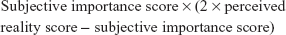Back to Journals » Patient Preference and Adherence » Volume 13
Achievement of patients’ preferences for participation in oncological symptom management and its association with perceived quality of care
Authors Lin C , Cohen E, Livingston PM , Mohebbi M , Botti M
Received 17 August 2018
Accepted for publication 5 November 2018
Published 31 December 2018 Volume 2019:13 Pages 83—90
DOI https://doi.org/10.2147/PPA.S184373
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Naifeng Liu
Cen Lin,1,2 Emma Cohen,3 Patricia M Livingston,2 Mohammadreza Mohebbi,4 Mari Botti2,5
1School of Nursing, Fudan University, Shanghai, China; 2School of Nursing and Midwifery, Faculty of Health, Deakin University, Geelong, VIC, Australia; 3Olivia Newton-John Cancer and Wellness Centre, Austin Health, Melbourne, VIC, Australia; 4Biostatistics Unit, Faculty of Health, Deakin University, Geelong, VIC, Australia; 5Deakin Centre for Quality and Safety Research – Epworth HealthCare Partnership, Melbourne, VIC, Australia
Purpose: The subjectivity of symptom experience and the recognized role of patients in symptom management highlight the need to understand cancer patients’ participation in symptom management and to identify the associations between patient participation and quality of care. However, research on patient participation has focused mostly on general healthcare activities, rather than symptom management, especially in cancer-care settings. This study aimed to compare the congruence between cancer patients’ preference for and actual perceived experience of participation in symptom management and identify the relationships between preferred and actual patient participation and perceived quality of care.
Methods: This was a cross-sectional study. Patient preference and actual experience of participation in symptom management were evaluated with the modified Control Preference Scale among patients recruited from a specialized cancer hospital in China. Patients’ perception of quality of care was assessed with the short-form Quality from the Patient’s Perspective questionnaire.
Results: A total of 162 patients were recruited. Their mean age was 47.5±12.2 years, and 51.9% were females. Patients’ perceived actual level of participation in symptom management substantially agreed with their preference (weighted κ-coefficient 0.61, 95% CI 0.45–0.77). There was no significant difference between patients’ perception of care quality and level of preference for participation (F=0.35, P=0.722) or actual experience of participation (F=0.76, P=0.519). Higher perceptions of quality of care were found among patients whose preferred roles were achieved (P=0.007) or surpassed (P=0.045).
Conclusion: This study identified substantial agreement between patients’ preferred and actual participation, given the generally passive preference. The findings indicated that supporting patients to achieve their preferred level of participation may be more important than focusing activities on encouraging increased desire to participate for the purpose of care-quality improvement.
Keywords: decision-making, quality of care, patient participation, symptom management, survey
Introduction
Patient participation has particular salience in the processes of care related to symptom management,1 because symptoms, as subjective evidence of disease, can only be perceived by patients themselves.2 In developed countries like the US, Australia, and the UK, there is growing recognition of the need to incorporate patients as participants in acute care, and this is reflected in the volume of research and health-care policy in the area.3,4 In China, although patients’ rights and responsibilities in care are recognized,5 the enactment of patient participation in clinical treatment and care has not been investigated or evaluated.
Patients’ actual experience of participation in health care has been identified as an indicator of patient satisfaction and the quality of care delivered.6,7 Investigation of patients’ preference for and achievement of participation has mostly focused on medical decision-making,8,9 and the findings are varied. For example, the proportion of patients whose experience matched their preferred level of participation ranged from 20% to 69%. Patients who achieved a more passive role than what they preferred ranged from 28% to 54% and a more active role ranged from 3% to 39%.10,11
Most Chinese studies that have investigated patients’ actual participation have found that scores of patients’ reported behaviors in relation to participation were lower than scores of their attitudes toward participation, which indicated that while patients were accepting and supportive of the notion of participation, they did not engage in behaviors required for them to participate.12–14 Whether or not patients’ attitudes (support of the notion of participation) and preferences (desire to participate and display of behaviors characteristic of participation) are consistent is not yet clear, so comparisons between attitudes, behavior, and preference, and actual participation should be interpreted with caution. The investigation of congruence between preferred level and actual achieved level of participation in different clinical contexts in China is necessary.
