Back to Journals » Patient Preference and Adherence » Volume 16
Adherence to Insulin Therapy Among Children with Type 1 Diabetes: Reliability and Validity of the Arabic Version of the 4-Item Morisky Medication Adherence Scale
Authors Elhenawy YI, Abdelmageed RI, Zaafar DK , Abdelaziz AW
Received 24 September 2021
Accepted for publication 15 December 2021
Published 7 June 2022 Volume 2022:16 Pages 1415—1421
DOI https://doi.org/10.2147/PPA.S341061
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Johnny Chen
Yasmine I Elhenawy,1 Reham I Abdelmageed,2 Dalia K Zaafar,3 Asmaa W Abdelaziz2
1Pediatric and Adolescent Diabetes Unit (PADU), Department of Pediatrics, Faculty of Medicine, Ain Shams University, Cairo, Egypt; 2Department of Pediatrics, Faculty of Medicine, Ain Shams University, Cairo, Egypt; 3Pharmacology Department, Faculty of Pharmacy, Modern University for Technology and Information, Cairo, Egypt
Correspondence: Yasmine I Elhenawy, Pediatric and Adolescent Diabetes Unit (PADU), Department of Pediatrics, Faculty of Medicine, Ain Shams University, 26 Hassan Ibrahim Hassan Street, Nasr City, Cairo, Egypt, Tel +201006714334, Email [email protected]
Background: Inadequate adherence to insulin is a major concern, necessitating the use of reliable and valid metrics for assessing adherence. Up to date, there are no Arabic validated tools assessing adherence to insulin therapy among children with type 1 diabetes (T1DM). Thus, the aim of this study is to evaluate the psychometric properties of an Arabic version of the four-item Morisky Green Levine Medication Adherence Scale (MGLS-4) as a self-reported measure of adherence to insulin among a cohort of Egyptian children with T1DM.
Methods: The MGLS-4 was translated using forward and backward translation. The Cronbach’s alpha was used to assess reliability. Criterion validity of the scale was tested by examining the correlation coefficients between the compliance score (level of adherence) and the HbA1c levels.
Results: A total of 400 patients completed the Arabic version of MGLS-4. 26.25% of the studied cohort was found to be non-adherent to insulin therapy; non-adherent patients were significantly older (P=0.001). Decreased maternal education level, decreased frequency of blood glucose monitoring and prolonged disease duration best predicted the occurrence of non-adherence among the studied cohort. The internal consistency of the current version showed good reliability (Cronbach’s alpha = 0.857). The adherence score and adherence level showed very strong correlation with HbA1c level (rho = 0.830, P < 0.001 and rho = 0.808, P < 0.001, respectively).
Conclusion: The Arabic version of MGLS-4 showed good reliability and validity as a self-administered tool for assessing adherence to insulin in pediatric patients with T1DM.
Keywords: adherence to insulin, Morisky–Green–Levine Medication Adherence Scale, type 1 diabetes
Introduction
Adherence to long-term therapy is defined by the World Health Organization as ‘the amount to which a person’s behavior—taking drugs, following a diet, and/or implementing lifestyle modifications, matches with approved recommendations from a healthcare professional.1 Management of diabetes possess a real challenge, making adherence to treatment more problematic and worse than adherence to treatment for other chronic disorders.2,3
Low adherence is a frequent symptom among children and adolescents with type 1 diabetes.4 As a result, achieving glycemic control remains a difficult task for patients. Endothelial dysfunction and microvascular consequences, as well as macrovascular problems and atherosclerotic hazard, are all risks associated with poor glycemic management.5
Furthermore, in countries with high prevalence of diabetes, such as many Arab countries, inadequate adherence to therapy, especially insulin is a major concern, necessitating the use of reliable and valid metrics assessing adherence to treatment. Among these measures, patient questionnaires are often employed since they are simple, inexpensive, and easy to administer.6 Simple, reliable, and valid self-reported tools are needed to assess medication adherence levels that could lead to a better considerate of low-adherence and settle the basis for interventions aimed at improving health consequences and decreasing the global healthcare costs.7
The four-item Morisky Green Levine Medication Adherence Scale (MGLS) questionnaire is one of the most widely used self-reported measures of pharmaceutical non-adherence. The scale’s psychometric qualities have been proven.8 The scale is simple to use and understand, and may be easily incorporated into routine care of patients. It has been used to assess drug adherence in different chronic conditions.9–11
Several studies evaluated the validity and reliability of different versions of MGLS-4 among patients with type 2 diabetes.11–14 However, to the best of our knowledge, data evaluating adherence to insulin among children and adolescents with type 1 diabetes is limited. Thus, the aim of the current study is to evaluate the psychometric properties of an Arabic version of the four-item Morisky Green Levine Medication Adherence Scale (MGLS-4) as a self-reported measure of adherence to insulin among a cohort of Egyptian children and adolescents with type 1 diabetes.
