Back to Journals » OncoTargets and Therapy » Volume 9
Accelerated partial breast irradiation in an Asian population: dosimetric findings and preliminary results of a multicatheter interstitial program
Authors Koh V, Tan PW, Buhari S, Iau P , Chan C, Shen L, Tan S, Tang JI
Received 19 February 2016
Accepted for publication 7 May 2016
Published 8 September 2016 Volume 2016:9 Pages 5561—5566
DOI https://doi.org/10.2147/OTT.S106758
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Li
Yaling Vicky Koh,1 Poh Wee Tan,1 Shaik Ahmad Buhari,2 Philip Iau,2 Ching Wan Chan,2 Liang Shen,3 Sing Huang Tan,4 Johann I-Hsiung Tang1
1Department of Radiation Oncology, National University Cancer Institute Singapore, 2Department of Surgery, National University Hospital, 3Department of Medicine, Biostatistics Unit, National University of Singapore, 4Department of Medical Oncology, National University Cancer Institute Singapore, Singapore
Introduction: Accelerated partial breast irradiation (APBI) using the multicatheter method has excellent cosmesis and low rates of long-term toxicity. However, there are few studies looking at the feasibility of this procedure and the outcomes in an Asian population. This study aims to look at outcomes at our hospital.
Methods: We identified 121 patients treated with APBI at our center between 2008 and 2014. The median follow-up for our patient group was 30 months (range 3.7–66.5). The prescribed dose per fraction was 3.4 Gy in 10 fractions. In this study population, 71% of the patients were Chinese while 15% (n=19) were of other Asian ethnicity.
Results: In this study, the median breast volume was 850 cc (range 216–2,108) with 59.5% (n=72) patients with a breast volume of <1,000 cc. The average planning target volume was 134 cc (range 28–324). The number of catheters used ranged from 8 to 25 with an average of 18 catheters used per patient. We achieved an average dose homogeneity index of 0.76 in our patients. The average D90(%) was 105% and the average D90(Gy) was 3.6 Gy per fraction. The median volume receiving 100% of the prescribed dose (V100) was 161.7 cc (range 33.9–330.1), 150% of the prescribed dose (V150) and 200% of the prescribed dose (V200) was 39.4 cc (range 14.6–69.6) and 14.72 cc (range 6.48–22.25), respectively. Our dosimetric outcomes were excellent even in patients with breast volume under 1,000 cc. There were no cases of grade 3 skin toxicity or acute pneumonitis. Two patients had a postoperative infection and two patients had fat necrosis postprocedure.
Conclusion: Multicatheter high dose rate APBI is a safe and feasible procedure that can be carried out with minimal toxicity in Asian patients with breast volumes under 1,000 cc.
Keywords: breast, Asian, multicatheter, brachytherapy
Introduction
Breast-conserving surgery followed by whole breast irradiation (WBI) has become the standard of care for early breast carcinoma.1–4 However, the need for WBI has been questioned and several centers have evaluated the feasibility and efficacy of accelerated partial breast irradiation (APBI).5–7 APBI represents a technique that allows for the delivery of adjuvant therapy after breast-conserving surgery, in 1 week or less, with multiple techniques being available at this time. To date at least five Phase III trials comparing different techniques of APBI to conventional WBI have been initiated.8–12 Polgár et al13 demonstrated excellent long-term local tumor control, survival, and cosmetic results with a low-rate of long-term toxicity in a 12-year randomized prospective study using multicatheter interstitial brachytherapy. In the US, the Pooled Registry of Multicatheter Interstitial Sites study group also showed excellent long-term local control and cosmesis in 1,356 patients who underwent multicatheter APBI.14 These studies have encouraged many centers around the world to explore and to set up APBI programs. However, most studies have been done in Europe and in the US. Little is known about the cosmesis and the feasibility of this procedure in Asian women. There is also concern among radiation oncologists that the procedure may be technically difficult in patients with smaller breast volume. Due to the smaller build of Asian women, many of our patients have breast volume of <1,000 cc.15 Otani et al16 had previously found the use of multicatheter APBI in Japanese women with A and B cup-sized breasts feasible. However, breast volume was not mentioned in the study and quality assurance and plan assessment in Otani’s study differed from other studies. The aim of this study was to present our findings in relation to a more quantifiable breast volume and to use the Radiation Therapy Oncology Group (RTOG) plan assessment to more accurately assess the feasibility of this technique. In this retrospective study, we report the dosimetric and toxicity outcomes of multicatheter high dose rate (HDR) APBI in our Asian population with breast volumes under 1,000 cc.