Patient participation is vital to enhancing quality of care. Research findings have shown that participation in health consultations enhances patients’ perceptions of quality of care,6,15 contributes to patient satisfaction,7 improves patient–clinician relationships,16 and reduces decisional regret.17 Patients’ collaboration with health professionals promotes the achievement of positive health outcomes. There is an increasing body of literature indicating that high levels of participation are associated with more recall of treatment information discussed during interactions with clinicians,18 better understanding of information provided,19 and improved adherence to treatment.20 However, the evidence for better health outcomes remains limited and controversial.19,21
In cancer-care settings, effective cancer and/or treatment-related symptom management is a priority.22 The promotion of patient participation in symptom management is based on the assumption that the subjectivity of symptom experience requires patients to contribute their experience and preferences to treatment decision-making. In the Chinese acute-cancer-care setting, there is limited understanding of the congruence between patients’ preference for and actual experience of participation in symptom management. There is also limited understanding of whether patients perceive higher quality of care when they participate in their symptom treatment or care. This study aimed to compare the congruence between cancer patients’ preference for and actual perceived experience of participation in symptom management and identify the relationships between preferred and actual patient participation and perceived quality of care.
Methods
Study design
This was a cross-sectional study. Patients’ reported preference for and actual experience of participation in symptom management and perceived quality of care were investigated.
Participants
Patients were eligible if they were >18 years of age and admitted with a cancer diagnosis to one of the two medical oncology units in a specialized cancer hospital in Shanghai, China, a university-affiliated teaching hospital. Patients were recruited into the study if they had been in hospital for 7 days or longer. One of the reasons for recruiting patients at this time point was to ensure that they had sufficient time to become familiar with hospital processes, such as routines of care, and to the ward environment in general. This period also provided an opportunity for patients and clinicians to establish a therapeutic relationship after their treatment started. The proportion of patients whose actual participation met their preference was reported as 40% in Cohen’s study.4 Sample size was calculated for prevalence rate (ie, percentage of participation that met their preference) in descriptive survey designs.23 We aimed to calculate the sample size with 95% precision (CI level for prevalence), and considering a margin of error of 8%, a sample size of 142 was required accordingly.
Patients were recruited between November 2013 and March 2014. A total of 300 patients met the eligibility criteria, and 162 (54%) participated in the study initially. Reasons for patient nonrecruitment were discharge before being approached (n=56), feeling unwell (n=42), refusal (n=31), and lacking confidence in their literacy (n=9). Data on nonparticipants’ sex, age, and type of cancer were compared with participants. The mean age of nonparticipants was 54±12 years, significantly older than participants (t=4.64, P<0.001). Approximately two-thirds of patients who refused to participate were male (χ2=11.26, P=0.01). The distribution of patients’ cancer diagnoses was also different between these two groups (χ2=16.24, P=0.013).
Although 162 patients participated in the control-preference survey, 23 patients did not complete the quality-of-care survey, because they felt tired or did not want to continue. Comparisons between the respondents and the nonrespondents showed there were no significant differences in sex (χ2=1.74, P=0.187), time since diagnosis (t=0.81, P=0.421), or number of symptoms (t=−0.02, P=0.983). Nonrespondents were older than respondents (t=−2.77, P=0.006).
Procedures
Primary nurses were all provided information about the study. Each day, the nurses reviewed the eligibility of the patients for whom they were providing care and asked patients who met the inclusion criteria for their permission to be approached by the researcher. The primary nurses provided the final list of eligible patients to the researcher. At the first meeting with eligible patients, the researcher introduced herself and explained the study. Written consent was obtained from patients. All patients who consented were recorded in the research ledger in terms of their name and time/date of consent. Every patient was assigned an ID number that was also recorded in the research ledger.
