Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Acne Keloidalis Nuchae is Associated with Cutis Verticis Gyrata
Authors Umar S , Lullo JJ, Carter MJ , Shitabata PK , Lee DJ
Received 11 April 2022
Accepted for publication 3 July 2022
Published 26 July 2022 Volume 2022:15 Pages 1421—1427
DOI https://doi.org/10.2147/CCID.S369243
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Sanusi Umar,1– 3 Jenna J Lullo,2 Marissa J Carter,4 Paul K Shitabata,2,5 Delphine J Lee1,2
1Department of Medicine, University of California at Los Angeles, Los Angeles, CA, USA; 2Division of Dermatology, Department of Medicine, Harbor-UCLA Medical Center, Torrance, CA, USA; 3Dr. U Hair and Skin Clinic, Manhattan Beach, CA, USA; 4Strategic Solutions, Inc., Bozeman, MT, USA; 5Dermatopathology Institute, Torrance, CA, USA
Correspondence: Sanusi Umar, Dr. U Hair and Skin Clinic, 2121 N. Sepulveda Avenue, Suite 200, Manhattan Beach, CA, 90266, USA, Tel +1-310 318-1500, Fax +1-310 318-1590, Email [email protected]
Purpose: Both acne keloidalis nuchae (AKN) and cutis verticis gyrata (CVG) are scalp conditions predominantly affecting men. Both are characterized by dermal thickening and fibroblast hyperactivity. AKN typically occurs in the nuchal area, often involving the naturally occurring folds in the occipital region. The aim of this study was to determine the relationship between excessive scalp folding (CVG) and AKN.
Patients and methods: A total of 108 patients with AKN seen over 11 years from July 2009 and November 2020 were retrospectively evaluated. Patients with AKN concomitant with CVG were selected for analysis.
Results: Seven of the 108 AKN patients had scalp-wide (widespread) AKN lesions, including 4 with CVG. In 3 of the 4 patients with concomitant AKN and CVG, the AKN was widespread, and its onset had preceded CVG by 1– 2 years. In the fourth CVG patient, AKN lesions were confined to the nuchal area, and the CVG preceded AKN onset by several years. All patients were male, with a mean age of 35.8 years (overall) and 38.0 years (CVG group).
Conclusion: We describe a previously unreported relationship between widespread AKN and CVG, with the development of AKN preceding CVG formation.
Keywords: transforming growth factor, cicatricial alopecia, fibroblasts, syndromic
Corrigendum for this paper has been published.
Plain Language Summary
This study adds to our knowledge of acne keloidalis nuchae and cutis verticis gyrata by suggesting a significant relationship between the two conditions. Dermatologists can now be aware of the association of these two conditions, with implications for treatment.
Introduction
Cutis verticis gyrata (CVG) is characterized by thickening of the scalp skin with excessive folds.1 CVG is considered rare (approximately 1/100,000 men) and can be primary or secondary in origin.2 Primary CVG can be non-essential (associated with neuropsychiatric and ophthalmic disorders) or essential (not associated with any other abnormality). Secondary CVG can result from inflammatory skin conditions, including psoriasis and eczema, or systemic diseases like acromegaly, myxedema, and pachydermoperiostosis. Acquired CVG has also been attributed to prolonged hair traction.3,4
Acne keloidalis nuchae (AKN) is a form of primary cicatricial alopecia, typically confined to the occipital area and in men of color. The chief precipitating factors are microtraumas in predisposed individuals that accompany hair regrowth following head shaving,5 posterior hairline carving, traction injuries from hair weaving,4,5, and trauma. Chronic rubbing of the nuchal area by shirt collars, caps, helmets, and wooden combs is also a common initiating factor.5 The association between AKN and CVG has not been studied previously. A review of the literature reveals only one case report of CVG in a patient who had AKN and folliculitis decalvans.4 Because AKN is often first observed in the nuchal area where scalp folds typically occur and the precipitating event might be skin friction within these folds,5 we decided to investigate the potential relationship between CVG (a condition of excessive scalp skin folds) and AKN.
