Back to Journals » Patient Preference and Adherence » Volume 16
Adherence to Antithrombotic Therapy for Patients Attending a Multidisciplinary Thrombosis Service in Canada – A Cross-Sectional Survey
Authors Bonsu KO, Young S, Lee T, Nguyen H, Chitsike RS
Received 25 March 2022
Accepted for publication 27 June 2022
Published 26 July 2022 Volume 2022:16 Pages 1771—1780
DOI https://doi.org/10.2147/PPA.S367105
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Kwadwo Osei Bonsu,1 Stephanie Young,1,2 Tiffany Lee,1,2 Hai Nguyen,1 Rufaro S Chitsike3,4
1School of Pharmacy, Memorial University of Newfoundland and Labrador, St John’s, NL, A1B 3V6, Canada; 2Pharmacy Program, Eastern Region Health Authority, St John’s, NL, A1B 3V6, Canada; 3Division of Medicine (Hematology), Memorial University of Newfoundland and Labrador, St John’s, NL, A1B 3V6, Canada; 4Division of Hematology, Eastern Region Health Authority, St John’s, NL, A1B 3V6, Canada
Correspondence: Kwadwo Osei Bonsu, School of Pharmacy, Memorial University of Newfoundland and Labrador, 300 Prince Philip Drive, St John’s, NL, A1B 3V6, Canada, Email [email protected]
Background: Poor medication adherence puts patients who require antithrombotic therapy at greater risk of complications. We started a multidisciplinary Adult Outpatient Thrombosis Service in 2017 in a Canadian health authority and were interested in the level of medication adherence in the population attending.
Aim(S): The aim of this study is to assess adherence to antithrombotic medications for patients attending a multidisciplinary Thrombosis Service.
Methods: We conducted a cross-sectional survey of outpatients seen at the Thrombosis Service between 2017 and 2019 using the 12-item validated Adherence to Refills and Medications Scale (ARMS) to assess adherence to antithrombotic (anticoagulants and antiplatelet) therapy. Linear regression analysis examined the factors associated with adherence to antithrombotic therapy.
Results: Of 1058 eligible patients, 53.2% responded to the survey. Seventeen were excluded from the analysis for missing more than 6 responses to the 12 items on the ARMS. About 55% (n = 297) were on direct oral anticoagulants (DOACs), 19% (n = 102) on warfarin, 5.0% (n = 27) on low molecular weight heparin, 3.3% (n = 18) on antiplatelet therapy and 18% (n = 96) were no longer on antithrombotic therapy. Nearly half (47%, n = 253) had taken antithrombotic therapy for 1– 5 years while 28% (n = 150) and 25% (n = 137) had taken antithrombotic treatments for < 1 and > 5 years, respectively. Most patients (87%, n = 475) were ≥ 50 years and half (51%, n = 277) were male. The mean adherence score was 13.9 (SD± 2.2) and 88% (n = 481) of participants were adherent to antithrombotic treatment (ARMS = 12– 16). Multivariable linear regression showed that patients with post-graduate education had 0.4% lower adherence to antithrombotic therapy as compared with elementary education (β = 0.0039, p = 0.048). Patients with prior antithrombotic agent use > 5 years had 0.5% lower adherence to antithrombotic treatment compared to patients with < 1 year (β = 0.0047, p = 0.0244).
Conclusion: Self-reported adherence to antithrombotic therapy was high (88%) within a multidisciplinary Thrombosis Service. Patients with advanced education and prolong duration of antithrombotic therapy were more likely to have lower self-reported adherence to antithrombotic treatment.
