI https://doi.org/10.2147/NDT.S248453
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Haiyan Jin,1,2,* Bin Zhang,3,4,* Huiru Cui,1 Wei Li,1 Hui Li,1 Qiang Hu,1 Jijun Wang,1,5,6 Chunbo Li1,5,6
1Shanghai Key Laboratory of Psychotic Disorders, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 3The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, People’s Republic of China; 4Guangdong Engineering Technology Research Center for Translational Medicine of Mental Disorders, Guangzhou, People’s Republic of China; 5Center for Excellence in Brain Science and Intelligence Technology (CEBSIT), Chinese Academy of Sciences, Shanghai, People’s Republic of China; 6Brain Science and Technology Research Center, Shanghai Jiao Tong University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chunbo Li
Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Wanpingnan Road 600, Shanghai 200030, People’s Republic of China
Email [email protected]
Bin Zhang
The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Mingxin Road 35, Guangzhou 510370, People’s Republic of China
Tel +862281268203
Email [email protected]
Background: Biased fear-related perception is one main characteristic in patients with panic disorder (PD) and their prominent cardiovascular symptoms associated with enhanced heartbeat perception.
Patients and Methods: We investigated interoceptive perception in 18 first-onset drug-naïve PD patients and 21 age- and gender-matched healthy controls (HC). Moreover, we compared blood oxygen level-dependent (BOLD) responses between the two groups during a heartbeat perception (interoception) task to assess task-evoked activity and its relationship with heartbeat perception scores (HPSs).
Results: We found that patients with PD compared to HCs revealed a trend higher but insignificant HPSs. Higher activity in the bilateral superior parietal lobule (SPL) was observed in PD patients compared to HCs during the perception of both heartbeats and pure tones compared to rest. Furthermore, patients with PD exhibited a significant positive correlation between BOLD activity in the left SPL during heartbeat > resting-state and HPS.
Conclusion: Using a sample of first-episode drug-naïve patients, our study reports that patients with PD show altered activation in the bilateral SPL during both interoceptive and exteroceptive perception. The increased activation during interoceptive stimuli might render PD patients more engaged in processing information associated with their internal states.
Keywords: panic disorder, MRI, perception, superior parietal lobule
Introduction
Panic disorder (PD) is characterized recurrent unexpected panic attacks with symptoms like palpitation, sweating, and feeling of impending death.1 The enhanced interoceptive processing of cardiovascular states such as rapid heartbeat is considered to play an important role in the maintenance of PD, which triggers the vicious circle of fear and increases the probability of a panic attack.2
Recent studies support the role of the biased perception in the development and maintenance of PD.3,4 Patients with PD suffer from high number of cardiovascular symptoms, which increases cardiac liability and heightens sensitivity to bodily responses.5 Since the PD patients are afraid that the bodily (cardiovascular) symptoms might lead to catastrophic consequences (eg heart attack), they monitor excessively their cardiovascular state, especially the heartbeat. Heartbeat perception score (HPS) was developed to measure the interoceptive sensitivity.1 Previous studies have reported higher HPS in PD,6 indicating their abnormal interoceptive processing, including visceroception and proprioception.1
Despite several behavioral studies in PD reporting altered interoceptive awareness,1,6,8 the neurofunctional correlates of biased interoception in PD remains poorly understood. Neuroimaging had been applied to explore brain activation patterns in PD. Based on recent research, patients with PD showed decreased activation in the superior parietal lobule (SPL) and middle frontal gyrus (MFG) in the alert state, while cognitive behavioral therapy (CBT) results in increased activation of these two regions.9 However, another study reported decreased parietal lobe activation in remitted PD patients, with improvements in severity of panic symptoms correlating negatively with changes of activity in the right SPL.10 Further, the SPL is activated during self-related tasks.11–13 Considering the above findings, SPL may be a key region for investigating the neurofunction of PD, especially during interoceptive awareness task.
In the current study, we investigated brain activity during interoceptive awareness in PD patients, using task fMRI, during which the patients silently counted the number of heartbeats and number of tones. In order to avoid confounding factors related to drug treatment, we chose first-episode drug-naïve patients with PD. We aimed to find the neural correlates of altered interoceptive awareness, observed in PD.