Methods
All patients provided written consent to publish the data and images in this report. The study was conducted in accordance with the Declaration of Helsinki (revised 2013). An institutional review board exemption was obtained from the Western Institutional Review Board. We used a database of 108 AKN patients whose AKN and other characteristics were compiled over 11 years at a single clinic in Los Angeles and reported in a previous investigation.6 The primary criterion used to initially identify the presence of CVG in these patients from photos and medical records was the presence of excessive and atypical scalp furrows. Patients found to have CVG and scalp-wide (widespread) AKN lesions were analyzed further with radiographs, urinary levels of prostaglandin E2 (PGE2), insulin growth factor (IGF), and thyroid functions to differentiate pachydermoperiostosis, acromegaly, and myxedema. Scalp-wide (widespread) AKN is defined when the distribution of AKN lesions significantly exceeds the occipital area to involve most sections of the scalp.6
After case identification, the incidence of CVG was calculated in the study population in terms of widespread AKN6 and AKN in general and compared to other population estimates.
Results
The incidence of CVG in the AKN population in our clinic was 4/108 (3.7%), versus 0.001% in the general population.2 Seven of 108 patients had widespread AKN, and 3 of 4 patients with CVG had widespread AKN (75%) (the fourth CVG patient had a large AKN plaque confined to the nuchal area) (Table 1). In all cases of concomitant widespread AKN and CVG, the CVG appeared within 2 years of AKN onset. These data suggested that CVG is associated with widespread AKN.
 |
Table 1 Cross-Tabulation of AKN, Widespread AKN, and CVG |
Patient 1
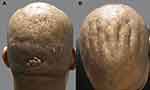
A 33-year-old Afro-Caribbean American man reported AKN lesions in the nuchal area since 27 years of age. He noted abnormal folds in his scalp, coincident with the rapid spread of AKN lesions within 2 years. He had a history of acne requiring isotretinoin treatment and pseudofolliculitis barbae. Examination revealed merged AKN papules and nodules involving all the scalp segments with CVG folds (Figure 1). The recesses of the CVG grooves were lined with papules consistent with AKN on histologic examination (Figure 2). He had no neuropsychiatric, ophthalmic, or skeletal abnormalities. Radiographs, urinary levels of PGE2, IGF level, and thyroid function tests showed no evidence of pachydermoperiostosis, acromegaly, or deposition diseases. His condition was refractory to prior treatments (isotretinoin, topical and intralesional steroids, and antibiotics). He is currently responding to a regimen of neodymium-doped yttrium aluminum garnet laser treatment for the worst affected areas of the occipital scalp.
Patient 2
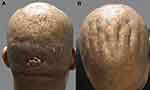
A 39-year-old Asian Pacific (Indonesian descent) man reported the onset of AKN lesions confined to the nape of the neck at 10 years of age. Within 2 years, he developed exaggerated scalp folds concurrent with AKN lesion proliferation, which spread to his entire scalp but notably spared the CVG grooves. He exhibited leonine facies and an inflamed plaque involving the whole scalp, except for AKN masses in the temporal areas. Tufted hair was observed on the scalp, along with several scalp folds consistent with CVG (Figure 3). The patient had a history and clinical evidence of severe acne involving the face and torso, rhinophyma, and pseudofolliculitis barbae. He had marked obesity with type 2 diabetes, acanthosis nigricans, keratosis pilaris, and hypertension. Radiological, histologic, and laboratory tests revealed findings similar to those in Patient 1. His condition was refractory to prior treatment with isotretinoin, topical and intralesional steroids, antibiotics, colchicine, and finasteride. He was successfully treated with low-dose radiation therapy, followed by excision of the residual lesions.7
Patient 3
A 38-year-old African man presented with a history of AKN bumps that commenced four years earlier in the nape area, followed by the appearance of deep grooves over the top of his head 2 years later, concomitant with the spread of the nape lesions to involve the crown and top of his head. There was no history of acne, pseudofolliculitis barbae, or systemic diseases. Radiologic, histologic, and laboratory test findings were similar to those in the 2 patients mentioned above. Examination revealed merging AKN papules involving the occipital, crown, vertex, and top of his head. In addition, the top of his head exhibited deep, sagittally disposed CVG grooves, all of which showed linear formations of merging AKN papules (Figure 4). Treatments with topical and intralesional steroids and antibiotics were unsuccessful.
Patient 4
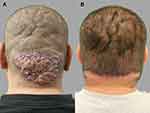
A 38-year-old Hispanic man developed a single large AKN plaque confined to the mid-three-quarters of his nape area (Figure 5A). He also reported excessive folding of his scalp involving the crown area, which preceded his AKN by over 2 decades. There was no history of acne, pseudofolliculitis barbae, or systemic diseases. Prior treatments of his AKN with antibiotics and topical and intralesional steroids were unsuccessful. Because his AKN lesion was wholly localized to the nape areas, it was amenable to complete excision with no recurrence noted (Figure 5B).