Keywords: medication adherence, self-reported adherence, multidisciplinary care, thrombosis service, anticoagulation management program, antithrombotic therapy
Background
Poor adherence or non-adherence to treatments for medical conditions presents a significant public health problem due to increased risk of complications, morbidity and mortality.1 Among patients with chronic diseases, poor medication adherence is associated with high treatment costs from ineffective management of the underlying disease.2 In Canada, medication non-adherence accounts for 5% of hospital admissions and physician visits, resulting in an additional $4 billion in health care costs annually.3 Moreover, a recent Canadian survey has shown that 30% of patients stop taking their medications before instructed, and about one in four people do not fill their prescriptions or take less medication than prescribed.4
Antithrombotic agents include anticoagulants (such as vitamin K antagonists (VKA) and direct oral anticoagulants (DOACs)) as well as antiplatelet agents (such as acetylsalicylic acid, clopidogrel and ticagrelor). Antithrombotic agents are the mainstay therapies for the prevention and treatment of arterial and venous thromboembolic disease.5–11 While these agents (primarily anticoagulants) have shown significant reduction in risk of adverse outcomes in venous thromboembolism (VTE) and non-valvular atrial fibrillation (NVAF) patients in clinical trials,5–12 their effectiveness in real-world practice is impacted by patients’ adherence.13,14 Non-adherence to antithrombotic therapy increases the risks of adverse outcomes and healthcare costs.15,16 The risk of complications associated with antithrombotic agents coupled with complexities surrounding the management of thromboembolic disease may influence medication taking behaviours. Compared to warfarin, the DOACs have more predictable pharmacokinetics, which obviate routine laboratory monitoring to prevent adverse events.12 The consequences of non-adherence in VTE and NVAF can potentially be more significant for DOACs, given their short half-lives.14,17,18 Thus, identifying the factors associated with adherence to antithrombotic treatment may help characterize the populations at risk of non-adherence and allow for implementation of appropriate measures to improve adherence.
Aside from medication and patient-related factors, poor access to healthcare and long wait times are associated with poor medication adherence.19 Care fragmentation and lack of coordination lead to discontinuation of care and ultimately affect medication adherence.19 Multidisciplinary care models draw on the expertise of various healthcare professionals in a specialized discipline and have the potential to improve clinical outcomes through provision of improved access to medical care, delivering more efficient and coordinated care, and improving guideline adherence and health outcomes.20–22
A multidisciplinary Adult Outpatient Thrombosis Service (Thrombosis Service) was established in October 2017. The Thrombosis Service is located in the Eastern Region Health Authority, which is the largest integrated health authority in the province of Newfoundland and Labrador (NL), Canada23 and provides healthcare for about two-thirds (~300,000 people) of NL’s population. The Thrombosis Service is a comprehensive thrombosis and anticoagulation management service, which utilizes separate but interrelated clinics to deliver a spectrum of care required by patients with or at risk of thromboembolic disease. The Thrombosis Service is staffed during weekdays by full- and part-time clinical pharmacists, a Medical Director (Thrombosis Physician/Hematologist), additional hematologist support as needed, and a clerical support. The Thrombosis Service includes an Emergency Thrombosis Clinic for care of post-acute episode of venous thromboembolism; Thrombosis Clinics for general thrombosis or anticoagulation-related questions and follow-up; Anticoagulation Management Clinics for management of patients requiring long-term anticoagulants; and a Perioperative Anticoagulation Management Clinic for patients on anticoagulants requiring surgery or procedures. The service model is designed with pharmacists as the first point of patient contact. A pharmacist completes the initial patient assessment, presents the case for discussion with the Thrombosis Physician/Hematologist, provides patient-centred education on medication therapy, and facilitates medication access and insurance coverage as required. Patients on long-term anticoagulants are followed up in pharmacist-led clinics. Pharmacists also respond to patient questions regarding their anticoagulant management, as well as healthcare providers’ questions regarding the Thrombosis Service.
The increased risk of complications associated with antithrombotic therapy and the complexities surrounding thromboembolic disease management may influence medication adherence. There are limited data regarding adherence among patients receiving antithrombotic treatment in a multidisciplinary thrombosis setting. This study aims to assess adherence to antithrombotic medications for patients attending a multidisciplinary Thrombosis Service.