Patients and Methods
Subjects
Eighteen subjects with PD and 21 age- and gender-matched healthy control (HC) subjects without mental disorders were recruited (Table 1). Patients were recruited as outpatients in Shanghai Mental Health Center (SMHC). HCs were recruited through advertisements. Blood pressure measurements and an electrocardiogram were conducted to ensure normal cardiovascular function of all study participants.
 |
Table 1 Demographic and Clinical Characteristics of the Participants |
Diagnosis of PD patients was confirmed by an expert psychiatrist based on DSM-5 criteria, and all subjects were further examined by two research doctors using the Mini International Neuropsychiatric Interview (MINI), Chinese version (citation). Inclusion criteria included: 1) age range of 18–60 years; 2) ≥ 6 years education; 3) scores on the Hamilton Anxiety Scale (HAMA) ≥ 14 and on the Hamilton Depression Scale (HAMD) < 14; and 4) first episode of PD. Exclusion criteria included: 1) intellectual disability, dementia, and other neurological illnesses; 2) head trauma leading to loss of consciousness; 3) severe somatic disease, such as cancer, heart failure, or pneumonia; 4) current substance abuse or dependence; 5) presence or history of other mental disorders; 6) contraindication to magnetic resonance scanning; 7) receiving any kind of drugs for syndromes of anxiety; and 8) PD patients with comorbid physical diseases.
HCs were screened using the MINI to rule out mental disorders. The inclusion and exclusion criteria used for HCs were identical to PD subjects except the third and fourth inclusion criteria.
This study was approved by the Research Ethics Committee at the SMHC and was conducted in accordance with the Declaration of Helsinki. All participants signed a written informed consent.
Assessment of Heartbeat Perception
Heartbeat perception assessment was performed using the mental tracking paradigm.14 All participants were asked to feel their heartbeat, count and report the number of heartbeats. During the measuring period outside the MRI scanner, a portable electrocardiograph was applied to record the number of occurrences of R waves and determine the true number of heartbeats. The experiment was repeated three times, and the heartbeat counting was performed for the length of 26, 21, and 36 s, randomly. Then, data were analyzed using the following formula. The HPS was calculated to measure the accuracy of heartbeat perception, and the full score was 1. Higher scores indicated higher heartbeat perception level. In the current study, four patients with PD and 10 HCs were not tested for HPS.
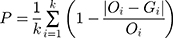
where “P” indicates HPS, “i” the number of times each experiment was performed, “O” the actual heartbeat number of each subject during the experiment, and “G” the heartbeat number counted by each subject.
Task Paradigm
The fMRI task was designed and administered using the E-Prime software package (Psychology Software Tools INC, US). Initially, the fMRI Hardware System (IFIS-SA, Invivo Corporation, US) was used to project experimental stimuli through a beamer into a mirror that was located in the head coil. The auditory stimuli were presented to the participants through Sensimetrics S14 insert earphone (Huth, de Heer, Griffiths, Theunissen, and Gallant, 2016) inside the soundproof earmuff. This promised high-quality stimuli presentation and avoided the noise impact from the MRI scanner.
An event-related functional MRI design for exploring the awareness of interoceptive and exteroceptive was used in the current study, based on the paradigm introduced by Pollatos and Critchley and modified by Wiebking.15–17 The experiment consisted of four scanning runs, and each run consisted of three conditions (9–13 s each), which presented 48 times in a pseudo-randomized order. The total experiment lasted 9.6 min. The three conditions included a heartbeat task (interoception), a pure-tone task (exteroception), and a rest state. Visual stimuli were projected onto a projection screen using an LCD projector through an adjustable mirror, angled 45° to the individual’s eyesight. Before each scanning session, subjects were instructed to adjust the tone volume to the same level of their heartbeat in order to equalize the difficulty of the pure-tone and heartbeat tasks. During interoceptive conditions (heartbeat), a dark colored heart was presented for 9–13 s. During this time, participants were asked to count their heartbeat silently. Afterwards, they reported the number of heartbeats on one rating scale (4 s) via pressing buttons. This feedback allowed the monitoring of each participant’s attention to the task. During the exteroceptive conditions (pure-tone), a dark colored musical note was presented for 9–13 s. During this time, participants were asked to hear pure-tones with one loudspeaker and count the number of pure-tones silently. Afterwards, they reported the number of pure-tones on a rating scale. During the rest conditions (resting-state), a dark cross was displayed for 9–13 s. Participants were instructed to remain relaxed in the resting-state. These rest conditions served as baseline activity and were the inter-trial intervals.
MRI Data Acquisition
All images were acquired on a 3.0-T SIMENS MAGNETOM Verio syngo MR scanner equipped with a 12-channel head coil (Siemens, Erlangen, Germany). Head motion was limited using form padding, and scanner noises were reduced using earmuff. Parameters for Sagittal three-dimensional T1-weighted images were: repetition time (TR), 1900 ms; echo time (TE), 2.46 ms; inversion time (TI), 900 ms; flip angle (FA), 9°; field of view (FOV), 256 mm × 256 mm; matrix, 256 × 256; slice thickness, 1 mm; 192 sagittal slices. Parameters for echo-planar imaging (EPI) sequence were: TR/TE, 2000/32 ms; FA, 70°, FOV, 240 mm × 240 mm; matrix, 64 × 64; slice thickness, 5 mm; 30 interleaved transverse slices; voxel size, 3.8 × 3.8 × 5 mm3.