Patients’ preferences for participation and actual perception of participation were elicited by giving them five participatory-role statement cards, and patients were asked to rank the cards in order of their most preferred role to least preferred role. Patients then selected the card that best represented their actual participation in symptom management. On completion of the preference survey, patients were provided with a hard copy of the survey questionnaire related to their symptom assessment and perception of quality of care, had the instructions read to them by the researcher, and were then asked to self-administer the questionnaire. The researcher was available if patients had questions or did not understand the instructions. Some patients, due to low literacy or sight problems, required assistance to complete the questionnaire. Questionnaires were reviewed for completeness on collection to minimize missing data.
Survey measurement tools
Patients’ symptoms were assessed by the Memorial Symptom Assessment Scale, which is a multidimensional instrument used to assess 32 common cancer-related symptoms experienced during the past week.24 This scale has been translated into Chinese and its validity tested with Hong Kong cancer patients by Cheng et al.25
Patients’ preference for and perception of actual participation in symptom management-related decision-making was assessed using the Control Preference Scale (CPS).10,26 This comprises five cards. Each card has a separate statement that portrays a role that patients could have in treatment decision-making, ranging from the patient making the decision alone (active role), the patient making his/her own decision after considering the doctor’s opinion (active–shared role), the patient sharing the responsibility with doctors (collaborative role), doctors making the decision after considering a patient’s opinion (passive–shared role), and doctors making the decision alone (passive role). The CPS has reported reliability in cancer populations.27,28 It has been shown to be easily understood by patients and has been translated into different languages.10,29 The CPS was modified for this study so that each role statement asked patients about their preference for participation in “symptom management” with “doctors and nurses”. For example, the passive-role statement was modified to “I prefer to leave all decisions regarding treatment(s) of my symptoms to my doctors and nurses”.
Patients’ perceptions of quality of care were assessed with the short-form (modified) Quality from the Patients Perspective (QPP) questionnaire.30 This has four dimensions: medical–technical competence of the caregivers, physical–technical conditions of the care organization, degree of identity orientation in attitudes and actions of the caregivers, and sociocultural atmosphere of the care organization. The scale of physical–technical conditions of the care organization was deleted, because it was irrelevant to the research questions. There are 24 items in the modified version. Some items in the original tool were modified by highlighting symptom-management activities. The evaluation of each dimension includes two steps: one is to assess what has been experienced in terms of quality of care using a 4-point Likert scale ranging from 1 (do not agree at all) to 4 (completely agree), and the other step is to examine the subjective importance of various aspects of care on a 4-point Likert scale from 1 (of little or no importance) to 4 (of the very highest importance). The QPP has been used mostly in Sweden among patients admitted in surgical and medical wards31 and elder patients in different care environments.32 The Chinese version of the QPP was produced after forward translation, back translation and revision by the research team.
In order to derive a score representing the overall perception of quality of care, Personal Quality of Care Index (PQCI) score was calculated:
|
This formula is based on the principles that the highest personal quality-of-care score is obtained if a person gives the highest rating (ie, 4) on both perceived reality and subjective importance, and the lowest personal quality-of-care score is obtained if a person gives the lowest rating (1) on perceived reality and the highest rating (4) on subjective importance. The range of the PQCI on a given item is from −8 (lowest quality) to 16 (highest quality).33
Statistical methods
Data were coded and entered into SPSS version 21. Frequencies and descriptive statistics were used to summarize the demographic characteristics and control-preference data. Agreement between patients’ preferred and actual participation in decision-making was identified through weighted κ-statistics.34 Quadratic weighting was used to calculate the coefficient of weighted κ-values with Stata version 12. Cutoffs for strength of agreement for the weighted K (kw) coefficient are: poor agreement (≤0), slight agreement (0.01–0.20), fair agreement (0.21–0.40), moderate agreement (0.41–0.60), substantial agreement (0.61–0.80), and almost-perfect agreement (0.81–1).35 One-way ANOVA was used to examine the link between participation level and quality-of-care perception (α=0.05). When significant differences were identified across the groups, pairwise comparisons were conducted with Bonferroni-adjusted significance (α=0.017).