Method
Patient Population and Study Settings
The study was conducted in a multidisciplinary Thrombosis Service established in October 2017 in Eastern Health, NL.23 Patients were eligible for the study if they were aged ≥18 years; had attended at least one appointment at the Thrombosis Service between October 10, 2017 and May 31, 2019, and had a valid mailing address within the hospital records. A total of 1058 patients were mailed an anonymous survey, which consisted of 24 questions, available in English, with a cover letter and self-addressed return stamped envelope. A follow-up reminder letter and survey was mailed about 2 weeks after the first survey. Adherence to antithrombotic treatment was assessed by the 12-item Adherence to Refills and Medications Scale (ARMS)25 and satisfaction with Thrombosis Service using Short Assessment of Patient Satisfaction (SAPS) instrument.24 In addition, data were collected on patient demographic characteristics and anticoagulation therapies. Return of the completed questionnaire was considered informed consent. The SAPS utilizes a 5-point scale (0–4), with the continuous score ranging from 0 to 28 and categorical responses defined as 0–10 very dissatisfied, 11–18 dissatisfied, 19–26 satisfied, and 27–28 very satisfied.
Assessment of Medication Adherence
Adherence to antithrombotic medications was assessed using the 12-item Adherence to Refills and Medications Scale (ARMS)25 which consisted of 2 subscales–adherence with taking medications (8 items) and adherence with the refilling of prescriptions (4 items). The 12 items on the scale each require a 1–4 Likert scale (1 = none of the time, 4 = all of the time) response. The item scores of the ARMS were summed to produce an overall adherence score with a possible range of 12–48, with lower scores indicating better adherence. The ARMS scores were dichotomized into high (≤16) or low (>16) adherence based on evidence that self-reported adherence is comparable to other measures of adherence such as Proportion of Days Covered (PDC) or Medication Possession Ratio (MPR).26 While there are no validated thresholds for measuring adherence using PDC or MPR, widely available evidence suggests the use of thresholds ≥0.8 or 80% as acceptable cut off point for high adherence to medications. Thus, on the ARMS range of 12–48 with lower scores indicating high or better adherence, a cut-off of ≤16 was used as a reasonable equivalence of PDC or MPR ≥ 0.9 or 90% if adherence was measured using pharmacy claims data.26 We used the ARMS score equivalent of MPR or PDC cut off ≥0.9 or 90% which is 10% greater than the acceptable and commonly used MPR or PDC thresholds of 0.8 or 80%, to account for possible overestimation of medication adherence characteristics of self-reported measures.2,26,29 Patients with ARMS score ≤16 were considered adherent and those with score >16 as non-adherent. The internal consistency reliability for the ARMS was acceptable (Cronbach’s alpha = 0.70).
Statistical Analysis
The primary outcome was adherence to antithrombotic medications determined by the 12-item ARMS. Patient satisfaction with the Thrombosis Service was calculated as the sum of responses to each of the seven items of the SAPS scale. Sum responses to SAPS scale were dichotomized into a continuous range 0–18 indicating patient is dissatisfied and 19–26 as at least satisfied with the Thrombosis Service. Descriptive statistics were used to characterize study participants–frequencies and percentages for categorical variables, and means with standard deviations (SDs) for continuous variables. We used chi-square tests to compare differences in proportions of patient characteristics by adherent versus non-adherent groups. Although a small number of potential explanatory variables were collected, stepwise linear regression was used for statistical model selection. All variables were entered as independent variables into a stepwise linear regression model to identify factors associated with adherence to antithrombotic medications. Patient data with more than 6 missing responses of the 12-item ARMS were excluded from the analysis. Listwise deletion was applied where missing responses occurred in sociodemographic variables. Data were analysed using the Statistical Package for Social Sciences V.26.0 (SPSS).
The study protocol was reviewed by the Health Research Ethics Board of NL, and it was determined that the project was a quality assurance/quality improvement project, and as such did not require ethics review.
Results
Of 1058 eligible patients, 563 responded to the survey representing a response rate of 53.2%. Seventeen were excluded, as they had failed to provide responses to more than 6 ARMS items. Out of remaining 546 patients with complete responses, 55% (n = 297) were on DOACs, 19% (n = 102) on warfarin, 5.0% (n = 27) on low molecular weight heparin (LMWH), 3.3% (n = 18) on antiplatelet therapy and 18% (n = 96) were no longer on antithrombotic therapy at the time of the survey. Nearly half (47%, n = 253) had taken antithrombotic medications for 1–5 years, while 28% (n = 150) and 25% (n = 137) for <1 year and >5 years, respectively.