Data Analysis
Demographic and clinical data were analyzed using SPSS 19.0 (SPSS Inc., Chicago, US), and were compared using two sample t-tests or chi-square test. Statistical significance was set at p < 0.05. Before using t-tests, a normality test (Kolmogorov–Smirnov) was performed for each group.
The fMRI data were preprocessed using Statistical Parametric Mapping Software (SPM12, http://www.fil.ion.ucl.ac.uk/spm). The 247 volumes of each run were corrected for time delay between different slices and realigned to the first volume. Parameters of head motion were computed with estimating translation in each direction and the angular rotation on each axis for each volume. Each participant had a maximum displacement of less than 3 mm in any cardinal direction, and a maximum spin less than 3°. Individual T1 images were linearly co-registered to the mean EPI image; and the transformed T1 images were segmented into white matter, grey matter (GM), and cerebrospinal fluid. The GM maps were then linearly co-registered to tissue probability maps in MNI space. The functional images with motion correction were linearly normalized to the individual’s structural image using the parameters estimated with linear co-registration. The functional images were resampled into 3 × 3 × 3 mm3 voxels. Finally, all datasets were smoothed with a Gaussian kernel of 8 × 8 × 8 mm3 FWHM (full-width half maximum).
We used SPM12 to do the analysis and modeled three regressors of interest: resting-state, heart-beats, and pure-tones, which were convolved with the canonical hemodynamic response function. The voxel time series were high-pass filtered at 1/128 Hz to account for non-physiological slow drifts in the measured signal and modeled for temporal autocorrelation across scans using an autoregressive model. We conducted two sample t-tests to determine the differently activated regions involved in interoception (heartbeats > resting-state) and exteroception (pure tones > resting-state). Effects were compared between HC and PD participants, including age and gender as covariates. Multiple comparisons were performed using whole brain voxel-wise family wise error (FWE) correlation, resulting in a corrected threshold of p < 0.05.
We further investigated the correlation of HPS with hemodynamic activity in regions showing significant group differences during heartbeats > resting-state using Pearson correlation analysis. Before using t-tests, a normality test (Kolmogorov–Smirnov) was performed for each variable.
Results
Demographic and Clinical Characteristics
All PD subjects were first-onset drug-naïve. PD patients and the corresponding HCs did not significantly differ in age or gender (Table 1), and were right-handed. The HPSs were higher in PD than in HC (not statistically significant, p = 0.07). Please also report the behavior results during the fMRI experiment, since you have the count heartbeat and the tones conditions. How are the accuracies and HPS in PD and HC in your experiment?
Task fMRI Findings
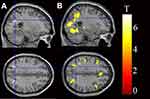
We studied all contrasts for the comparison “PD vs HC”. When comparing activity of heartbeats with resting-state, PD patients showed higher activity in the bilateral SPL (p < 0.05, FWE corrected) than HCs (Figure 1A and Table 2). When comparing activity of pure-tones with resting-state, PD patients also showed higher activity in the bilateral SPL (p < 0.05, FWE corrected; Figure 1B and Table 2).
 |
Table 2 Brain Regions Exhibiting Differences in Hemodynamic Responses Between Two Groups (PD > HC) in the Two Test Conditions |
Activation Correlation with the HPS
Patients exhibited a positive correlation between the HPS and the BOLD activity in the left SPL (r = 0.47, p = 0.04; Figure 2A) and in the right SPL (r = 0.41, p = 0.07; Figure 2B) during the heartbeats over resting-state condition, although the latter was not statistically significant. However, there was no correlation between BOLD activity in the SPL and HPS in HC (Figure 2C and D). Furthermore, according to the Z test for assessing the difference in correlation coefficients no significant difference between two groups was found.
Discussion
The present study found that the accuracy of heartbeat perception, although not statistically significant, was higher in patients with first-episode drug-naïve PD than in HCs, which is consistent with previous studies.6,8,18 We also investigated brain activity for perceptive awareness and found altered function of the SPL during interoceptive and exteroceptive processing in PD patients. Furthermore, this hyperactivity correlated positively with HPS in patients with PD but not in HCs. To our knowledge, this is the first study to observe altered brain activity during perceptive awareness in first-episode drug-naïve PD patients.
