Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
Adequate nutrition status important for bone mineral density improvement in a patient with anorexia nervosa
Authors Nakamura Y, Kamimura M, Koiwai H, Kato H
Received 19 December 2017
Accepted for publication 9 February 2018
Published 18 May 2018 Volume 2018:14 Pages 945—948
DOI https://doi.org/10.2147/TCRM.S160280
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Yukio Nakamura,1,2 Mikio Kamimura,3 Hidefumi Koiwai,4 Hiroyuki Kato1
1Department of Orthopaedic Surgery, Shinshu University School of Medicine, Matsumoto, Japan; 2Department of Orthopedic Surgery, Showa-Inan General Hospital, Komagane, Japan; 3Center of Osteoporosis and Spinal Disorders, Kamimura Orthopedic Clinic, Matsumoto, Japan; 4Koiwai Orthopedic Clinic, Komoro, Japan
Abstract: Low bone mineral density (BMD) is one of the most frequent complications of anorexia nervosa (AN). We report the clinical outcomes of a female patient with severe AN, whose chest had become deformed due to thoracic fracture. Lumbar BMD was 0.358 g/cm2 (T-score = −6.3), and total hip BMD was 0.411 g/cm2 (T-score = −4.4). Active vitamin D increased these parameters by 81.0% and 57.4%, respectively, but a drop in her nutrition status afterward resulted in a sharp decrease in BMD values. These findings suggest that adequate nutrient intake is essential for effective osteoporosis treatment in patients with AN.
Keywords: anorexia nervosa, bone mineral density, daily teriparatide, osteoporosis
Introduction
Anorexia nervosa (AN) is a severe eating disorder characterized by low body weight, intense fear of weight gain, and undue influence of weight and shape on self-evaluation.1 The overall age- and sex-adjusted incidence rate of AN is reportedly 4.1 per 100,000 person-years (95% CI: 2.4–5.9), with a female-to-male ratio of age-adjusted rates of 1.2:1.2 AN tends to manifest during adolescence3 and has the highest mortality rate of any psychiatric disorder, with no gold standard treatment4 and high therapy dropout and relapse rates.
One of the first treatment approaches that were based on Bruch’s observations was the “Maudsley model” of family therapy, in which the aim was to put patients in control of eating behavior.5 Approximately two-thirds of patients who receive this treatment as a form of early intervention show good recovery after 1 year, and this intervention is now recommended as a first-line treatment for adolescents with AN.6 However, it is important to note that this approach was not found to be effective if the illness had persisted for more than 3 years, or if the onset of the disorder occurred after the age of 18 years.
Numerous reports on AN and osteoporosis (OP) have surfaced over the past 3–4 decades. El Ghoch et al7 recently reviewed that diminished bone mineral density (BMD) was one of the most frequent medical complications in AN; nearly 85% of females with AN have very low BMD, and consequently, a sevenfold increase in the risk of spontaneous fracture compared with healthy controls. McAnarney et al8 described multiple rib fractures in a patient with AN as a result of forced vomiting in an individual with fragile bones. Khosla et al2 reported a preponderance of cancellous bone fractures in AN individuals (vertebral: 81%; rib: 37%; wrist: 13%), with 13% experiencing hip fracture.
The current first-line drugs for OP are bisphosphonates (BPs). Third-generation nitrogen-containing BPs inhibit farnesyl pyrophosphate synthetase in the mevalonate pathway in osteoclasts.9 Various other drugs have been described for patients with OP, such as the active vitamin D analog and 1α (OH) vitamin D3 (alfacalcidol; ALF), frequently used in Japan.
Concerning the treatment of adolescents with AN and OP, most methods tested (eg, hormone replacement, oral contraceptive pills, and BPs) have yielded only modest or negligible BMD improvements,10 with no data on the effectiveness of other strategies, such as physical activity intervention and/or the available nutritional supplementation (calcium, vitamin D, etc).8 The only promising pharmacological treatment to date has been physiological estrogen replacement by means of transdermal estradiol associated with cyclic progesterone, which, despite comparable weight gains, was associated with a significantly greater increase in spine and hip BMD than a placebo in non-severely underweight adolescents with AN.11 However, these findings require replication in patients with more severe malnutrition.
Case report
The patient was a 32-year-old female with a weight of 43 kg and a height of 157 cm. Since she has refused to know her body weight at all times, we have not measured it after she visited us for the first time. She had a medical history of pubic fracture and had been an outpatient at Shinshu University School of Medicine for 10 years following hospitalization for severe weight loss.
Her chief complaints were bilateral thoracic pain that had suddenly manifested 1 month prior and a common cold persisting for 2 months. The pain was obvious when taking a deep breath, coughing, or rolling over. The rib cage had become deformed to resemble the circumference of a barrel.
