Back to Journals » Clinical Interventions in Aging » Volume 15
Abaloparatide and the Spine: A Narrative Review
Authors Thompson JC, Wanderman N , Anderson PA , Freedman BA
Received 3 March 2020
Accepted for publication 23 April 2020
Published 29 June 2020 Volume 2020:15 Pages 1023—1033
DOI https://doi.org/10.2147/CIA.S227611
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Jeremy C Thompson,1 Nathan Wanderman,1 Paul A Anderson,2 Brett A Freedman1
1Department of Orthopedic Surgery, Mayo Clinic, Rochester, MN, USA; 2Department of Orthopaedic Surgery, Department of Orthopedics Surgery & Rehabilitation, University of Wisconsin, Madison, WI 53705-2281, USA
Correspondence: Brett A Freedman
Mayo Clinic, 200 First Street S.W., Rochester, MN 55905, USA
Tel +507 284-2884
Fax +507 266-4234
Email [email protected]
Abstract: Osteoporosis is a common and debilitating condition characterized by diminished bone mass and architecture leading to bone fragility. Antiresorptive medicines like bisphosphonates (and less commonly denosumab) are the typical first-line agents for the medical treatment of osteoporosis. However, newer anabolic agents have been shown to improve bone mass and architecture, as well as reduce fracture risk, to a greater degree than traditional antiresorptive therapies. Teriparatide (human recombinant parathyroid hormone (PTH) 1– 34, Forteo, Ely Lilly, Indianapolis, IN), which was the first in class to be approved in the United States, is the most widely used anabolic osteoporosis medicine and has shown significant benefit over traditional antiresorptive therapies. However, abaloparatide (synthetic parathyroid-related peptide (PTHrP), Tymlos, Radius Health, Waltham, MA), the second drug in this family, has recently become available for use. In this narrative review, we review the mechanism, effects, and benefits of abaloparatide compared to alternative treatments as well as discuss the current literature in regard to its effect on osteoporosis-related complications in the spine.
Keywords: abaloparatide, Tymlos, anabolic, osteoporosis, spine, teriparatide
Introduction
Osteoporosis is a common, debilitating condition characterized by diminished bone mass and architecture leading to bone fragility. It is estimated that over 10.2 million Americans have osteoporosis with an additional 43.4 million living with osteopenia.1–3 Approximately 16% of men and 30% of women older than 50 years have osteoporosis.3 Osteoporosis places patients at a significant risk of sustaining fractures most commonly involving the hip, wrist, and thoracolumbar spine with over 2 million osteoporosis-related fractures per year.1,2,4 Further, there is a 20% incidence of vertebral compression fracture in osteoporotic patients.5 Osteoporosis and osteoporotic fragility fracture are associated with significant complications including decreased quality of life, reduced independence, risk of additional fracture and increased mortality.1–4,6,7
Antiresorptive medicines like bisphosphonates (and less commonly denosumab) are the typical first-line agents for the medical treatment of osteoporosis.1,4 However, newer anabolic agents have been shown to improve bone mass and architecture, as well as reduce fracture risk, to a greater degree than traditional antiresorptive therapies. Teriparatide (human recombinant parathyroid hormone (PTH) 1–34, Forteo, Ely Lilly, Indianapolis, IN), which was the first in class to be approved in the United States, is the most widely used anabolic osteoporosis medicine and has shown significant benefit over traditional antiresorptive therapies.8–19 However, abaloparatide (synthetic parathyroid-related peptide (PTHrP), Tymlos, Radius Health, Waltham, MA), the second drug in this family, has recently become available for use.
The purpose of this review is two-fold. First, we will review the mechanism, effects and benefits of abaloparatide compared to alternative treatments. Second, we will review the current literature regarding the effect of abaloparatide on osteoporosis-related complications in the spine.
