Back to Journals » The Application of Clinical Genetics » Volume 12
ACE gene polymorphism and its association with serum erythropoietin and hemoglobin in Iraqi hemodialysis patients
Authors Al-Radeef MY , Fawzi HA , Allawi AA
Received 20 December 2018
Accepted for publication 10 April 2019
Published 1 July 2019 Volume 2019:12 Pages 107—112
DOI https://doi.org/10.2147/TACG.S198992
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Martin Maurer
Mohanad Yasir Al-Radeef,1 Hayder Adnan Fawzi,2 Ali Abdulmajid Allawi3
1Department of Clinical Pharmacy, College of Pharmacy, Tikrit University, Tikrit, Iraq; 2Department of Clinical Pharmacy, Baghdad Medical City Hospital, Baghdad, Iraq; 3Department of Internal Medicine, College of Medicine, Baghdad University, Baghdad, Iraq
Purpose: To evaluate the frequencies of angiotensin-converting enzyme gene polymorphism in Iraqi hemodialysis patients and to examine the association between this polymorphism and serum erythropoietin and hemoglobin levels.
Methods: In this study, 70 chronic renal failure Iraqi patients on maintenance hemodialysis (patient group) and 20 healthy subjects (control group) were genotyped for angiotensin-converting enzyme gene polymorphism. The distribution of genotype and allele frequencies of this polymorphism in these subjects were also evaluated.
Results: The distribution of angiotensin-converting enzyme genotypes between groups was similar, and the ID genotype was the most frequent, followed by DD and II genotypes (50%, 37%, and 13%). The control group had a nonsignificant difference in serum erythropoietin levels among different angiotensin-converting enzyme genotypes, while patients with ID and DD genotypes displayed significant elevation in serum erythropoietin with time. No significant differences in hemoglobin levels were observed in patient and control groups. A significant positive correlation was observed between serum erythropoietin and hemoglobin in the control group with different angiotensin-converting enzyme genotypes, while a nonsignificant negative correlation was observed in the patient group throughout the study.
Conclusions: Chronic kidney disease did not significantly alter angiotensin-converting enzyme genotypes, and angiotensin-converting enzyme gene polymorphism had a significant effect on serum erythropoietin levels and a nonsignificant effect on hemoglobin levels.
Keywords: ACE gene, polymorphisms, hemodialysis, erythropoietin, hemoglobin
Introduction
Chronic kidney disease (CKD) is common and continues to rise universally. It is a risk factor for end-stage renal disease (ESRD) and is also a strong risk factor for cardiovascular disease (CVD) and mortality.1 ESRD is a complex disorder with a variety of phenotypes starting from a diversity of underlying disorders of the kidney in conjunction with genetic and environmental factors in addition to other preexisting or secondary clinical entities.2
Renin-angiotensin–system (RAS) is a multifunctional system. A number of genetic variations linking RAS components have clinical or physiological influences. Among the candidate genes of RAS, the angiotensin-converting enzyme (ACE), angiotensinogen (AGT) and angiotensin II type 1 receptor (AGTR1) genes which appear to be mainly biologically and clinically relevant to renal disease. The genetic polymorphisms of these key components of RAS provide a basis for studying the relationship between genetic variants and the development of CVD and/or renal damage.3
Despite the enormous number of studies looking for candidate genes, the ACE gene stills the unique, well-characterized locus that clearly associated with pathogenesis and progression of CKD.4 ACE gene polymorphism (rs1799752) characterized by the deletion (D) or insertion (I) of a 287 base pair (bp) fragment in the 17q23 chromosome was identified in 1990.5
Angiotensin II stimulates the proliferation of early erythroid progenitors in-vitro. Consequently, ACE DD carriers may exhibit greater erythropoietic activity. Some researchers reported an elevation in erythropoietin (EPO) requirement in patients with the ACE II genotype undergoing peritoneal dialysis, but others found no effect.6
Hence, this study was designed to evaluate the frequencies of ACE gene polymorphism in Iraqi patient with CKD on maintenance hemodialysis, compare them with a group of healthy subjects, and to examine the association between this polymorphism and serum EPO and hemoglobin (Hb) levels.
Materials and methods
This study was conducted at Medical City Complex, Baghdad Teaching Hospital, Iraqi center of kidney dialysis from November 2015 until June 2016. The study protocol approved by the committee of local ethics in the college of medicine, University of Baghdad, Iraq with written informed consent taken from the participants, and the study and all its procedure were done by the Helsinki Declaration of 1975, as revised in 2000.
Those who satisfied the following criteria participated in this study: (1) treatment with hemodialysis for at least six months; (2) age >18 years; and (3) receiving injections of methoxy polyethylene glycol epoetin-beta (MPGE-β) for renal anemia. Exclusion criteria were: (1) recent symptoms and signs of bleeding that required a blood transfusion; (2) acute renal failure; (3) malignant disease; (4) hematologic disease; and (5) acute infectious disease.