Methods
Participants
A total of 400 patients, ages 6 to 18 years, were randomly recruited from the Pediatric and adolescent Diabetes Unit (PADU), at Ain Shams University. If a participant’s cognitive disability interfered with the study, they were ruled out. The study was approved by the Research Ethical Committee at the Faculty of Medicine, Ain Shams University (Ethical Committee No. FMASU R 64) and was conducted in accordance with the Helsinki Declaration of 1975. Written consent was taken from the participants and/or their caregivers before enrolment in the study after being informed about the purpose of the study.
Procedure
A cross-sectional study using face-to-face interview, the participants fulfilled a self-administered questionnaire that contained three parts: sociodemographic data (age and gender of the child, marital status, education level, and employment status of the parents); disease profile (duration of diabetes, current diabetes medications, frequency of glucose monitoring, and last HbA1c result); and the Arabic version of the four-item Morisky Green Levine Medication Adherence Scale (MGLS-4).
HbA1 c was measured by HPLC based on the charge of the glycated molecule by cation exchange high performance liquid chromatography (CE-HPLC) and the value of the last 3 months prior to enrolment in the study was reported.
Measures
The MGLS-4 scale is a four questions questionnaire with yes/no answers. The MGLS-4 score ranged from 0 to 4. Based on the score, adherence is categorized into high, medium, and low adherence. A score of 4 denotes high adherence, a score of 2 or 3 denotes medium adherence and a score of 0 or 1 denotes low adherence.8
Translation
The MGLS-4 was translated from English to Arabic with the agreement of the scale’s owner, Professor Morisky.8 This version was created utilising a forward and backward translation process, in which two pairs of linguistic specialists worked separately to complete the translation. Researchers looked through the two primary copies and decided on an Arabic draft copy. The draft was re-translated into English by a bilingual specialist. In terms of conceptual comparability, translators compared the backward-translated English draft to the initial. The translated questionnaire was then distributed to 30 Egyptian patients with type 1 diabetes mellitus, who finished it and provided feedback on the questions. The researchers took care of the patients’ feedback, and a final Arabic version was developed and prepared for reliability and validity testing.
Statistical Analysis
IBM SPSS Statistics version 23 (IBM Corp., Armonk, NY) was used to analyse the data. To describe sample characteristics, descriptive statistics and frequencies were used. Means and SDs were used to represent continuous demographic data such as age, diabetes duration, HbA1c, and insulin dose, which were then compared using Tukey’s post hoc test. Percentage was used to describe category qualities, followed by Dunn test for multiple comparisons.
The four questions of the scale were subjected to maximum likelihood principal component analysis (PCA). To determine the adequacy of data for component analysis, the Kaiser-Meyer-Olkin (KMO) Measure of Sampling Adequacy and Bartlett’s Test of Sphericity were utilized. As criteria for sample adequacy and practicality, a KMO value of at least.6 and a P-value of at least.05 for Bartlett’s Test were identified.
Reliability Assessment
The Cronbach’s alpha coefficient was used to assess internal consistency reliability. Cronbach’s alpha of >0.5 can be acceptable for newly created measures; otherwise, 0.7 should be the cut-off.15
Criterion Validity
Criterion validity of the scale was tested by examining the correlation coefficients between the compliance score (level of adherence) and the HbA1c levels (or adequacy of glycemic control). Inadequate glycemic control was defined as HbA1c level > 7% (53 mmol/mol). P-value < 0.05 is considered statistically significant.
Diagnostic Accuracy (Sensitivity and Specificity)
Sensitivity and specificity were assessed to identify the efficacy of MGLS-4 to identify patients with inadequate glycemic control. Only two sets of adherence scores were included in the sensitivity and specificity analysis: low adherence patients as one group, and medium and high adherence patients as the second group.