The mean adherence to antithrombotic medications measured by ARMS was 13.9 (SD±2.2, range 12–25). The mean responses to each of the 12 items of ARMS ranged from 1.05 ± 0.22 to 1.81 ± 1.12 (Table 1). About 457 (88.0%) of patients were adherent (ARMS ≤16) to their antithrombotic medications. Adherence did not significantly differ by most of the patient characteristics except age categories. Patients aged 50 years and older were more likely to be adherent to their antithrombotic therapy compared those <50 years (p = 0.032) (Table 2).
 |
Table 1 Mean Responses to the Items on Adherence to Refills and Medications Scale (ARMS) |
 |
Table 2 Relationship Between Patient Characteristics and Adherence to Antithrombotic Therapy |
About 82.4% (460) of patients were either satisfied or very satisfied with the Thrombosis Service and >88% (408) of those patients were adherent to their antithrombotic medications. The univariate analysis showed that post-secondary education (β = 0.0052, p = 0.006) and satisfaction with Thrombosis Service (β = 0.0004; p = 0.019) were significantly correlated with adherence to antithrombotic medications. In multivariable linear regression, patients with post-graduate education had a 0.4% lower mean adherence to antithrombotic medications compared to patients with elementary school education (β = 0.0039, p = 0.048). Patients with prior antithrombotic agent use for >5 years had a 0.5% lower mean adherence to antithrombotic agents compared to patients with <1 year of use (β = 0.0047, p = 0.0244) while holding all other factors constant. The correlation between satisfaction with Thrombosis Service and adherence to antithrombotic treatment observed in univariate analysis did not persist in the multivariable linear regression (Table 3).
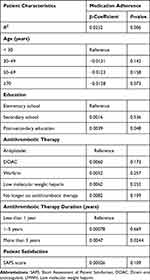 |
Table 3 Multivariate Linear Regression Showing Factors Associated with Adherence to Antithrombotic Therapy in Patients Attending a Multidisciplinary Thrombosis Service |
Discussion
The present research is one of the first to assess self-reported adherence to antithrombotic therapy using the 12-item ARMS among patients attending at least one appointment in a multidisciplinary Thrombosis Service. Adherence to antithrombotic therapy was high (88%). Multivariable linear regression showed that patients with post-graduate education had lower adherence to antithrombotic treatment compared with those with elementary education. Patients with prior antithrombotic agent use for >5 years had lower adherence to antithrombotic medications compared to patients with <1 year.
A number of methods have previously been used to measure adherence in patients on various medications, including those on antithrombotic agents. Of those, the self-reported adherence measure presents a practical way to assess adherence because of its low cost. Self-reported adherence has reliably predicted clinical outcomes and appears to be the most practicable approach to assessing adherence to antithrombotic treatments in real-world settings where a considerable number of patients are expected to be on DOACs.27–29 While several validated instruments on self-reported adherence exist in literature, the ARMS has been demonstrated to be reliable in chronic disease populations, and has good performance characteristics even among low-literacy patients.25 In addition, the ARMS has correlated strongly with the Morisky Medication Adherence Scale (MMAS)30 and has demonstrated good internal consistency and test–retest reliability.25 The 12-item ARMS has recently been used to assess adherence to anticoagulants and its predictors in NVAF.29 The ARMS has several advantages over other methods of evaluating adherence, including its ease of use, quick administration, and low-cost approach to measuring adherence in busy ambulatory care settings.