Basic Science
Abaloparatide is a synthetic 34-amino acid peptide analogue of PTHrP. PTHrP is a protein with similar function to PTH that is expressed in almost all human tissues and has many regulatory functions. While not normally detectable under physiologic conditions (except in pregnancy and lactation), PTHrP can be detected in malignancy where it is associated with humoral hypercalcemia of malignancy.20,21
PTH and PTHrP influence bone turnover via the receptor activator of nuclear factor kappa-β ligand (RANKL) pathway in osteoblasts. In this pathway, PTH and PTHrP stimulate PTH receptors in osteoblasts, inducing cyclic adenosine monophosphate (cAMP) activation, osteoblastic bone formation, and later osteoclast activation. However, there are differences in activation between PTHrP and PTH. The PTH receptor has at least 2 conformations that activate the RANKL pathway: R0 and RG. R0 is a G-protein independent receptor that is the primary target of teriparatide (Forteo). The R0 receptor binds PTH with high affinity and results in prolonged binding and activation leading to an initial burst of bone formation followed by bone resorption due to downstream osteoclast activation. On the contrary, RG, a G-protein dependent receptor targeted by abaloparatide, reversibly binds to PTHrP with high affinity resulting in transient activation that maximizes initial bone formation while limiting late bone resorption and osteoclast differentiation [Figure 1].22,23
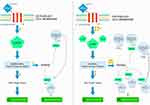 |
Figure 1 Model of parathyroid hormone (PTH)/teriparatide and PTH-related peptide (PTHrP/abaloparatide activation of parathyroid hormone type 1 receptor (PTH1R)). |
The effectiveness of abaloparatide has been demonstrated in multiple rodent and primate studies. Varella et al demonstrated that long-term daily administration of abaloparatide resulted in dose-dependent gains in bone mass, bone architecture, and up to 2.7-fold greater peak load to failure over controls in the lumbar spine of ovariectomized osteopenic rats.24 Similarly, Berhardsson et al confirmed these findings and further showed increases in bone formation markers without associated increases in bone resorption factors associated with hypercalcemia.25 In orchiectomy-induced osteoporotic male mice, Chandler et al showed reversal of osteoporosis with 8-weeks of abaloparatide therapy.26 Doyle et al translated the rodent model to primates and showed that 16 months of abaloparatide treatment resulted in increased whole body bone mass and greater lumbar vertebral strength in ovariectomized monkeys.27 Moreover, Sahbani et al compared the effects of abaloparatide and teriparatide in wild-type female mice. His findings showed an increase in bone formation marker, P1NP, and decrease in bone resorption marker, TRAcP-5b when compared to teriparatide. Additionally, although the study showed that abaloparatide demonstrated an increase in cortical thickness when compared to teriparatide, it is not known whether the increase in cortical thickness was due to the difference in endocortical resorption or in periosteal bone formation.28
Clinical Results
The beneficial effects of abaloparatide in human studies have been consistent with those demonstrated in animal models. Additionally, while teriparatide has been shown to significantly improve bone mineral density and architecture when compared to placebo and bisphosphonates therapy, abaloparatide therapy has shown superior clinical results. In a multi-center, multi-national, double-blind placebo-controlled clinical trial, Leder et al observed lumbar BMD increases up to 6.7% over 24 weeks with abaloparatide vs only 5.5% and 1.6% in the teriparatide and placebo groups, respectively (p = <0.001)29. The increase in lumbar BMD tripled increases in hip BMD. Similarly, Bilezikian et al, in a Phase 2 randomized control trial of postmenopausal women aged 55–85 years, demonstrated consistently greater dose-dependent improvements in lumbar trabecular bone score by 12 weeks with abaloparatide when compared to teriparatide and placebo.6 Improvement in lumbar TBS indicates improvement in bone microarchitecture and corresponds to decrease risk of fracture and improvement in pedicle screw strength.30,31
Abaloparatide therapy increases biomechanical strength and bone-formation, which results in a substantial reduction in fragility fractures. The ACTIVE randomized control trial (RCT) compared the clinical effects of abaloparatide to teriparatide and placebo. This trial was an 18-month double-blinded RCT in 2463 patients with osteoporosis. Subjects were randomized to receive daily injections of 80 micrograms of abaloparatide, 20 micrograms of teriparatide, or placebo (both the abaloparatide and teriparatide dosages are the standard recommended dosages for these medications). The primary endpoint of the study was new vertebral compression fractures which occurred in 0.58%, 0.84%, and 4.22% in the abaloparatide, teriparatide, and control groups, respectively (p = <0.001). Both active treatments had a significantly lower risk than placebo. Subjects in the abaloparatide group showed a significantly greater improvement of BMD in the lumbar spine, femoral neck, and total hip compared to teriparastide and placebo at all time points beginning at 6 months a significantly lower incidence of hypercalcemia in the abaloparatide group (3.4%) versus teriparatide group (6.4%) (p = 0.01). Overall, Miller and colleagues concluded that abaloparatide treatment reduced the risk of new vertebral and nonverbal fractures over 18 months compared to placebo. However, the study was inadequately powered to make a definitive claim regarding comparison between abaloparatide and teriparatide.32,33