Discussion
Widespread AKN is defined as AKN lesions that spread beyond the typical confines of the occipital zones, affecting most sections of the scalp.6 Here, we retrospectively evaluated a cohort of 108 patients with AKN, including 3 patients with AKN that first appeared in the nape area. These three patients experienced the emergence of CVG within 2 years of the onset of AKN and subsequently had rapid scalp-wide spread of the AKN lesions. CVG preceded the manifestation of AKN in one patient in our cohort, whose CVG was concomitant with localized AKN. Although our findings are based on a small sample, the results in the context of a cohort of 108 patients suggest a strong association between AKN and CVG, as patients with widespread scalp AKN showed a propensity for CVG. This association is probably due to a common causal denominator. Both conditions are histologically associated with dermal collagen thickening and fibroblast hyperactivity,4 and can be induced by trauma or inflammation (shaving or tight weaving).3–5 Furthermore, both conditions predominantly affect men,5,8,9 while CVG is a hyperandrogenic condition,10 AKN typically affects post-pubertal men and spares older men past their testosterone prime.8
The upregulation of transforming growth factor-beta (TGF-β) activity has been implicated in pachydermoperiostosis, a cause of CVG.11,12 Furthermore, 2 cases of CVG were reported following treatment with vemurafenib and radiation for brain cancer.13 In both patients, dermal collagen thickening was noted on pathological examination of the CVG-affected skin. Vemurafenib can directly upregulate fibroblast activity by inducing paradoxical mitogen-activated protein (MAP) kinase signaling in primary human skin fibroblasts and inducing the production of TGF-β in melanoma cells.14 Both processes can cause dermal collagen thickening,4,15 which is found in both CVG and AKN. Notably, both patients described in the study13 exhibited scalp-wide lesions described by the authors as erythematous hypertrophic plaques. On closer examination of the published photographs, however, the lesions resembled widespread merging papular lesions similar to the AKN lesions in patients 1 and 3 of our case series. There was no report of a lesion biopsy done by the authors to rule out AKN. Thus, the case report could have inadvertently described a condition that upregulates fibroblast activity and potentially increases TGF-β activity to cause CVG and scalp-wide AKN papular lesions.
AKN is associated with elevated TGF-β activity (akin to hypertrophic and keloidal scarring), which leads to increased fibroblastic activity upregulation by TGF-β in AKN compared to the non-affected lesions.16
We suspect that the higher than the previously reported incidence of CVG in AKN patients (3.7% in our cohort vs 0.0001% in the general male population) may be attributed to the upregulated activity of the members of the TGF-β family, which may cause both conditions. In addition, in a subset of patients with AKN, TGF-β activity may be robust enough to both precipitate CVG and exacerbate the spread of AKN lesions.
Although it has been speculated that physical friction within the skin folds might be responsible for AKN, because AKN lesions classically occur in the occipital area in the vicinity of the naturally occurring folds,5 the sparing of CVG folds in one of our patients with widespread AKN suggests that the spread may not be caused by the physical phenomenon of friction between the skin walls forming the CVG folds. Since the treatment of scalp-wide AKN is more challenging, recognizing this association favors the need to intervene early with more effective treatments when CVG appears within a couple of years’ onset of AKN lesions while still localized to the nape area.17–20 Our findings may also suggest a possible therapeutic target for AKN and CVG that involve antagonists of TGF-β associated activities.
The main limitation of our study is its retrospective nature. However, its main strength is the size (given the low prevalence of AKN and CVG) of the enrolled patients. A much larger prospective study will help to validate our findings.
Conclusion
Our study reports the first evidence of a high association between AKN and CVG. Furthermore, our data support a syndromic association between AKN and CVG, predicting a high chance of scalp-wide spreading of AKN lesions that preceded the onset of CVG within 2 years.
Abbreviations
AKN, Acne Keloidalis Nuchae; CVG, Cutis Verticis Gyrata; TGF-β, Transforming growth factor-beta.
Ethics Approval and Informed Consent
All patients provided written consent to publish the data and images in this report. The study was conducted in accordance with the Declaration of Helsinki (revised 2013). Exemption was received from Western IRB.
Acknowledgments
We thank our patients.
Disclosure
Dr Delphine J Lee is a stock holder of Biogen/IDEC. The authors report no other conflicts of interest in this work.