Results
Demographic Characteristics
The demographic data and disease characteristics of studied cohort are highlighted in Table 1. The mean age of the studied cohort was 8.1 ±2.07 years with female predominance (66%), most of the patients were having diabetes for at least one year with average duration of 1.21±0.94 years. All the patients were on multiple daily injections with the majority receiving 3 daily injections of insulin. The educational levels were diverse among the caregivers. The MGLS-4 scale was used to categorize the respondents’ adherence to insulin medication into low, medium, and high adherence levels. 26.25% of the studied cohort was found to be non-adherent to insulin therapy; non-adherent patients were significantly older. Following the completion of the multiple regression analysis, decreased maternal education level, decreased frequency of self-monitoring of blood glucose and prolonged disease duration best predicted the occurrence of non-adherence to insulin therapy among the studied cohort (Table 2).
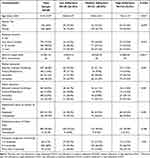 |
Table 1 Socio-Demographic Data and Disease Characteristics Based on Patients’ Level of Adherence |
 |
Table 2 Multivariate Regression Analysis for Determinants Predicting Non- Adherence to Insulin Among Studied Cohort |
Reliability
The internal consistency of the 4 items comprising the scale indicated good reliability (Cronbach’s alpha = 0.857). The reliability index dropped, when any item was removed, as a result, all four objects were kept. The overall correlation coefficient for items 1 to 4 varied from 0.63 to 0.73 (Table 3).
 |
Table 3 The Cronbach’s Alpha Coefficient Assessing Internal Consistency Reliability of the Arabic Version of MGLS-4 |
Validity
The criterion validity of MGLS-4 is illustrated in Table 4. The adherence score and adherence level showed very strong correlation with HbA1c level (Spearman’s rho = 0.830, P-value < 0.001 and 0.808, P-value < 0.001, respectively). Similarly, the adherence score and adherence level showed strong correlation with inadequacy of glycemic control (rank biserial correlation coefficient = 0.740, P-value < 0.001 and 0.697, P-value < 0.001, respectively).
 |
Table 4 Criterion Validity of MGLS-4 |
Sensitivity and Specificity
The sensitivity and specificity of MGLS were 75% and 27%, respectively. Positive and negative predictive values were 25% and 73%, respectively.
Discussion
Despite the importance of adherence to treatment regimens, assessment of adherence is not easy to measure in clinical practice.16 Several studies successfully assessed adherence to insulin therapy among a cohort of patients with diabetes using the 4-item Morisky–Green–Levine Medication Adherence Scale.16,17 To date, there is no Arabic validated measure assessing adherence to insulin in patients with diabetes. This is the first study to assess the Arabic version of MGLS-4 among a cohort of children and adolescents with type 1 diabetes.
Data from the current study showed that almost 26% of the studied cohort was not adherent to insulin therapy. These findings are in agreement with studies showing that non-adherence is common among pediatric patients with T1DM, with 16–49% being non-adherent to insulin therapy.4,18 Additionally, Chu et al similarly observed that non-adherent patients were significantly older.18
The current study explored various factors that could affect and predict adherence to insulin therapy. Similar to findings by Chua et al,18 duration of diabetes was an important and significant predictor of non-adherence among the studied cohort. This finding could be attributed to treatment fatigue occurring with longer diabetes durations, which requires more frequent assessment of adherence among patients with longer duration of diabetes.19 Maternal education with better knowledge of diabetes was another significant predictor of adherence. Mariye et al, reported that better understanding of diabetes could positively impact adherence to treatment.17
In this study, the reliability of the Arabic version of the 4-item Morisky– Green–Levine Medication Adherence Scale was good and was found to be higher than the reliability reported by Morisky et al (Cronbach’s alpha = 0.61).8
Compared with other translated versions of MGLS evaluating medication adherence in patients with diabetes, the reported alpha coefficient reported in this study was higher than those reported for the Singaporean (0.62),12 Thai (0.61)13 and Korean (0.66)20 versions.