Additional post hoc studies have shown that teriparatide therapy is also beneficial in special populations. McClung et al demonstrated a 12% increase in BMD at 12 months with abaloparatide therapy which was similar to the results found in younger patients.34 Cosman et al further demonstrated those with high risk of osteoporotic fracture including those with T-score < −3.0, history of non-vertebral osteoporotic fracture, and age >75 years had significantly greater major osteoporotic fracture risk reduction with abaloparatide vs teriparatide (78% reduction with abaloparatide vs 23% with teriparatide) (p = 0.007).35 She concluded that abaloparatide results in improvement in BMD across all age groups and risk factors. Additionally, Reginster et al added that abaloparatide is potentially a more effective therapy than teriparatide based on the number needed to treat comparisons showing that the NNT for ABL in ACTIVE was 28 for vertebral, 55 for non-vertebral, 37 for clinical, and 34 for major osteoporotic fracture. NNT for these fracture types for teriparatide in ACTIVE were 30, 92, 59, and 75, respectively.36
While studies have shown that anabolic therapy provides substantial benefit in BMD and fracture risk, these therapies can result in loss of bone mass soon after they are discontinued, particularly if an antiresorptive agent is not subsequently administered. Leder et al showed BMD decreases of −4.2 ± 4.3% in the femoral neck, −4.5 ± 3.6% in the hip, and −10 ± 5.4% in the spine of patients 1–2 years after completing a 24-month course of abaloparatide therapy. Leder and colleagues also found that subjects who were treated with denosumab for 1–2 years after completion of abaloparatide did not show the same loss of BMD with only −0.6 ± 2.7% in the femoral neck, −0.8 ± 3.1% in the total hip, and −1.2 ± 4.7% in the spine.37
Bone et al further studied this phenomenon in the ACTIVExtented trial. In this trial, patients in the abaloparatide and placebo arms of the ACTIVE trial were re-enrolled at the completion of the trial to receive 24 months of alendronate therapy. Authors found that the continued protection against new fracture was substantial in the abaloparatide/alendronate (ABL/ALN) group as observed by an 84% relative risk reduction for sustaining a new radiographic vertebral fracture (0.9% incidence in the ABL/ALN group vs 5.6% Placebo/ALN group). Additionally, Kaplan–Meier incidence rates for other reported fracture types were significantly lower for abaloparatide/alendronate vs placebo/alendronate and gains in BMD achieved during ACTIVE were further increased in the abaloparatide/alendronate group.38 A summary of the study characteristics is included in Table 1, and a summary of the study findings is included in Table 2.
 |
Table 1 Summary of Study Characteristics |
 |  |  |
Table 2 Summary of Study Findings |
Initiating Abaloparatide (Tymlos)
Abaloparatide is currently approved by the FDA for the treatment of postmenopausal women with osteoporosis who are at high risk for fracture. “Risk” factors that qualify patients for the use of abaloparatide include a history of previous osteoporotic fracture, multiple risk factors for fracture, or patients who have failed or are intolerant to other available osteoporosis therapy. The typical dosing regimen is 80 micrograms/day subcutaneous injection for up to 18 months. Prior to initiation of therapy and calcium and vitamin D replenishment is recommended. Abaloparatide undergoes renal metabolism so dosing may need to be adjusted for patients with renal impairment.39
There are multiple contraindications to abaloparatide therapy. The most concerning to patients may be related to its dose-dependent increase in the incidence of osteosarcoma in F344 rats that are predisposed to osteogenic sarcoma. Of note, this relationship was observed at dosages 4–28 times the 80 mcg/day dose given to humans. While it is unknown if abaloparatide will cause osteosarcoma in humans at clinically relevant doses, it is not recommended for patients at increased risk for osteosarcoma. This includes those with Paget’s disease, unexplained elevations of alkaline phosphatase, open epiphyses, bone metastases or other skeletal malignancies, hereditary disorders predisposing to osteosarcoma, or prior external beam radiation or implant radiation of the skeletal system.