The majority of patients (88%) in the present study were adherent to antithrombotic therapy measured by the ARMS. Our self-reported adherence estimate differed from previous studies27–29 conducted in similar patient populations. The adherence to antithrombotic agents seen in our study is higher than that of a Canadian study of 500 consecutive outpatients with VTE or NVAF,28 which reported that 56.5% of participants were adherent using a validated 4-item MMAS.28 In addition, the adherence rate in the present study was higher than previous research by Rossi et al,27 Chen et al29 and Zhao et al,31 which were cross-sectional studies of consecutive outpatients diagnosed with NVAF. According to Rossi et al, 73.8% of the patients were adherent to anticoagulants.27 Zhao et al reported a non-adherence rate of 32.3% with the use of a validated 8-item MMAS,31 while Chen et al showed relatively less non-adherence to anticoagulants using a validated 8-item ARMS29 in NVAF patients seen at tertiary centers.
Our study demonstrated a significant association between higher levels of education and lower adherence to antithrombotic medications. A number of previous studies have shown no association,27–29,32 while others have demonstrated positive33–35 or negative36 associations between educational level and medication adherence. Our results are in line with the findings of Jaam et al’s study which reported a significant association between higher education and lower medication adherence with a validated ARMS in diabetes.36 A recent study also showed a marginal but significant association between higher education and nonadherence to medications.37 Higher level of education has been shown to be associated with greater rates of postponing medication taking and reducing doses of prescribed medications38 and could plausibly explain the results seen in our study.
Our multivariate linear regression showed a significant but marginal association between prolonged duration of antithrombotic therapy and lower adherence to treatment. Patients who had taken antithrombotic treatment for >5 years were more likely to have lower adherence compared with those who had <1 year of antithrombotic therapy. This finding is consistent with a cross-sectional study conducted by Miyazaki et al.39 Miyazaki et al examined the association between medication adherence and illness perceptions, and further explored the factors associated with poor medication adherence in patients diagnosed with NVAF receiving DOACs using the MMAS-8.39 Miyazaki et al reported a significant association between the longer duration of DOAC exposure and poor medication adherence.39 Conversely, previous studies, which assessed medication adherence and its predictors using the pharmacy claims data, appear incongruent with our findings. Feng Lai et al40 and Salmasi et al18 have recently demonstrated a significant association between the longer duration of therapy and the adherence to anticoagulants measured using PDC or MPR. A study authored by Manzoor et al reported that adherence was lower and worsened over time in patients who were previously anticoagulant naïve, an indication that patients with previous experience of anticoagulant use may fare better in terms of adherence to DOAC therapy than those who are newly initiated on DOAC therapy.41 However, in the present survey, a significant number of patients had used antithrombotic medications for a short duration (<5 years) and may plausibly have been undergoing more intensive monitoring with more frequent contact with care providers compared to antithrombotic-experienced patients with longer duration of antithrombotic agent use.42
While adherence to antithrombotic treatment within the Thrombosis Service was generally high, marginal but statistically significant lower mean adherence was seen in patients with more advanced education and prolonged duration of antithrombotic agent use compared to those with elementary school education and <1 year on antithrombotic treatment. Given that associations between adherence and high education and longer duration of antithrombotic therapy are marginally significant and have not been consistently reported in previous studies,18,27–29,32,39,40 it is unclear what the true significance of these findings is. However, it is worth noting that, more advanced education and prolonged duration of antithrombotic therapy were not linked to a greater adherence to antithrombotic treatment; and so it may not be safe to assume that patients with advanced education or have been on antithrombotic medications for a prolonged period are very adherent to their medication. These patient populations may require measures to facilitate ongoing adherence to antithrombotic therapy.
Our study has a number of strengths. First, our study is the largest in terms of sample size to assess self-reported adherence to antithrombotic therapy among patients requiring these medications. Second, we used a stringent adherence threshold with a comparable cut-off ≥0.9 or 90% for PDC or MPR to evaluate the prevalence of antithrombotic medication adherence in the present study.26 Despite the stringent threshold used in the present study, adherence to antithrombotic therapy was high. Third, we are among the first to report on adherence to antithrombotic medications in patients seen within a specialized multidisciplinary Thrombosis Service. Finally, the use of validated tools to assess medication adherence constitutes another strength of the study.