Those who completed the study courses successfully were grouped into:
Patients Group: Includes 70 CKD patients (40 males and 30 females).
Control Group: Includes 20 healthy subjects (10 males and ten females) without any medical problems.
Three milliliters of venous blood samples were collected in the morning after an overnight fast before the dialysis session. Serums were extracted from two ml of blood sample while the remaining one ml was collected in EDTA vials for the extraction of genomic DNA. Samples were collected from each patient at the beginning of the study (baseline sample), and then after three months and after six months from the baseline sample to follow-up the changes in the studied parameters. Samples then were stored at (–80 C) until the time of the assay. A single blood sample drawn from each subject of the control group for comparison.
The dose of MPGE-β was titrated by 25% every two weeks in an attempt to maintain a target Hb level between 10 and 11 g/dl, and serum EPO concentrations measured by ELISA (using DEMEDITEC EPO immunoassay, Germany).
According to the manufacturer’s protocol, genomic DNA extracted from blood samples (baseline samples only) by using genomic DNA extraction kit , and the quality of DNA was analyzed by agarose gel electrophoresis.
The ACE gene polymorphism was detected by polymerase chain reaction (PCR) according to the method described by Mattu et al7 with some modification. The template genomic DNA (0.5 μg per sample) was amplified using the following primers:
Forward: 5’CTG GAG ACC ACT CCC ATC CTT TCT 3’
Reverse: 5’ GAT GTG GCC ATC ACA TTC GTC AGA T 3’
These primers (10 pmol of each) were added to a mixture containing 10 mmol/L Tris-HCl (pH 9.0); 30 mmol/L KCl; 250 μmol/L dNTP (each of dATP, dCTP, dGTP, and dTTP); 1.5 mmol/L MgCl2; and 1 U Taq DNA polymerase in a final volume of 20 μL.
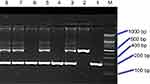
The PCR was initiated with a denaturation by first heating the samples for 3 minutes at 95 °C. Thirty-five cycles of denaturation for 30 seconds at 95 °C, annealing for 30 seconds at 58 °C, primer extension for 30 seconds at 72 °C, and the last extension for 5 mins at 72 °C applied for amplification. The PCR products of the ACE gene locus (The 490 bp in case of insertion and 190 bp in case of deletion products) were run on 2% agarose gel electrophoresis at 150 V for 60 min and visualized at room temperature under UV light after ethidium bromide (0.5 μg/mL) staining.
Statistical calculations were made using the SPSS program version 20, and Minitab version 17 software. A -value<0.05 was considered statistically significant in all comparisons. Anderson Darling test was made to check the adherence of continuous variables to a normal distribution. Discrete variables were presented using their number and percentages. The chi-square test used for comparisons of discrete variables between each study group. Genotypes frequencies of the ACE gene obtained by direct count. Linear regression analysis was made to assess the relationship between serum EPO and Hb.
Results
Demographic and laboratory data of this study groups are expressed in Table 1. The PCR product of ACE gene polymorphism is schematized in Figure 1. The distribution of ACE genotypes and their alleles between the study groups is similar (p-value>0.05), so no differences in distribution between CKD patients and healthy subject as presented in Tables 2 and 3.
 |
Table 1 Demographic data of the study groups at a baseline level |
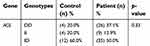 |
Table 2 Distribution of ACE gene polymorphism in the study groups |
 |
Table 3 Distribution of individual ACE gene alleles in the study groups |
The prevalence of ACE genotypes in the patients group is varied in which ID genotype is the most common (50%), followed by DD genotype (37%) and II genotypes (13%).
As shown in Table 4, the control group had a nonsignificant difference in serum EPO levels among different ACE genotypes (p-value>0.05), as well as, in patients group all different ACE genotypes had nonsignificant differences in serum EPO levels at all periods. According to individual ACE genotypes, patients with ID genotype displayed a more significant (p-value<0.05) elevation in serum EPO levels with time, followed by DD genotype, while II genotype had a nonsignificant elevation.
 |
Table 4 Serum erythropoietin levels (mIU/ml) divided by ACE genotypes. Data expressed as median (IQR) |
As shown in Table 5, nonsignificant differences (p-value>0.05) in Hb levels were observed for control and patients groups among different ACE genotypes. According to individual ACE genotypes, patients with different ACE genotypes exhibited a nonsignificant change in Hb levels with the time.