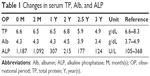
On presentation, her lumbar 1–4 BMD (L-BMD) was 0.358 g/cm2 (T-score = −6.3), and bilateral total hip BMD (H-BMD) was 0.411 g/cm2 (T-score = −4.4). Spinal plain radiographs showed no apparent fractures. Serum albumin was 4.3 g/dL, and 1,25(OH)2 D3 was 35.1 pg/dL; both were within normal range. Serum bone alkaline phosphatase (BAP) was 212.0 U/L, and urinary N-terminal telopeptide of type I collagen was 226.0 nmol BCE/mmol Cr, which indicated extremely accelerated bone metabolism (Tables 1 and 2). She was diagnosed as having OP based on BMD measured by dual-energy X-ray absorption, for which ALF treatment was soon commenced. Thoracic pain subsided thereafter.
  | Table 1 Changes in serum TP, Alb, and ALP |
The values of bone turnover markers were gradually decreased after the therapy. At 3 years of treatment, alkaline phosphatase was 124 U/L (89.6% decrease from peak value), and BAP was 11.6 U/L (94.9% decrease from peak value). 25(OH)D and deoxypyridinoline values were slightly elevated, and tartrate-resistant acid phosphatase-5b was within normal range, showing no obvious acceleration of bone metabolism.
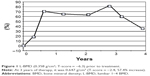
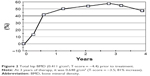
In 8 months of treatment, the percentage changes of L-BMD or H-BMD were increased to 70.7% or 41.4%, respectively. With respect to the peak values, L-BMD was 0.647 g/cm2 (T-score = −2.4; 81.0% increase; Figure 1), and H-BMD was 0.648 g/cm2 (T-score = −3.5; 57.4% increase; Figure 2).
  | Figure 2 Total hip BMD (0.411 g/cm2; T-score = −4.4) prior to treatment. |
She started to complain of deterioration of unbalanced diet and weight loss after the 2.5-year treatment. Her nutritional status soon degenerated, and serum albumin decreased to 3.4 g/dL. At 4 years of treatment, L-BMD had fallen to 0.484 g/cm2 (T-score = −4.7; 25.2% decrease from peak value), and H-BMD was 0.605 g/cm2 (T-score = −2.7; 6.6% decrease from peak value).
This patient gave written informed consent to publication of the patient’s personal medical information prior to her inclusion in this report.
Discussion
There have been numerous reports of accelerated bone resorption and inhibited bone formation in AN.12,13 In the current case, both bone resorption and bone formation were initially accelerated. We previously reported that bone turnover markers were significantly increased with accompanying back pain in elderly women, presumably due to insufficiency fracture,12 and that various bone fragility fractures might increase bone turnover markers.13 Our patient had suffered multiple fractures prior to her visit to our facility, and her thoracic circumference resembled that of a barrel. Thus, her fractures might have caused a remarkable increase in bone turnover markers. Despite the nonuse of bone anti-resorptive drugs, her BAP decreased from 228.0 to 11.6 U/L (94.9%) over 3 years, suggesting that bone metabolism improved from fracture healing. In patients with AN showing exceptional bone metabolism, especially the enhancement of bone formation marker, very severe AN complicated with fracture and pain is highly probable.
Evidence from a large number of studies has suggested that weight recovery in adolescents with AN may not be sufficient to fully reverse the detrimental effects of prolonged undernutrition on skeletal development. The benefits of exercise and calcium/vitamin D supplementation on BMD in patients with AN are also equivocal.14 Russell et al10 have reported that BPs can be an agent to treat AN except for premenopausal women and that the increase in L-BMD or H-BMD was 3%–4% or 2%, respectively. Isobe et al15 have recently reported that denosumab was effective for three patients with AN. In their report, the increases in L-BMD or H-BMD in those three cases were 15.7%, 18.6%, or none, and 35.2%, 11.6%, or 10.7%, respectively. Compared with those data, this study showed that the increase in L-BMD or H-BMD was 81% or 57.4% at the best during the study period, which showed a much better improvement than that in their study.17
In this article, the increase in L-BMD was 70.7% and that in H-BMD was 41.4% at only 8 months of the treatment, which was too excellent considering her therapy being vitamin D alone. In our report on pregnancy and lactation-associated OP, we described two cases occurring in the early postpartum period that led to multiple spinal compression fractures.16 BMD gains were impressive in the early phases of treatment by combined vitamins D and K as follows: 19.7% at 1 year, 23.3% at 2 years, and 36.1% at 4 years in one case and 13.3% at 1 year, 17.3% at 2 years, and 26.3% at 3 years in the other (unpublished data). Thus, combination vitamin D and K therapy enabled a marked gradual increase in BMD in pregnancy and lactation-associated OP. In these cases, T-score of L-BMD was −3.6 SD and −3.7 SD, respectively, revealing that the BMD values were decreased markedly. In this study, the T-score of L-BMD or H-BMD was −6.3 SD or −4.4 SD, those of which were greatly decreased compared with those in their cases.16 Based on those findings and the results of the current case of AN, decreased BMD by repetitive fractures could be naturally recovered to some extent? We speculate that vitamin D and/or K may be instrumental in this process.