Ethics
Approval to conduct the research project was obtained from the Human Research Ethics Committee of Deakin University and the Clinical Pharmacology Base and Clinical Research Ethics Committee of Fudan University Shanghai Cancer Center. The authors confirm that the study was carried out in accordance with the principles of the Declaration of Helsinki.
Results
Participants
The mean age of the 162 patients was 47.5±12.2 (21–75) years. There were slightly more females (n=84, 51.9%) than males (n=78, 48.1%). Among those responding to demographic questions, 49 (32.2%) patients had completed at least an undergraduate education. The majority of patients were married (n=137, 90.1%). Almost half (n=70, 46%) the patients were still employed, 7.2% (n=11) were still working during their hospitalization, and the remainder were on sick leave.
The most common types of cancer were head and neck cancer (n=60, 37.0%), breast cancer (n=39, 24.1%), bowel cancer (n=27, 16.7%), and lymphoma (n=21, 13.0%). The majority of patients were admitted to the ward for radiotherapy alone (n=90, 59.2%) or in combination with chemotherapy (n=49, 32.2%). The majority of patients receiving treatment for their cancer had been diagnosed <1 year prior. The median time since diagnosis was 0.32 years (P25=0.19, P75=0.67), the minimum <1 month, and the maximum 11.59 years. All except two patients reported that they had at least one symptom during the investigation period and the average number of symptoms was 10.7±6.7 (0–27). The five most prevalent symptoms reported by patients were dry mouth (n=102, 68.0%), pain (n=88, 58.7%), difficulty sleeping (n=76, 50.7%), lack of energy (n=75, 50.0%), and changes in taste (n=73, 48.7%).
Preference for participation and perceptions of actual role
The distribution of patients’ preferences for participation in symptom management-related decision-making and their perceptions of the role they were able to achieve when interacting with doctors and nurses is presented in Figure 1. Three of the 162 patients did not report their achieved roles because they felt that there had not been any occasion that had required decision-making during their admission.
  | Figure 1 Patients’ preferred and experienced roles of participation. |
The extent to which patients’ preferences for participation were congruent with their actual experience during interactions with doctors and nurses is presented in Table 1. There were 64.8% (n=103) of patients who reported their actual experience of participation was consistent with their preference, whereas 24.5% (n=39) of patients experienced a more passive role than they preferred and 10.7% (n=17) experienced a more active role than they preferred. An agreement of substantial strength was found between patients’ preferred roles and actual roles in decision-making (kw=0.61, SE 0.08, 95% CI 0.45–0.77).
  | Table 1 Relationships between patients’ preferred and actual level of participation |
Perception of quality of care
The results of the PQCI mean scores compared within four categories of control preference (passive to active–shared) for participation are shown in Table 2. The active category was deleted in the analysis, because there was only one patient in it. There were no differences in mean scores of PQCI between control-preference categories (F3=0.35, P=0.722). Similarly, no significant differences were found when comparing mean PQCI scores within categories of actual participation achieved (F3=0.76, P=0.519).
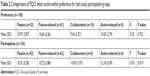  | Table 2 Comparisons of PQCI mean scores within preference for and actual participation groups |
Table 3 shows the mean PQCI scores for patients grouped according to their participation outcomes, specifically whether patients were able to achieve their preference for participation or their experience of participating was either less active or more active than their preference. The null hypothesis of similar means for PQCI scores was rejected (F=4.16, P=0.018). Further Bonferroni pairwise comparisons were conducted, revealing that mean PQCI scores were significantly higher for patients whose actual participation was commensurate with their preferred role (P=0.007) or for patients who experienced more active participation than they preferred (P=0.045) when compared to patients whose participatory role was less active than their preference.