Methods
Between November 2008 and August 2014, 121 patients treated with APBI with multicatheter HDR interstitial brachytherapy were included in the study. All patients underwent lumpectomy and axillary nodal evaluation either by sentinel node biopsy or axillary clearance. Clips were placed in the cavity postsurgery at the superior, inferior, anterior, posterior, and lateral aspects to help define the cavity for APBI. Patient selection criteria were as follows: 1) tumors of <3 cm in size, 2) no lymph node involvement, 3) negative surgical margins, and 4) no multicentric disease or extensive intraductal component. The study protocol was reviewed and approved by the Domain Specific Research Board (DSRB number 2007/00368) and written informed consent was obtained from the patients.
Implant technique
Within 8 weeks of lumpectomy and axillary nodal evaluation, patients underwent an interstitial implant using the template method performed in the supine position.17,18 Once the cavity was localized, the ipsilateral breast area was surgically prepared under sterile conditions. To avoid injuring the underlying chest wall structures or causing a pneumothorax, the overlying breast was pinched and gently lifted off the chest wall before applying the template and securing it. Three to five anchoring needles were then placed in an asymmetric pattern usually involving C-12. C-12 was the grid coordinates of the template that corresponded to the center of the template. The purpose of the asymmetric pattern was to aid easy orientation of the template in reference to the patient’s anatomy as well as to secure and prevent slipping of the template from the breast.
A computed tomography scan was then obtained with the anchoring catheters in situ. These images were reconstructed on the Oncentra planning system Version 4.3 (Elekta AB, Stockholm, Sweden).
Planning technique
The planning target volume (PTV) was formed by expanding 15–20 mm from the contrast enhanced tumor cavity and any surrounding surgical clips. The PTV-evaluation (PTV-Eval) was that formed by excluding the 5 mm skin rind as well as the underlying chest wall muscle layer. The skin was also contoured on the sagittal slice depicting the largest breast contour (usually the slice showing the nipple) and the most anterior chest wall surface was also contoured. The images were then reoriented in Oncentra to depict the “template view” to determine the location of the remaining catheters. Next an overlying photocopy of the template on a transparency was placed on the top of the screen and the “template view” template magnification was matched 1:1 to the overlaid template transparency. The corresponding anchoring needle positions were then marked on the overlaid transparency and the chest wall and PTV-Eval contour were outlined onto the transparency (Figure 1). Once done, the catheter placement for the remaining catheters was easily determined.
  | Figure 1 (A) Overlaying and tracing the contours onto the transparency template. (B) Picture showing the transparency template with the positions of the catheter placement. |
The final step required replacing the needles with polyethylene tubing with a hemispheric button at each end. Extra attention was given to make sure that the button on the connector side of the remote afterloader was flush to the skin. After each catheter was trimmed and numbered, an en face picture of the implant showing the catheter numbers with respect to the breast anatomy was obtained to aid in the reconstruction of the catheters. Computed tomography-based simulation of the patient was performed with the patient in the supine position and the images were then transferred to the Oncentra treatment planning system.