When our patient’s AN status deteriorated after year 3, serum albumin became decreased from 4.3 to 3.4 g/dL and 25(OH)D3 remained at 5.0 ng/mL regardless of active vitamin D administration. Serum 25(OH)D3 is the major and main storage form of vitamin D whose sufficiency level is estimated as 20–30 ng/mL. Therefore, 5.0 ng/dL represented a serious vitamin D deficiency, and BMD rapidly decreased irrespective of vitamin D continuation. Jáuregui-Lobera et al17 reported that the most effective strategy to recover BMD in AN seems to be weight gain and menstrual recovery. Indeed, the improvement in nutrition may supersede all other treatment modalities for OP associated with AN.
Overall, it appeared that worsened AN causing inadequate nutrition and a rapid decline in BMD that was refractory to active vitamin D therapy may have increased our patient’s risk of fracture. Accordingly, anti-resorption drugs may be advocated for OP complicated with AN, especially when nutrition status may become compromised.
Disclosure
The authors report no conflicts of interest in this work.
References
APA. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, TX: American Psychiatric Publishing; 2013. | ||
Khosla S, Lufkin EG, Hodgson SF, Fitzpatrick LA, Melton LJ 3rd. Epidemiology and clinical features of osteoporosis in young individuals. Bone. 1994;15(5):551–555. | ||
Micali N, Hagberg KW, Petersen I, Treasure JL. The incidence of eating disorders in the UK in 2000–2009: findings from the General Practice Research Database. BMJ Open. 2013;3:e002646. | ||
National Institute for Health and Clinical Excellence. Eating Disorders: Core Interventions in the Management of Anorexia Nervosa, Bulimia Nervosa and Related Eating Disorders. Leicester: British Psychological Society; 2004. | ||
Russell GF, Szmukler GI, Dare C, Eisler I. An evaluation of family therapy in anorexia nervosa and bulimia nervosa. Arch Gen Psychiatry. 1987;44:1047–1056. | ||
Lock J. An update on evidence-based psychosocial treatments for eating disorders in children and adolescents. J Clin Child Adolesc Psychol. 2015;44:707–721. | ||
El Ghoch M, Gatti D, Calugi S, Viapiana O, Bazzani PV, Dalle Grave R. The association between weight gain/restoration and bone mineral density in adolescents with anorexia nervosa: a systematic review. Nutrients. 2016;8(12):E769. | ||
McAnarney ER, Greydanus DE, Campanella VA, Hoekelman RA. Rib fractures and anorexia nervosa. J Adolesc Health Care. 1983;4(1):40–43. | ||
Ste-Marie LG, Brown JP, Beary JF, et al. Comparison of the effects of once-monthly versus once-daily risedronate in postmenopausal osteoporosis: a phase II, 6-month, multicenter, randomized, double-blind, active-controlled, dose-ranging study. Clin Ther. 2009;31:272e85. | ||
Sullivan PF. Mortality in anorexia nervosa. Am J Psychiatry. 1995;152:1073–1074. | ||
Misra M, Golden NH, Katzman DK. State of the art systematic review of bone disease in anorexia Nervosa. Int J Eat Disord. 2016;49:276–292. | ||
Kamimura M, Uchiyama S, Takahara K, Hashidate H, Kawaguchi A, Nakagawa H. Urinary excretion of type I collagen cross-linked N-telopeptide and serum bone-specific ALP in patients with osteoporosis. Age-related changes in elderly women with back pain. J Bone Miner Metab. 2005;23:495–500. | ||
Takahara K, Kamimura M, Nakagawa H, Uchiyama S. Changes in biochemical markers of bone in patients with insufficiency fractures. J Bone Miner Metab. 2004;22:618–625. | ||
Bonjour JP, Kohrt W, Levasseur R, Warren M, Whiting S, Kraenzlin M. Biochemical markers for assessment of calcium economy and bone metabolism: application in clinical trials from pharmaceutical agents to nutritional products. Nutr Res Rev. 2014;27:252–267. | ||
Isobe F, Nakamura Y, Suzuki T, Kato H. Effects of denosumab on osteoporosis in three cases with anorexia nervosa and a review of the literature. Mod Rheumatol Case Rep. 2018;2:104–106. | ||
Nakamura Y, Kamimura M, Ikegami S, et al. A case series of pregnancy- and lactation-associated osteoporosis and a review of the literature. Ther Clin Risk Manag. 2015;11:1361–1365. | ||
Jáuregui-Lobera I, Bolaños-Ríos P, Sabaté J. Bone mineral density in anorexia nervosa: only weight and menses recovery? Endocrinol Nutr. 2016;63:458–465. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.