Discussion
In this study, only six patients preferred to make decisions by themselves with or without discussion with their doctors and nurses. Similar findings can be found in another Chinese study in which eight of 113 colorectal cancer patients preferred active participation in surgical treatment decisions.36 In an Australian study, more cancer patients (30 of 171) with active preference were reported.4 The high proportion of patients with passive preference in China might be explained by the paternalistic style of interactions in Chinese healthcare settings.37 In this care environment, patients may not fully perceive the participatory roles in cancer care, especially in treatment decision-making.38
The findings of this study identified a substantial agreement between patients’ preferred roles and actual participatory roles, with nearly two-thirds of patients achieving their preference for participation in symptom management. This result is consistent with previous studies in which patients’ participatory roles were assessed using the CPS tool in acute-care settings. Zhang et al10 investigated 178 Chinese patients with chronic hepatitis, and found 69% of patients’ actual experience of participation matched their preference. Moderate agreement was also indicated in a study of 153 elderly inpatients in Sweden, with 44% total agreement between preference and actual participation related to medical decision-making.11 Another Swedish study found a similar relationship among 39 medical patients in decisions related to patient care.39
Given the high percentage of reported passive roles, both in patients’ preferred and actual experience, the moderate agreement might not mean that patients collaborated well with clinicians in the care setting. The congruence may in fact reflect that the majority of patients in this and other studies had a more passive preference for participation. Patients with a preference for passive participation may be easier for clinicians to interact with than patients who want a more active role, particularly when models of care and interactions are based on a paternalistic framework. Supporting evidence can be found in Pardon et al’s findings that high levels of congruence between preferred and actual participation were found only in patients who preferred an absolute passive or absolute active role, while those who preferred more collaborative or shared decision-making roles with their clinicians often reported lower achievement of their preference. The authors concluded that in the latter, patients were more critical and achievement of preferences was more open to nuances.40
Among patients who did not report congruence between preferred and actual experience of participation, 24.5% experienced a more passive role than their expectation, which was higher than the proportion of patients whose actual participation exceeded their preference. Previous studies also found that patients wanted more participation in decision-making than they actually achieved.10,11,41 This gap between preferred and actual participation reveals a significant issue in patients’ experience of care in acute-care environments.
The positive effects of patient participation in improving care quality have been identified in the literature.6,15 The significance of gaps in preferred and actual roles in decision-making is illustrated by the finding that patients perceive they had received higher quality of care when their actual participation roles agreed with their preferred participation roles. There have been few studies to investigate the impact of achieving participation preferences on the perception of quality of care, as most studies have explored differences between level of actual participation and quality of care,6,15 which in this Chinese study was found not to be significantly correlated. The finding that most patients in this study experienced passive participation in symptom-related decision-making may have contributed to this noncorrelation.
The findings indicate that improving patients’ actual experience of participation to agree with or be more active than their preferred level of participation may be more important in achieving good care quality than focusing on activities encouraging patients to adopt a more active preference. Health professionals need to recognize each patient’s unique preferences and understandings and respect the individual’s description of his or her situation, in order to assess patients’ preference accurately and provide opportunities for true patient participation.42 This is consistent with the notion of patient-centered care, where the patient is a respected and autonomous individual and their individual needs are embodied in care planning.43
Limitations
Only 54% of eligible patients were recruited into the study. Patients who were older, male, and unwell were more likely to decline to participate. In addition, this study focused on symptom management in patients with cancer, and a third were diagnosed with head and neck cancer. This may influence the external validity of the findings to other clinical groups and other aspects of care decision-making.
Conclusion
This study identified substantial agreement between patients’ preferred and actual participation when overall preference for participation in decision-making was passive in the Chinese acute-care setting. Patients perceived higher quality of care when their actual participation agreed with or was more active than their preferred participation. These findings indicate that it is important to support patients to achieve or surpass their preferred level of participation based on their individual needs for the purpose of care-quality improvement.
Acknowledgments
We acknowledge all participating patients who reported their perceptions and nurses who helped patient recruitment in the study.