Earlier Ashur et al evaluated the reliability and validity of the Arabic version of the 8-item Morisky Medication Adherence among a cohort of patients with type 2 diabetes on oral hypoglycemic. They postulated that the Arabic version of MGLS - 8 is a reliable and valid tool for assessing adherence to oral hypoglycemic among patients with type 2 diabetes.14
Insulin therapy is the cornerstone in the management of T1DM. Insulin non-adherence was found to be associated with increased risk of complications and increasing costs and expenses by the health-care system.18 Therefore, this signifies the importance of validating simple tool for assessing adherence to insulin therapy and incorporating it as a part of the standard of care offered to pediatric patients with type 1 diabetes.18
In spite of different versions validating the usage of MGLS among patients with type 2 diabetes,12–1420 data about versions evaluating adherence to insulin in patients with type 1 diabetes is scares. To the best of our knowledge this is the first study in Egypt to validate a patient-reported measure in patients with type 1 diabetes addressing adherence to insulin therapy.
In concordance with previous findings,12,14,21–24 higher scale scores were associated with better glycemic control, as evidenced by better HbA1c levels. Hood et al in their meta-analysis supported the adherence-glycemic control link in pediatric type 1 diabetes.25 Patients with higher medication adherence are more likely to be concerned with their disease and more aware of the importance of achieving a target glycemic control.12
The current version of MGLS −4 successfully differentiated between pediatric patients with poor and adequate glycemic control, supporting the criterion validity of the Arabic version of MGLS −4.
Although the sensitivity of the current Arabic version is lower than that reported with the original MGLS (93%),14 however the sensitivity is comparable to that reported among other versions of the MGLS.12–14,20 The current reported sensitivity highlights the efficacy of the Arabic version of MGLS-4 to identify patients with type 1 diabetes non- adherent to their insulin therapy. Although the sensitivity of the Arabic version of MGLS-4 was comparable to that reported among other versions of the MGLS, the specificity of Arabic version of MGLS-4 among patients with type 1 diabetes was lower than that reported among other versions of the MGLS12–14 and this is an important limitation of the current study.
Insulin adherence is an important pillar in diabetes care and it is essential to have simple and reliable tools aiming to assess adherence in patients with type 1 diabetes.12 This study represents a comprehensive insight for the validation of the Arabic version of MGLS-4 as a measure of adherence to insulin in pediatric patient with type 1 diabetes, providing sufficient psychometric properties of the Arabic version of MGLS-4.
The convenience sampling method may have subjected the study to a sort of selection bias which may impose a limitation on the generalizability of the findings. An important limitation of the current study is the low reported specificity of the Arabic version of MGLS-4. Absence of assessing the celling effect and confirmatory factor analysis of the Arabic version of MGLS-4 is another important limitation. The sample size in the current study is an important strength factor of this study.
Conclusion
The Arabic version of MGLS-4 showed good reliability and validity as a self-administered tool for assessing adherence to insulin in pediatric patients with type 1 diabetes. Arabic version of MGLS-4 could be a simple and feasible screening tool for assessing insulin adherence paving the way for implementing intervention strategies, which could positively impact both glycemic control and economic outcomes.
Acknowledgments
We are grateful to Professor Donald E. Morisky for approving and permitting the usage of the 4-items Morisky-Green-Levine Medication Adherence Scale (MGLS-4).
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Adherence to long-term therapies: evidence for action; 2003. Available from: http://whqlibdoc.who.int/publications/2003/9241545992.pdf.