 |
Table 5 Hb levels (g/dl) divided by ACE gene polymorphism. Data expressed as mean ± SD |
Results presented in Table 6 and Figure 2 showed that the overall correlation between EPO and Hb in the control group was significant (p-value<0.05) and moderately positive. However, different ACE genotype groups showed a nonsignificant correlation, despite the high correlation coefficient for DD and II polymorphism this can be attributed to low number of subjects had DD (4 subjects) and II (4 subjects) polymorphism in control group.
 |
Table 6 Correlation between serum erythropoietin and Hb levels in the control group for each ACE genotype at baseline |
 |
Figure 2 Scatterplot for control group describing the correlation between serum erythropoietin (EPO) and Hb. |
Results presented in Table 7 and Figure 3 showed that the overall correlation between EPO and Hb in patients group at baseline was nonsignificant (p-value>0.05) and weakly negative; however, only DD genotype had a mild and significant inverse correlation, while II genotype had a nonsignificant and direct correlation.
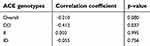 |
Table 7 Correlation between serum erythropoitinand Hb in patients group for each ACE genotype at baseline |
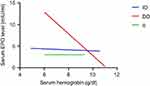 |
Figure 3 Scatterplot for patient group describing the correlation between serum erythropoietin (EPO) and Hb at baseline. |
Results presented in Table 8 showed that the overall correlation between EPO and Hb in patients group at three months interval was nonsignificant (p-value>0.05) and weakly negative, and different ACE genotypes had an inverse and nonsignificant correlation.
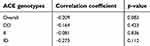 |
Table 8 Correlation between serum erythropoietin and Hb in patient group for each ACE genotype at three months interval |
Results presented in Table 9 showed that the overall correlation between EPO and Hb in patients at six months interval was nonsignificant (p-value>0.05) and weakly negative, and different ACE genotypes had an inverse and nonsignificant correlation.
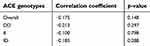 |
Table 9 Correlation between serum erythropoietin and Hb in patient group for each ACE genotype at six months interval |
Discussion
This study was done in an attempt to provide some evidence of the association between ACE gene polymorphism with serum EPO and Hb in Iraqi patients in view of the high prevalence of CKD. The current study showed that ID genotype was the most frequent, followed by DD and II of total cases of CKD, with nonsignificant difference in these genotypes between CKD and healthy subjects. These results approximately consistent with Kiss et al in Hungary which they found that the frequency of ID, DD, and II in CKD patients were 41.5%, 38.5% and 20.0% respectively.8 On the other hand, Shanmuganathan et al in an Indian cohort found the frequency of ID, DD and II genotypes in patients with CKD without hypertension to be 6.67%, 80.0%, and 13.33%, respectively and the frequency of ID, DD and II genotypes in patients with CKD with hypertension was 93.33%, 6.67%, and 0.0%, respectively,9 while in a study conducted in an Egyptian CKD pediatric population on maintenance hemodialysis the frequency was 40.91%, 56.82%, and 2.27% for ID, DD and II genotypes respectively.10
These differences are attributed chiefly to different ethnic population. The direct association between some gene polymorphisms and the diseases remains a controversy among various human ancestries reported. Some earlier studies approved the importance of human genetic variation in complex disease which can cause alleles to occur at a greater frequency in people from specific geographic areas.11
This study for the first time presents a quantitative analysis of serum EPO, Hb, and ACE gene polymorphism in CKD patients receiving MPGE-β therapy also in normal healthy subjects. The main findings regarding this issue were: (1) a significant elevation in serum EPO levels was observed in CKD patients’ with DD and ID genotypes throughout the study (ie, a significant effect of ACE gene polymorphism on serum EPO levels). (2) A significant positive correlation was observed between serum EPO and Hb in normal healthy subjects with different ACE genotypes. This indicates that Hb levels will increase when serum EPO increased. (3) A nonsignificant and negative correlation was observed between serum EPO and Hb in CKD patients with different ACE genotypes throughout the study. This indicates that Hb levels will decrease even though serum EPO increased.
A linear relationship is usually observed between the mass of red blood cells and Hb saturation with oxygen in healthy peoples, while an inverse linear relationship between serum EPO and Hb presents in chronically anemic patients.1 Panjeta et al investigated 562 individual subjects for the correlation between EPO, Hb and/or hematocrit based on the degree of renal insufficiency. A correlation between EPO and Hb and/or hematocrit found in healthy subjects, and EPO is significantly correlated (p<0.0005) with Hb and with hematocrit. The correlation is strong and negative, and the value of EPO increases with the decrease of Hb and/or hematocrit. On the other hand, subjects with a third and fourth degree of renal insufficiency EPO was not in correlation with Hb and hematocrit (p>0.05).12 The use of recombinant human erythropoietin has led to anemia correction and improvement in the quality of life for the majority of CKD patients. Nevertheless, there is considerable variability in patient response, and various factors have been linked to this variation.13 However, even after the exclusion of such factors, some patients continue to express suboptimal response.14
Conclusions
CKD especially ESRD did not significantly alter the distribution of ACE genotypes. Furthermore, ACE gene polymorphism had a significant effect on serum EPO levels, and patients with DD and ID genotypes exhibited a significant elevation in serum EPO levels with time. On the other hand, ACE gene polymorphism did not have a significant effect on Hb levels in these patients.