Author contributions
CL, EC, PML, and MB were involved in managing the project, designing the study, and analysis and interpretation of data. CL was also responsible for collecting the data and drafting the manuscript. MM was involved in designing the study, preparing a statistical analysis plan, and interpreting the results obtained. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Heyn L, Finset A, Eide H, Ruland CM. Effects of an interactive tailored patient assessment on patient-clinician communication in cancer care. Psychooncology. 2013;22(1):89–96. | ||
MedicineNet [homepage on the Internet]. Medical definition of symptom; 2016. Available from: https://www.medicinenet.com/script/main/art.asp?articlekey=5610. Accessed February 2, 2017. | ||
Prey JE, Woollen J, Wilcox L, et al. Patient engagement in the inpatient setting: a systematic review. J Am Med Inform Assoc. 2014;21(4):742–750. | ||
Cohen E. Patient Participation in Symptom Management in an Acute Oncology Setting. Australia: School of Nursing and Midwifery, Melbourne: Deakin University; 2012. | ||
Chinese Hospital Association [homepage on the Internet]. Notice on the issurance and implementation of Patient Safety Goals in 2017; 2016. Available from: http://www.cha.org.cn/. Accessed April 15, 2017. | ||
Weingart SN, Zhu J, Chiappetta L, et al. Hospitalized patients’ participation and its impact on quality of care and patient safety. Int J Qual Health Care. 2011;23(3):269–277. | ||
Thyssen GD, Beck A. How patients experience the surroundings in relation to patient participation: a qualitative study of inpatients with intestinal failure. Patient Prefer Adherence. 2014;8:585–592. | ||
Brom L, Hopmans W, Pasman HRW, Timmermans DR, Widdershoven GA, Onwuteaka-Philipsen BD. Congruence between patients’ preferred and perceived participation in medical decision-making: a review of the literature. BMC Med Inform Decis Mak. 2014;14(1):25. | ||
Say R, Madeleine M, Richard T. Patients’ preference for involvement in medical decision making: a narrative review. Patient Educ Couns. 2006;60(2):102–114. | ||
Zhang YH, Su H, Shang L, et al. Preferences and perceived involvement in treatment decision making among Chinese patients with chronic hepatitis. Med Decis Mak. 2011;31(2):245–253. | ||
Ekdahl AW, Andersson L, Wiréhn AB, Friedrichsen M. Are elderly people with co-morbidities involved adequately in medical decision making when hospitalised? A cross-sectional survey. BMC Geriatr. 2011;11:46. | ||
Luo L, Wang GR, Liang HX. Investigation on cognition and health information needs of hospitalized patients’ involvement in patient safety. Chin J Mod Nurs. 2015;19:2285–2287. Chinese. | ||
Yue GJ. Study on Inpatients’ Participation Status of Intravenous Infusion Safety [master’s thesis]. Nursing College, Henan: Zhengzhou University; 2014. Chinese. | ||
Yu JJ. Exploring the Experience and Expectations of Surgical Informed Consent: from Patients’ and Doctors’ Perspective [master’s thesis]. Shanghai: The Second Military Medical University; 2014. Chinese. | ||
Jangland E, Carlsson M, Lundgren E, Gunningberg L. The impact of an intervention to improve patient participation in a surgical care unit: a quasi-experimental study. Int J Nurs Stud. 2012;49(5):528–538. | ||
Tan AK Jr. Emphasizing caring components in nurse-patient-nurse bedside reporting. Int J Caring Sci. 2015;8(1):188–193. | ||
Sawka A, Straus S, Gafni A, et al. Thyroid cancer patients’ involvement in adjuvant radioactive iodine treatment decision-making and decision regret: an exploratory study. Support Care Cancer. 2012;20(3):641–645. | ||
Dillon PJ. Assessing the influence of patient participation in primary care medical interviews on recall of treatment recommendations. Health Commun. 2012;27(1):58–65. | ||
Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. | ||
Arnold CL, Coran JJ, Hagen MG. Revisiting patient communication training: an updated needs assessment and the AGENDA model. Patient Educ Couns. 2012;88(3):399–405. | ||