References
1. Walia R, Bhansali A. Cutis verticis gyrata. BMJ Case Rep. 2011;2011:bcr0120113763. doi:10.1136/bcr.01.2011.3763
2. Snyder MC, Johnson PJ, Hollins RR. Congenital primary cutis verticis gyrata. Plast Reconstr Surg. 2002;110:818–821. doi:10.1097/01.PRS.0000019720.50128.7F
3. Kanwar AJ, Ghosh S, Thami GP, et al. Alopecia and cutis verticis gyrata due to traction presenting as headache. Int J Dermatol. 1992;31:671–672. doi:10.1111/j.1365-4362.1992.tb03997.x
4. Alonso Pereira L, Teixeira M, Andrade J, et al. An overlap of secondary cutis verticis gyrata, folliculitis decalvans, folliculitis keloidalis nuchae and the use of dreadlocks: the role of inflammation due to traction. Skin Appendage Disord. 2017;2:130–134. doi:10.1159/000449006
5. Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483–489. doi:10.2147/CCID.S99225
6. Umar S, Lee DJ, Lullo JJ. A retrospective cohort study and clinical classification system of acne keloidalis nuchae. J Clin Aesthet Dermatol. 2021;14:E61–E67.
7. Umar S, Sila CR. Acne keloidalis nuchae: a role for low-dose radiotherapy. JAAD Case Rep. 2021;13:90–93. doi:10.1016/j.jdcr.2021.05.008
8. Ogunbiyi AO, Adedokun B. Perceived etiological factors of folliculitis keloidalis nuchae and treatment options amongst Nigerian men. Br J Dermatol. 2015;173:22–25. doi:10.1111/bjd.13422
9. Ennouhi MA, Guerrouani A, Moussaoui A. Idiopathic cutis verticis gyrata in a female. Cureus. 2018;10:e2105. doi:10.7759/cureus.2105
10. Palazzo R, Schepis C, Ruggeri M, et al. An endocrinological study of patients with primary cutis verticis gyrata. Acta Derm Venereol. 1993;73:348–349. doi:10.2340/0001555573348349
11. Ninomiya S, Hara T, Tsurumi H, et al. Myelofibrosis successfully treated with prednisolone in a patient with pachydermoperiostosis. Intern Med. 2011;50:2207–2211. doi:10.2169/internalmedicine.50.5717
12. Kabashima K, Sakabe J, Yoshiki R, et al. Involvement of Wnt signaling in dermal fibroblasts. Am J Pathol. 2010;176:721–732. doi:10.2353/ajpath.2010.090454
13. Harding JJ, Barker CA, Carvajal RD, et al. Cutis verticis gyrata in association with vemurafenib and whole-brain radiotherapy. J Clin Oncol. 2014;32:e54–e56. doi:10.1200/JCO.2013.49.3528
14. Fedorenko IV, Wargo JA, Flaherty KT, et al. BRAF inhibition generates a host-tumor niche that mediates therapeutic escape. J Invest Dermatol. 2015;135:3115–3124. doi:10.1038/jid.2015.329
15. Na K, Oh SH, Kim SK. Acne keloidalis nuchae in Asian: a single institutional experience. PLoS One. 2017;12:e0189790. doi:10.1371/journal.pone.0189790
16. Okoye GA, Rainer BM, Leung SG, et al. Improving acne keloidalis nuchae with targeted ultraviolet B treatment: a prospective, randomized, split-scalp comparison study. Br J Dermatol. 2014;171:1156–1163. doi:10.1111/bjd.13119
17. Umar S, David CV, Castillo JR, Queller J, Sandhu S. Innovative surgical approaches and selection criteria of large acne keloidalis nuchae lesions. Plast Reconstr Surg Glob Open. 2019;7:e2215. doi:10.1097/GOX.0000000000002215
18. Umar S, Castillo JR, David CV, Sandhu S. Patient selection criteria and innovative techniques for improving outcome and cosmesis in acne keloidalis nuchae lesion excision and primary closure. JAAD Case Rep. 2019;5:24–28. doi:10.1016/j.jdcr.2018.10.002
19. Umar S. Selection criteria and techniques for improved cosmesis and predictable outcomes in laser hair removal treatment of acne keloidalis nuchae. JAAD Case Rep. 2019;5:529–534. doi:10.1016/j.jdcr.2019.02.034
20. Maranda EL, Simmons BJ, Nguyen AH, Lim VM, Keri JE. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther. 2016;6:363–378. doi:10.1007/s13555-016-0134-5
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.