Acknowledgments
The authors acknowledge the contribution and cooperation of the patients’ enrolled in this study. We would also like to thank both the College of Pharmacy Tikrit University and Baghdad Medical City staff for their help in carrying out this work. This work is funded privately by the researchers alone; it was without institutional or grant support.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Al-Radeef MY, Allawi AAD, Fawzi HA. Interleukin-6 gene polymorphisms and serum erythropoietin and hemoglobin in hemodialysis Iraqi patients. Saudi J Kidney Dis Transpl. 2018;29(5):1042–1049. doi:10.4103/1319-2442.243952
2. Braliou GG, Grigoriadou AM, Kontou PI, Bagos PG. The role of genetic polymorphisms of the renin-angiotensin system in renal diseases: a meta-analysis. Comput Struct Biotechnol J. 2014;10(16):1–7. doi:10.1016/j.csbj.2014.05.006
3. Su SL, Lu KC, Lin YF, et al. Gene polymorphisms of angiotensin-converting enzyme and angiotensin II type 1 receptor among chronic kidney disease patients in a Chinese population. J Renin Angiotensin Aldosterone Syst. 2012;13(1):148–154. doi:10.1177/1470320311430989
4. Ha SK. ACE insertion/deletion polymorphism and diabetic nephropathy: clinical implications of genetic information. J Diabetes Res. 2014;2014:846068. doi:10.1155/2014/846068
5. Wong C, Kanetsky P, Raj D. Genetic polymorphisms of the RAS-cytokine pathway and chronic kidney disease. Pediatr Nephrol. 2008;23(7):1037–1051. doi:10.1007/s00467-008-0816-z
6. Jeong KH, Lee TW, Ihm CG, Lee SH, Moon JY. Polymorphisms in two genes, IL-1B and ACE, are associated with erythropoietin resistance in Korean patients on maintenance hemodialysis. Exp Mol Med. 2008;40(2):161–166. doi:10.3858/emm.2008.40.2.161
7. Mattu RK, Needham EWA, Galton DJ, Frangos E, Clark AJL, Caulfield M. A DNA variant at the angiotensin-converting enzyme gene locus associates with coronary artery disease in the caerphilly heart study. Circulation. 1995;91(2):270–274. doi:10.1161/01.CIR.91.2.270
8. Kiss Z, Ambrus C, Kulcsar I, Szegedi J, Kiss I. Effect of angiotensin-converting enzyme gene insertion/deletion polymorphism and angiotensin-converting enzyme inhibition on erythropoiesis in patients on haemodialysis. J Renin Angiotensin Aldosterone Syst. 2015;16(4):1021–1027. doi:10.1177/1470320314535276
9. Shanmuganathan R, Kumaresan R, Giri P. Prevalence of angiotensin converting enzyme (ACE) gene insertion/deletion polymorphism in South Indian population with hypertension and chronic kidney disease. J Postgrad Med. 2015;61(4):230–234. doi:10.4103/0022-3859.166510
10. Elshamaa MF, Sabry SM, Bazaraa HM, et al. Genetic polymorphism of ACE and the angiotensin II type1 receptor genes in children with chronic kidney disease. J Inflamm (Lond). 2011;8(1):20. doi:10.1186/1476-9255-8-20
11. Hua L, Li L, Zhou P, Yang Z. Combining geographic region with meta-analysis to map the potential association between three genetic polymorphisms and coronary artery disease. J Med Biochem. 2013;32(3):256. doi:10.2478/jomb-2013-0013
12. Panjeta M, Tahirovic I, Karamehic J, Sofic E, Ridic O, Coric J. The relation of erythropoietin towards hemoglobin and hematocrit in varying degrees of renal insufficiency. Mater Sociomed. 2015;27(3):144–148. doi:10.5455/msm.2015.27.144-148
13. Varagunam M, McCloskey DJ, Sinnott PJ, Raftery MJ, Yaqoob MM. Angiotensin-converting enzyme gene polymorphism and erythropoietin requirement. Peritoneal Dialysis Int. 2003;23(2):111–115. Available from: http://www.pdiconnect.com/content/23/2/111.long
14. Alves MT, Vilaca SS, Carvalho M, Fernandes AP, Dusse LM, Gomes KB. Resistance of dialyzed patients to erythropoietin. Rev Bras Hematol Hemoter. 2015;37(3):190–197. doi:10.1016/j.bjhh.2015.02.001
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.