Dosimetry
A total of 34 Gy in ten fractions, two fractions per day, 3.4 Gy per fraction, separated by at least 6 hours, given on five treatment days was delivered via a 192Ir remote afterloader in the supine position. Target coverage (PTV-Eval) was ≥90% of the prescribed dose covering ≥90% of the PTV-Eval (D90 ≥90%). In our study, the dose constraint for the skin was set as <100% of the prescribed dose. The skin was defined at the surface of the patient external contour. Care was taken to ensure that the 100% isodose line did not touch the patient contour.19
To ensure appropriate dose homogeneity throughout the implant, two parameters were used: the volume of tissue receiving higher doses and a dose homogeneity index (DHI). The actual volume of tissue receiving 150% (V150) and 200% (V200) of the prescribed dose was limited to ≤70 and ≤20 cc, respectively. The DHI, as represented by the volume ratio (1 – V150/V100), was constrained to ≥0.75. V150 represented the volume of tissue receiving 150% of the prescribed dose, and V100 represented the volume of tissue receiving the prescribed dose.
In addition, <60% of the ipsilateral whole breast reference volume should receive ≥50% of the prescribed dose. The online Breast Atlas from the RTOG website was used to target the whole breast reference volume.20
Toxicity
Patients were reviewed at 1 week, then 3 months, and thereafter every 6 months. Patients were assessed for skin toxicity, such as radiodermatitis and telangiectasia, subcutaneous fibrosis, and fat necrosis. They were also assessed for symptomatic acute pneumonitis. These toxicities were graded using the Common Terminology Criteria for Adverse Events v3.0 where applicable.21
Statistical analysis
The results were analyzed statistically using the Stata v11.0 (Statacorp, College Station, TX, USA). The two-sample Wilcoxon rank sum (Mann–Whitney U-test) was used in the analysis given the small sample size.
Results
We identified 121 patients treated with APBI at our center between 2008 and 2014. The median follow-up for our patient group was 30 months (range 3.7–66.5).
Patient and tumor characteristics
In our study population, 71% of the patients were Chinese, while 15% (n=19) were of other Asian ethnicity. Ninety percent (n=110) of our patients were at least 50 years old at the time of the implant. Eighty-two percent (n=100) were postmenopausal (Table 1).
  | Table 1 Patient characteristics |
Seventy percent (n=85) patients had T1 tumors, 12% (n=15) had T2 tumors, and 17% (n=21) had ductal carcinoma in situ. Most patients treated with APBI had estrogen receptor positive tumors (Table 2).
  | Table 2 Histological findings |
APBI in an Asian population
In this study, the median breast volume was 850 cc (range 216–2,108). The average lumpectomy cavity volume was 23.9 cc (range 3.1–61.5). The average PTV was 134 cc (range 28–324). The number of catheters used ranged from 8 to 25 with average of 18 catheters being used per patient.
We achieved an average DHI of 0.76 (range 0.57–0.84) in our patients. The average D90(%) was 105% and D90(Gy) was 3.6 Gy per fraction. The median volume receiving 100% of the prescribed dose (V100) was 161.7 cc (range 33.9–330.1), 150% of the prescribed dose (V150) and 200% of the prescribed dose (V200) was 39.4 cc (range 14.6–69.6) and 14.72 cc (range 6.48–22.25), respectively. The maximum skin dose was 3.0 Gy per fraction (range 2.8–3.4).
We found 59.5% (n=72) patients with a breast volume of <1,000 cc. In this group, the average number of catheters used was 16.7 and the average DHI and V100 was 0.74 and 134 cc, respectively. V150 was 38.5 cc and V200 was 14.2 cc. There was no increased incidence of toxicity in this group of patients.
Outcomes
In our study, one patient relapsed with bone metastases and passed away two and a half years from the implant date. Due to the short follow-up, 26 patients were not due for their follow-up mammograms at the time of the data analysis. For the remaining patient cohort, there were no local recurrences detected on follow-ups.