2. Lerman I. Adherence to treatment: the key for avoiding long term complications of diabetes. Arch Med Res. 2005;36:300–306. doi:10.1016/j.arcmed.2004.12.001
3. DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200–209. doi:10.1097/01.mlr.0000114908.90348.f9
4. Schwartz DD, Cline VD, Hansen JA, Axelrad ME, Anderson BJ. Early risk factors for nonadherence in pediatric type 1 diabetes: a review of the recent literature. Curr Diabetes Rev. 2010;6:167–183. doi:10.2174/157339910791162952
5. Nathan DM; DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37:9–16. doi:10.2337/dc13-2112
6. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi:10.1056/NEJMra050100
7. Garfield S, Clifford S, Eliasson L, et al. Suitability of measures of self-reported medication adherence for routine clinical use: a systematic review. BMC Med Res Methodol. 2011;11(1):149. doi:10.1186/1471-2288-11-149
8. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. PMID: 3945130. doi:10.1097/00005650-198601000-00007
9. Shalansky SJ, Levy AR, Ignaszewski AP. Self-reported Morisky score for identifying nonadherence with cardiovascular medications. Ann Pharmacother. 2004;38:1363–1368. doi:10.1345/aph.1E071
10. Erickson SR, Coombs JH, Kirking DM, Azimi AR. Compliance from self-reported versus pharmacy claims data with metered-dose inhalers. Ann Pharmacother. 2001;35:997–1003. doi:10.1345/aph.10379
11. Al-Qazaz HK, Hassali MA, Shafie AA, Sulaiman SA, Sundram S, Morisky DE. The eight-item Morisky Medication Adherence Scale (MMAS): translation and validation of the Malaysian version. Diabetes Res Clin Pract. 2010;90:216–221. doi:10.1016/j.diabres.2010.08.012
12. Wang Y, Lee J, Toh MP, Tang WE, Ko Y. Validity and reliability of a self-reported measure of medication adherence in patients with type 2 diabetes mellitus in Singapore. Diabet Med. 2012;29(9):e338–e344. doi:10.1111/j.1464-5491.2012.03733.x
13. Sakthong P, Chabunthom R, Charoenvisuthiwongs R. Psychometric properties of the Thai version of the 8-item Morisky Medication Adherence Scale in patients with type 2 diabetes. Ann Pharmacother. 2009;43(5):950–957. PMID: 19366872. doi:10.1345/aph.1L453
14. Ashur ST, Shamsuddin K, Shah SA, Bosseri S, Morisky DE. Reliability and known-group validity of the Arabic version of the 8-item Morisky Medication Adherence Scale among type 2 diabetes mellitus patients. East Mediterr Health J. 2015;21(10):722–728. PMID: 26750162. doi:10.26719/2015.21.10.722
15. Nunnally JC, Bernstein IH. Psychometric Theory.
16. Gomes MB, Negrato CA. Adherence to insulin therapeutic regimens in patients with type 1 diabetes. A nationwide survey in Brazil. Diabetes Res Clin Pract. 2016;120:47–55. PMID: 27513598. doi:10.1016/j.diabres.2016.07.011
17. Mariye T, Girmay A, Birhanu T, et al. Adherence to insulin therapy and associated factors among patients with diabetes mellitus in public hospitals of Central Zone of Tigray, Ethiopia, 2018: a cross-sectional study. Pan Afr Med J. 2019;33:309. doi:10.11604/pamj.2019.33.309.17547
18. Chua B, Lim XY, Poh KM, Stephanie J, Cheen M, Lim ST. Adherence to insulin in Singaporean pediatric type 1 diabetes patients and its association with glycemic control and healthcare utilization. Asian J Pharm Clin Res. 2019;12(12):176–182. doi:10.22159/ajpcr.2019.v12i12.35558
19. Borus JS, Laffel L. Adherence challenges in the management of type 1 diabetes in adolescents: prevention and intervention. Curr Opin Pediatr. 2010;22:405–411. doi:10.1097/MOP.0b013e32833a46a7
20. Lee WY, Ahn J, Kim JH, et al. Reliability and validity of a self-reported measure of medication adherence in patients with type 2 diabetes mellitus in Korea. J Int Med Res. 2013;41(4):1098–1110. PMID: 23860015. doi:10.1177/0300060513484433
21. Donnelly LA, Morris AD, Evans JM. Adherence to insulin and its association with glycaemic control in patients with type 2 diabetes. Q J Med. 2007;100:345–350. doi:10.1093/qjmed/hcm031
22. Schectman JM, Nadkarni MM, Voss JD. The association between diabetes metabolic control and drug adherence in an indigent population. Diabetes Care. 2002;25:1015–1021. doi:10.2337/diacare.25.6.1015
23. Rhee MK, Slocum W, Ziemer DC, et al. Patient adherence improves glycemic control. Diabetes Educ. 2005;31:240–250. doi:10.1177/0145721705274927
24. Krapek K, King K, Warren SS, et al. Medication adherence and associated hemoglobin A1c in type 2 diabetes. Ann Pharmacother. 2004;38:1357–1362. doi:10.1345/aph.1D612
25. Hood KK, Peterson CM, Rohan JM, Drotar D. Association between adherence and glycaemic control in paediatric Type 1 diabetes: a meta-analysis. Pediatrics. 2009;124(6):e1171–e1179. doi:10.1542/peds.2009-0207
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