Stiggelbout AM, Van der Weijden T, De Wit MPT, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256. | ||
Reilly C, Bruner D, Mitchell S, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21(6):1525–1550. | ||
Krishnamoorthy K, Peng J. Some properties of the exact and score methods for binomial proportion and sample size calculation. Commun Stat Simul Comput. 2007;36(6):1171–1186. | ||
Portenoy RK, Thaler HT, Kornblith AB, et al. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res. 1994;3(3):183–189. | ||
Cheng KK, Wong EM, Ling WM, Chan CW, Thompson DR. Measuring the symptom experience of Chinese cancer patients: a validation of the Chinese version of the memorial symptom assessment scale. J Pain Symptom Manage. 2009;37(1):44–57. | ||
Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res. 1997;29(3):21–43. | ||
Brown R, Butow P, Wilson-Genderson M, Bernhard J, Ribi K, Juraskova I. Meeting the decision-making preferences of patients with breast cancer in oncology consultations: impact on decision-related outcomes. J Clin Oncol. 2012;30(8):857–862. | ||
Degner LF, Sloan JA. Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol. 1992;45(9):941–950. | ||
Lee SYK, Degner LF, Knobf MT. Involvement in treatment decision-making among Chinese American women with early-stage breast cancer. Oncol Nurs Forum. 2007;34(1):251. | ||
Larsson BW, Larsson G. Development of a short form of the Quality from the Patient’s Perspective (QPP) questionnaire. J Clin Nurs. 2002;11(5):681–687. | ||
Fröjd C, Swenne CL, Rubertsson C, Gunningberg L, Wadensten B. Patient information and participation still in need of improvement: evaluation of patients’ perceptions of quality of care. J Nurs Manag. 2011;19(2):226–236. | ||
Wilde B, Larsson G, Larsson M, Starrin B. Quality of care from the elderly person’s perspective: subjective importance and perceived reality. Aging (Milano). 1995;7(2):140–149. | ||
Wilde B, Larsson G, Larsson M, Starrin B. Quality of care. Development of a patient-centred questionnaire based on a grounded theory model. Scand J Caring Sci. 1994;8(1):39–48. | ||
Cohen J. Weighted kappa: nominal scale agreement provision for scaled disagreement or partial credit. Psychol Bull. 1968;70(4):213–220. | ||
Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther. 2005;85(3):257–268. | ||
Hou XT, Xu Z, Zhou YJ, Lu Q, Pang L. Colorectal cancer patients’ participant roles during operation treatment decision making process: a cross-sectional study. Chin J Nurs. 2014;49(5):526–529. Chinese. | ||
Ling Y, Lun LI. The rationality of medical paternalism under a new type of physician-patient relationship. J Huaihua Univ. 2017;36(8):60–62. | ||
Lin C, Cohen E, Livingston PM, Botti M. Perceptions of patient participation in symptom management: a qualitative study with cancer patients, doctors, and nurses. J Adv Nurs. Epub 2018 Sep 12. | ||
Vestala H, Frisman GH. Can participation in documentation influence experiences of involvement in care decision-making? Open Nurs J. 2013;7:66–72. | ||
Pardon K, Deschepper R, Vander Stichele R, et al. Are patients’ preferences for information and participation in medical decision-making being met? Interview study with lung cancer patients. Palliat Med. 2011;25(1):62–70. | ||
Mohsin-Shaikh S, Garfield S, Franklin BD. Patient involvement in medication safety in hospital: an exploratory study. Int J Clin Pharm. 2014;36(3):657–666. | ||
Larsson IE, Sahlsten MJ, Segesten K, Plos KA. Patients’ perceptions of nurses’ behaviour that influence patient participation in nursing care: a critical incident study. Nurs Res Pract. 2011;2011:534060. | ||
Kitson A, Marshall A, Bassett K, Zeitz K. What are the core elements of patient-centred care? A narrative review and synthesis of the literature from health policy, medicine and nursing. J Adv Nurs. 2013;69(1):4–15. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.