Previous studies have shown that SPL is involved in attention.19,20 During self-related tasks, such as read own personality trait, the SPL and other brain regions are activated.11–13 In the current study, we found that patients with PD showed significantly higher activation of the bilateral SPL during perceptive processing, especially interoceptive awareness, because PD subjects could focus and involve the attention control to detect the signals much better than health controls. The bilateral SPL reflects a baseline state of brain function in the absence of external cognitive stimuli, and mediates self-referential thoughts.21,22 Furthermore, utilization of interoceptive cues aids intuitive decision-making in PD patients; however, interoception related to cardioceptive information constitutes a major source of threat to these patients.23 This suggests that increased activation of what during interoceptive stimuli processing renders PD patients more engaged in processing information related to their internal states, thus increasing the probability of panic attacks. Moreover, the positive correlation between SPL activation and HPS, ie, the accuracy of heartbeat perception, in PD patients further confirms this hypothesis.
Our results also revealed an increase in bilateral SPL activation during exteroceptive awareness in PD patients. This increase was observed for both interoception and exteroception. We hypothesized that the increased sensitivity of exteroceptive perception may be accompanied by changes in interoceptive awareness and may enhance the severity of panic syndrome. Wiebking and colleagues used a similar paradigm to investigate the perceptive awareness in patients with major depressive disorder (MDD) and found that depressed individuals showed reduced activation during exteroceptive processing.7 Although the SPL and insula are differentially altered during exteroceptive awareness, both regions are associated with interoception.12,24 During exteroceptive perception, PD and MDD patients have different responses, which may be associated with differences in the pathological mechanism of the two disorders. Future studies are required to investigate the causal relationship between interoceptive and exteroceptive perception among PD and MDD individuals.
Recently, Pollatos and colleagues used repetitive transcranial magnetic stimulation (rTMS) to inhibit specific locations associated with interoceptive facets in healthy individuals. The authors found that inhibition of the insula results in a decline in cardiac interoceptive accuracy.25 Furthermore, a previous study found that low-frequency rTMS on the right parietal lobe could relieve anxious syndrome in patients with anxiety disorder (Li et al, 2012). Thus, our study provides a hint for PD treatment, by which inhibition of the SPL in PD patients may decline cardiac interoceptive accuracy.
The current study should be considered in light of certain limitations. The small number of sample subjects constricted the statistical power, as shown in the case of group differences regarding HPSs. Additionally, four patients with PD and 10 HCs were not tested for HPS, and others were tested for HPS only once. Due to the small sample size, we did not separate PD patients for specific stimuli responsible for triggering panic symptoms, which may differ across the cohort. Moreover, during the Schandry heartbeat perception task, participants do not rely on external cues to count their heartbeats, and Zamariola et al reported that the interoceptive accuracy scores massively reflect under-reports and suggested undistinguishable interoceptive capacities within the top scores.26 In the current study, the patients we collected reported their heart complaint at the first visit and were not young, so our results hardly applied to young patients. Furthermore, we did not use an intervention method, such as rTMS, to verify our hypothesis. Therefore, future studies with a larger sample size and applying an intervention method are required.
Conclusions
In conclusion, this research is notable as the first study using fMRI to investigate the neural basis underlying perceptive awareness in PD patients not receiving any psychotropic medications, using fMRI. In these patients, we identified increased activation of the bilateral SPL during interoception and a positive correlation of SPL activation with the HPS. This indicates that PD patients may process information on their internal state more intensively, which may increase the probability of panic attacks. Our study provides insights to develop novel PD add-on treatment.