However, there are limitations to note. Given that the present study is observational and cross-sectional, causal inferences could not be made. Also, the use of ARMS may overestimate adherence to social-desirability biases common to self-reported instruments. However, we adopted strategies to reduce social desirability bias. First, surveys were anonymous to avoid pressure on participants to respond in a socially acceptable way. Secondly, we minimized the participation in data collection or survey coordination for researchers who were involved in direct patient care at the Thrombosis Service. This was to reduce any impact healthcare provider–patient relationships might have on participants’ responses to the survey. Our study may have been subject to recall bias, as some patients may have difficulty remembering the details of their encounter, particularly if there was a delay between the clinical encounter and the survey receipt. Finally, the study was conducted in a specialist setting, which may limit its generalizability. Nearly half (46.8%) of patients did not respond to the survey, and it is unknown if this would change the results. However, previous data from patients attending the Thrombosis Service had baseline characteristics (eg, age and sex distribution) similar to the population in this study.43 Moreover, our response rate is consistent with or higher than previous research which assessed adherence to antithrombotic treatments in patients with thromboembolic conditions using similar methods to ours.27–29 Thus, the results may be representative of the patients attending the various clinics of the Thrombosis Service and data from those who did not respond to the survey is unlikely to change the results.
Conclusion
Self-reported adherence to antithrombotic therapy was high (88%) in patients managed within a specialized multidisciplinary Thrombosis Service. Patients with more advanced education and those with prolonged duration of antithrombotic agents were marginally associated with lower adherence to antithrombotic therapy. Further research is required to evaluate the impact of model of care on adherence to antithrombotic therapy.
Acknowledgments
Our thanks to the survey authors for their permission to utilize the survey instrument, and to Callie Langmead, Research Assistant, for data entry. The abstract of this paper was presented at the American Society of Haematology Conference 2020 as a poster presentation with interim findings. The poster’s abstract was published as conference proceedings in the Blood Journal, volume 136, Issue Supplement 1: URL: https://ashpublications.org/blood/article/136/Supplement%201/38/472197/Patient-Adherence-to-Medication-within-A-New and, https://www.sciencedirect.com/science/article/pii/S0006497118716637
Funding
The development, implementation, and evaluation of the Eastern Region Health Authority Adult Outpatient Thrombosis Service, including this survey research, was supported by unrestricted grants from Sanofi Canada and Bayer Canada. The funders have no role in the design, data collection, analysis, interpretation and writing of the manuscript.
Disclosure
Kwadwo Osei Bonsu received a postdoctoral fellow salary from an unrestricted grant provided by Sanofi Canada. For research and clinical activities outside the work presented here, Dr Stephanie Young reports grants from Sanofi, grants from Bayer, during the conduct of the study; personal fees from Pfizer, outside the submitted work. Dr Rufaro Chitsike reports grants from Sanofi, grants from Bayer, personal fees from Pfizer, personal fees from Servier, during the conduct of the study, received speaking honoraria from Pfizer Canada. The authors report no other competing interests in this work.
References
1. Shore S, Carey EP, Turakhia MP, et al. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the veterans health administration. Am Heart J. 2014;167(6):810–817. doi:10.1016/j.ahj.2014.03.023
2. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi:10.1056/NEJMra050100
3. World Health Organization. Evidence for action World Health Organization 2003; 2003. Available from: https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1%0A.
4. Canada Pharmacists Association. Medication adherence - English. Available from: https://www.pharmacists.ca/advocacy/advocacy-activities/medication-adherence/.
5. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease. Chest. 2012;141(2 Suppl):e419S–e496S. doi:10.1378/chest.11-2301
6. Brenner B, Hoffman R. Emerging options in the treatment of deep vein thrombosis and pulmonary embolism. Blood Rev. 2011;25(5):215–221. doi:10.1016/j.blre.2011.04.003
7. Cohen AT, Dobromirski M. The use of rivaroxaban for short- and long-term treatment of venous thromboembolism. Thromb Haemost. 2012;107(06):1035–1043. doi:10.1160/TH11-12-0859
8. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi:10.1056/NEJMoa0905561
9. Connolly SJ, Wallentin L, Ezekowitz MD. The long-term multicenter observational study of dabigatran treatment in patients with atrial fibrillation (RELY-ABLE) study. Circulation. 2013;128(3):237–243. doi:10.1161/CIRCULATIONAHA.112.001139
10. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi:10.1056/NEJMoa1009638
11. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi:10.1056/NEJMoa1107039
12. Vo T, Vazquez S, Rondina MT. Current state of anticoagulants to treat deep venous thrombosis. Curr Cardiol Rep. 2014;16(3):463. doi:10.1007/s11886-013-0463-2
13. De Geest S, Zullig LL, Dunbar-Jacob J, et al. ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Ann Intern Med. 2018;169(1):30–35. doi:10.7326/M18-0543
14. Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25(9):2303–2310. doi:10.1185/03007990903126833
15. Deshpande CG, Kogut S, Willey C. Real-World Health care costs based on medication adherence and risk of stroke and bleeding in patients treated with novel anticoagulant therapy. J Manag Care Spec Pharm. 2018;24(5):430–439. doi:10.18553/jmcp.2018.24.5.430
16. Casciano JP, Dotiwala ZJ, Martin BC, Kwong WJ. The costs of warfarin underuse and nonadherence in patients with atrial fibrillation: a commercial insurer perspective. J Manag Care Pharm. 2013;19(4):302–316. doi:10.18553/jmcp.2013.19.4.302
17. Aronis KN, Hylek EM. Evidence gaps in the era of Non–Vitamin K oral anticoagulants. J Am Heart Assoc. 2021;7:e007338. doi:10.1161/JAHA.117.007338
18. Salmasi S, Loewen PS, Tandun R, Andrade JG, De Vera MA. Adherence to oral anticoagulants among patients with atrial fibrillation: a systematic review and meta-analysis of observational studies. BMJ Open. 2020;10(4):e034778. doi:10.1136/bmjopen-2019-034778
19. Kvarnström K, Westerholm A, Airaksinen M, Liira H. Factors contributing to medication adherence in patients with a chronic condition: a scoping review of qualitative research. Pharmaceutics. 2021;13(7):1100. doi:10.3390/pharmaceutics13071100
20. Choi BCK, Pak AWP. Multidisciplinarity, interdisciplinarity and transdisciplinarity in health research, services, education and policy: 1. Definitions, objectives, and evidence of effectiveness. Clin Investig Med. 2006;29:351–364.
21. Van Camp YP, Van Rompaey B, Elseviers MM. Nurse-led interventions to enhance adherence to chronic medication: systematic review and meta-analysis of randomised controlled trials. Eur J Clin Pharmacol. 2013;69(4):761–770. doi:10.1007/s00228-012-1419-y
22. Gillis AM, Burland L, Arnburg B, et al. Treating the right patient at the right time: an innovative approach to the management of atrial fibrillation. Can J Cardiol. 2008;24(3):195–198. doi:10.1016/s0828-282x(08)70583-x
23. Haché J, Bonsu KO, Chitsike R, Nguyen H, Young S. Assessment of a pharmacist-led direct oral anticoagulant monitoring clinic. Can J Hosp Pharm. 2021;74. doi:10.4212/CJHP.V74I1.3035
24. Hawthorne G, Sansoni J, Hayes L, Marosszeky N, Sansoni E. Measuring patient satisfaction with health care treatment using the short assessment of patient satisfaction measure delivered superior and robust satisfaction estimates. J Clin Epidemiol. 2014;67(5):527–537. doi:10.1016/j.jclinepi.2013.12.010
25. Kripalani S, Risser J, Gatti ME, Jacobson TA. Development and evaluation of the Adherence to Refills and Medications Scale (ARMS) among low-literacy patients with chronic disease. Value Health. 2009;12(1):118–123. doi:10.1111/j.1524-4733.2008.00400.x