Postprocedure complications and toxicity
There were two incidences of postoperative infection that resolved within a week with oral antibiotics. Two patients experienced fat necrosis that was diagnosed on routine ultrasound surveillance 36 months postimplant insertion. A summary of other toxicities observed in our study is shown in Table 3. No grade 3 toxicity was observed. Due to the low incidence of toxicity observed in our study group, we could find no relationship between the breast volume and the incidence of toxicity observed.
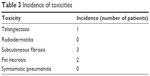  | Table 3 Incidence of toxicities |
Discussion
Our study shows that the multicatheter interstitial brachytherapy is dosimetrically feasible in the Asian population with good outcomes and with minimal acute toxicity. This is encouraging for centers in Asia that wish to start an APBI program.
Multicatheter interstitial breast brachytherapy has been gaining popularity as an adjuvant treatment for early-stage breast cancer due to its short treatment time, good outcomes, and excellent cosmesis.
As such, there is a need for sharing of expertise from various centers, especially in the use of this procedure in different ethnic populations. Studies documenting cosmesis and outcomes have been done in Europe and in the US. This is the first study that documents multicatheter interstitial APBI and the outcomes in an Asian population of largely Chinese ethnicity.
In the Asian population, it is fairly common to encounter breast volumes of <1,000 cc, which can make APBI challenging. Yoshida et al22 reported the Japanese experience in APBI and cited challenges including small breast volume and high incidence of infection postoperatively. Small breast size were found to make insertion of the catheters and planning within the limits of the organs at risk difficult. Also, the use of open cavity technique,23 large V100, V150, and V200 volumes and nonuse of prophylactic antibiotics during the brachytherapy were found to be risk factors for infection. In our patients, we used the closed cavity technique and used prophylactic antibiotics throughout the brachytherapy, thus avoiding the problems of infection. We also ensured that the V100, V150, and V200 were within the RTOG technical guidelines thereby decreasing the chance of infection in our patients. Our results compare favorably with both Japanese and European series (Table 4).
  | Table 4 Comparison of our results with other centers |
In the Japanese studies, breast cup size was used as a determinant of breast size. Our study used breast volume to report on the outcomes. We found this to be more accurate than breast cup size reporting from the patient. Furthermore, the dosimetric findings in our study in patients with breast volume under 1,000 cc compared favorably with the current RTOG trial RTOG 04-13 technical guidelines for interstitial brachytherapy. This shows that multicatheter interstitial brachytherapy is a good treatment option for this subset of patients with good acute toxicity profile. Patients in this group have generally been considered technically challenging for interstitial brachytherapy. Our study shows that this procedure is very effective in patients with this breast volume while meeting the RTOG guidelines for treatment planning. The limitations of this study are the short follow-up. However, our study shares the experience of APBI in an Asian population with more than half of the subjects having a breast volume of <1,000 cc. Future studies should focus on the long-term outcome of toxicity and recurrence in this study group.
Conclusion
Multicatheter HDR APBI is a dosimetrically feasible procedure that can be carried out with minimal acute toxicity in Asian patients with breast volumes under 1,000 cc.
Disclosure
The authors report no conflicts of interest in this work.