Acknowledgment
We want to acknowledge the subjects participating in the study and contributions of all investigators. The abstract of this paper was presented at the 2019 SOBP Annual meeting as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Biological Psychiatry.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Domschke K, Stevens S, Pfleiderer B, Gerlach AL. Interoceptive sensitivity in anxiety and anxiety disorders: an overview and integration of neurobiological findings. Clin Psychol Rev. 2010;30(1):1–11. doi:10.1016/j.cpr.2009.08.008
2. Ehlers A, Breuer P. How good are patients with panic disorder at perceiving their heartbeats? Biol Psychol. 1996;42(1–2):165–182. doi:10.1016/0301-0511(95)05153-8
3. Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychol Rev. 2001;108(1):4–32. doi:10.1037/0033-295X.108.1.4
4. Richards J, Klein B, Carlbring P. Internet-based treatment for panic disorder. Cogn Behav Ther. 2003;32(3):125–135. doi:10.1080/16506070302318
5. Ballenger JC. Somatic symptoms and physiologic responses in generalized anxiety disorder and panic disorder: an ambulatory monitor study Hoehn-Saric R, McLeod DR, Funderburk F, et al (Johns Hopkins Med Insts, Baltimore, Md) Arch Gen Psychiatry 61: 913–921,2004. Year Bk Psychiatr Appl Ment Health. 2006;2006:243–244. doi:10.1016/S0084-3970(08)70238-X
6. Hoehn-Saric R, McLeod DR, Funderburk F, Kowalski P. Somatic symptoms and physiologic responses in generalized anxiety disorder and panic disorder: an ambulatory monitor study. Arch Gen Psychiatry. 2004;61(9):913–921. doi:10.1001/archpsyc.61.9.913
7. Wiebking C, Bauer A, de Greck M, Duncan NW, Tempelmann C, Northoff G. Abnormal body perception and neural activity in the insula in depression: an fMRI study of the depressed “material me”. World J Biol Psychiatry. 2010;11(3):538–549. doi:10.3109/15622970903563794
8. Ehlers A. A 1-year prospective study of panic attacks: clinical course and factors associated with maintenance. J Abnorm Psychol. 1995;104(1):164–172. doi:10.1037/0021-843X.104.1.164
9. Neufang S, Geiger MJ, Homola GA, et al. Cognitive-behavioral therapy effects on alerting network activity and effective connectivity in panic disorder. Eur Arch Psychiatry Clin Neurosci. 2019;269(5):587–598. doi:10.1007/s00406-018-0945-8
10. Lai CH, Wu YT. Changes in regional homogeneity of parieto-temporal regions in panic disorder patients who achieved remission with antidepressant treatment. J Affect Disord. 2013;151(2):709–714. doi:10.1016/j.jad.2013.08.006
11. Ochsner KN, Beer JS, Robertson ER, et al. The neural correlates of direct and reflected self-knowledge. NeuroImage. 2005;28(4):797–814. doi:10.1016/j.neuroimage.2005.06.069
12. Kircher TT, Brammer M, Bullmore E, Simmons A, Bartels M, David AS. The neural correlates of intentional and incidental self processing. Neuropsychologia. 2002;40(6):683–692. doi:10.1016/S0028-3932(01)00138-5
13. Kircher TT, Senior C, Phillips ML, et al. Towards a functional neuroanatomy of self processing: effects of faces and words. Brain Res Cogn Brain Res. 2000;10(1–2):133–144. doi:10.1016/S0926-6410(00)00036-7
14. Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18(4):483–488. doi:10.1111/j.1469-8986.1981.tb02486.x
15. Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–195. doi:10.1038/nn1176
16. Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res. 2007;1141:178–187. doi:10.1016/j.brainres.2007.01.026
17. Wiebking C, Northoff G. Neural activity during interoceptive awareness and its associations with alexithymia-An fMRI study in major depressive disorder and non-psychiatric controls. Front Psychol. 2015;6:589. doi:10.3389/fpsyg.2015.00589
18. Ehlers A, Breuer P, Dohn D, Fiegenbaum W. Heartbeat perception and panic disorder: possible explanations for discrepant findings. Behav Res Ther. 1995;33(1):69–76. doi:10.1016/0005-7967(94)E0002-Z
19. Gruber O, Melcher T, Diekhof EK, Karch S, Falkai P, Goschke T. Brain mechanisms associated with background monitoring of the environment for potentially significant sensory events. Brain Cogn. 2009;69(3):559–564. doi:10.1016/j.bandc.2008.11.008
20. Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54(3):2492–2502. doi:10.1016/j.neuroimage.2010.10.014
21. Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30(2):625–637. doi:10.1002/hbm.20531
22. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi:10.1038/nrn2201
23. Wolk J, Sutterlin S, Koch S, Vogele C, Schulz SM. Enhanced cardiac perception predicts impaired performance in the Iowa Gambling Task in patients with panic disorder. Brain Behav. 2014;4(2):238–246. doi:10.1002/brb3.206
24. Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp. 2013;34(11):2944–2958. doi:10.1002/hbm.22113
25. Pollatos O, Herbert BM, Mai S, Kammer T. Changes in interoceptive processes following brain stimulation. Philos Trans Royal Soc B: Biol Sci. 2016;371(1708):20160016. doi:10.1098/rstb.2016.0016
26. Zamariola G, Maurage P, Luminet O, Corneille O. Interoceptive accuracy scores from the heartbeat counting task are problematic: evidence from simple bivariate correlations. Biol Psychol. 2018;137:12–17. doi:10.1016/j.biopsycho.2018.06.006
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.