26. Kini V, Ho PM. Interventions to improve medication adherence: a review. JAMA. 2018;320(23):2461–2473. doi:10.1001/jama.2018.19271
27. Rossi AP, Facchinetti R, Ferrari E, et al. Predictors of self-reported adherence to direct oral anticoagulation in a population of elderly men and women with non-valvular atrial fibrillation. J Thromb Thrombolysis. 2018;46(2):139–144. doi:10.1007/s11239-018-1679-1
28. Castellucci LA, Shaw J, van der Salm K, et al. Self-reported adherence to anticoagulation and its determinants using the Morisky medication adherence scale. Thromb Res. 2015;136(4):727–731. doi:10.1016/j.thromres.2015.07.007
29. Chen P-T, Wang T-J, Hsieh M-H, et al. Anticoagulation adherence and its associated factors in patients with atrial fibrillation: a cross-sectional study. BMJ Open. 2019;9(9):e029974. doi:10.1136/bmjopen-2019-029974
30. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi:10.1097/00005650-198601000-00007
31. Zhao S, Zhao H, Wang X, et al. Factors influencing medication knowledge and beliefs on warfarin adherence among patients with atrial fibrillation in China. Patient Prefer Adherence. 2017;11:213–220. doi:10.2147/PPA.S120962
32. Algabbani FM, Algabbani AM. Treatment adherence among patients with hypertension: findings from a cross-sectional study. Clin Hypertens. 2020;26(1):1–9. doi:10.1186/s40885-020-00151-1
33. Pietrzykowski Ł, Michalski P, Kosobucka A, et al. Medication adherence and its determinants in patients after myocardial infarction. Sci Rep. 2020;10(1):12028. doi:10.1038/s41598-020-68915-1
34. Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166(17):1842–1847. doi:10.1001/archinte.166.17.1842
35. Crowley MJ, Zullig LL, Shah BR, et al. Medication non-adherence after myocardial infarction: an exploration of modifying factors. J Gen Intern Med. 2015;30(1):83–90. doi:10.1007/s11606-014-3072-x
36. Jaam M, Mohamed Ibrahim MI, Kheir N, Hadi MA, Diab MI, Awaisu A. Assessing prevalence of and barriers to medication adherence in patients with uncontrolled diabetes attending primary healthcare clinics in Qatar. Prim Care Diabetes. 2018;12(2):116–125. doi:10.1016/j.pcd.2017.11.001
37. Dew MA, DiMartini AF, De Vito Dabbs A, et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83(7):858–873. doi:10.1097/01.tp.0000258599.65257.a6
38. Kugler C, Fischer S, Gottlieb J, et al. Symptom experience after lung transplantation: impact on quality of life and adherence. Clin Transplant. 2007;21(5):590–596. doi:10.1111/j.1399-0012.2007.00693.x
39. Miyazaki M, Nakashima A, Nakamura Y, et al. Association between medication adherence and illness perceptions in atrial fibrillation patients treated with direct oral anticoagulants: an observational cross-sectional pilot study. PLoS One. 2018;13(9):e0204814. doi:10.1371/journal.pone.0204814
40. Lai YF, Neo JK, Cheen MHH, Kong MC, Tai BC, Ng HJ. Comparison of medication adherence and treatment persistence between new oral anticoagulant and warfarin among patients. Ann Acad Med Singapore. 2016;45(1):12–17. doi:10.47102/annals-acadmedsg.V45N1p12
41. Manzoor BS, Lee TA, Sharp LK, Walton SM, Galanter WL, Nutescu EA. Real-world adherence and persistence with direct oral anticoagulants in adults with atrial fibrillation. Pharmacotherapy. 2017;37(10):1221–1230. doi:10.1002/phar.1989
42. Wang Y, Kong MC, Lee LH, Ng HJ, Ko Y. Knowledge, satisfaction, and concerns regarding warfarin therapy and their association with warfarin adherence and anticoagulation control. Thromb Res. 2014;133(4):550–554. doi:10.1016/j.thromres.2014.01.002
43. Young S, Ward R, Chitsike R. Pharmacists as front line clinicians in a new multidisciplinary thrombosis service. J Thromb Thrombolysis. 2019;47:600. doi:10.1007/s11239-019-01850-9AbstractA12
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