References
Litiere S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I–II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol. 2012;13(4):412–419. | ||
Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. | ||
Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. | ||
Morrow M. Rational local therapy for breast cancer. N Engl J Med. 2002;347(16):1270–1271. | ||
Shah C, Antonucci JV, Wilkinson JB, et al. Twelve-year clinical outcomes and patterns of failure with accelerated partial breast irradiation versus whole-breast irradiation: results of a matched-pair analysis. Radiother Oncol. 2011;100(2):210–214. | ||
Arthur DW, Winter K, Kuske RR, et al. A phase II trial of brachytherapy alone after lumpectomy for select breast cancer: tumor control and survival outcomes of RTOG 95-17. Int J Radiat Oncol Biol Phys. 2008;72(2):467–473. | ||
Benitez PR, Keisch ME, Vicini F, et al. Five-year results: the initial clinical trial of Mammosite balloon brachytherapy for partial breast irradiation in early stage breast cancer. Am J Surg. 2007;194(4):456–462. | ||
Polgár C, Strnad V, Major T. Brachytherapy for partial breast irradiation: the European experience. Semin Radiat Oncol. 2005;15(2):116–122. | ||
Vicini FA, Arthur DW. Breast brachytherapy: North American experience. Semin Radiat Oncol. 2005;15(2):108–115. | ||
Polgár C, Major T. Current status and perspectives of brachytherapy for breast cancer. Int J Clin Oncol. 2009;14(1):7–24. | ||
Gifford KA, Nelson CL, Kirsner SM, Kisling KD, Ballo MT, Bloom ES. On the feasibility of treating to a 1.5 cm PTV with a commercial single-entry hybrid applicator in APBI breast brachytherapy. J Contemp Brachytherapy. 2012;4(1):29–33. | ||
Skowronek J, Wawrzyniak-Hojczyk M, Ambrochowicz K. Review article: Brachytherapy in accelerated partial breast irradiation (APBI) – review of treatment methods. J Contemp Brachytherapy. 2012;4(3): 152–164. | ||
Polgár C, Major T, Fodor J, et al. Accelerated partial-breast irradiation using high-dose-rate interstitial brachytherapy: 12-year update of a prospective clinical study. Radiother Oncol. 2010;94(3):274–279. | ||
Kamrava M, Kuske RR, Anderson B, et al. Outcomes of breast cancer patients treated with accelerated partial breast irradiation via multicatheter interstitial brachytherapy: The Pooled Registry of Multicatheter Interstitial Sites (PROMIS) experience. Ann Surg Oncol. 2015;22(Suppl 3):S404–S411. | ||
Li FY, He ZY, Xue M, Chen LX, Wu SG, Guan XX. Feasibility and acute toxicity of 3-dimensional conformal external-beam accelerated partial-breast irradiation for early-stage breast cancer after breast-conserving surgery in Chinese female patients. Chin Med J (Engl). 2011;124(9):1305–1309. | ||
Otani Y, Nose T, Dokiya T, et al. A Japanese prospective multi-institutional feasibility study on accelerated partial breast irradiation using interstitial brachytherapy: treatment planning and quality assurance. Radiat Oncol. 2015;10(1):126. | ||
Tang JI, Tan PW, Koh YV, Buhari SA. Multi-catheter interstitial accelerated partial breast irradiation – tips and tricks for a good insertion. J Contemp Brachytherapy. 2014;6(1):85–90. | ||
Koh YV, Buhari S, Tan PW, et al. Comparing a volume based template approach and ultrasound guided freehand approach in multicatheter interstitial accelerated partial breast irradiation. J Contemp Brachytherapy. 2014;6(2):173–177. | ||
NSABP B-39: A randomized phase III study of conventional whole breast irradiation (WBI) versus partial breast irradiation (PBI) for women with Stage 0, I, or II breast cancer. Available from: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0413. Accessed November 10, 2013. | ||
RTOG Radiation Therapy Oncology Group. Breast Cancer Atlas. Available from: https://www.rtog.org/LinkClick.aspx?fileticket=vzJFhPaBipE%3d&tabid=236. Accessed November 10, 2013. | ||
DCTD, NCI, NIH, DHHS. Cancer Therapy Evaluation Program. Common terminology criteria for adverse events, Version 3.0 (CTCAE); 2006. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed June 23, 2016. | ||
Yoshida K, Nose T, Masuda N, et al. Preliminary result of accelerated partial breast irradiation after breast-conserving surgery. Breast Cancer. 2009;16(2):105–112. | ||
Nose T, Komoike Y, Yoshida K, et al. A pilot study of wider use of accelerated partial breast irradiation: intraoperative margin-directed re-excision combined with sole high-dose-rate interstitial brachytherapy. Breast Cancer. 2006;13(3):289–299. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