Patients should be informed of the possible side effects of abaloparatide. Orthostatic hypotension occurs in approximately 4% of patients on abaloparatide therapy. Because it typically occurs within 4 hours of injection, patients are advised to sit or lie during and immediately after administration to minimize this side effect. Additional side effects include dizziness (10%) and tachycardia (2%). While drug site reactions including redness (58%), edema (10%), and pain (9%) are common, severe reactions are rare and generally do not limit its use. Abaloparatide therapy may also cause laboratory abnormalities. Increase in serum uric acid is common (25%) but is not associated with increases in gout or arthralgia. Hypercalcemia, defined as albumin-corrected serum calcium ≥10.7 mg/mL, occurs in approximately 3% of patients but rarely causes discontinuation of therapy.33,39
Based on the wholesale acquisition cost for one abaloparatide pen-injection, the monthly cost of abaloparatide therapy is approximately $1721.40 While these costs are substantial and potentially burdensome for some patients, it is about one-half the monthly cost of teriparatide therapy (approximately $3295). Le and colleagues performed a cost-effectiveness analysis comparing abaloparatide and teriparatide. Using a discrete event simulation (DES) model and ACTIVE trial outcomes, the authors concluded that ABO/ALN therapy afforded patients 10-year average total discounted per-patient cost of $26,837 vs $46,783 for TPTD/ALN. Overall ABL/ALN provided a greater value, with higher quality-adjusted life-years at lower costs than for TPTD/ALN in general ($333,266/QALY vs $951,016/QALY) and high risk ($188,891/QALY vs $537,998/QALY) subgroups.40 Hiligsman performed a similar study with consistent results confirming that ABL/ALN therapy was more cost-effective than TPTD/ALN.41
Use in Spine Surgery
Osteoporosis plays a significant role in outcomes of spine surgery as successful spinal fusion requires adequate bone stock for implant fixation and bone physiology to support fusion. Numerous cadaveric and clinical studies have shown increased implant failure in patients with compromised BMD, which leads to complications such as pseudoarthrosis, progressive kyphosis, fracture, and implant subsidence and/or pullout.42,43 On the contrary, studies have shown general consensus regarding the benefits of anabolic osteoporosis medications, specifically teriparatide, on spinal fusion outcomes. These benefits include shorter time to fusion, higher insertional torque, pull-out strength, and lower rates of screw pullout and proximal junctional kyphosis.5,8,10-19 Additionally, Kong et al found that patients receiving teriparatide for 12 months after percutaneous kyphoplasty had a lower risk of new vertebral compression fracture and greater improvements in pain and quality of life than placebo at all time points up to 24 months after surgery.44 While published data has only evaluated teriparatide use in spinal surgery, similar work is ongoing and needed to demonstrate the clinical benefits of abaloparatide.
Conclusion
Abaloparatide is a second-generation anabolic therapy used for the treatment of osteoporosis. It differs from the 1st generation anabolic therapy, teriparatide, in that a reversibly binds to the RG configuration PTH receptor with high affinity resulting in transient activation of osteoblasts that maximizes initial bone formation while limiting late bone resorption and osteoclast differentiation. Animal and human studies have shown the beneficial effects to BMD, bone architecture, and fracture protection. Additional benefits to abaloparatide therapy are its relatively mild side effect profile and economic cost. Additional studies are needed to determine exhibit the effect of abaloparatide on spinal surgery outcomes.
Disclosure
Dr Paul A Anderson reports personal fees from Radius Medical, Amgen, Titan spine, Medtronic, Regeneration Technologies inc, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis - 2016. Endocr Pract. 2016;22(Suppl 4):1–42. doi:10.4158/EP161435.GL
2. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526. doi:10.1002/jbmr.2269
3. Wright NC, Saag KG, Dawson-Hughes B, Khosla S, Siris ES. The impact of the new National Bone Health Alliance (NBHA) diagnostic criteria on the prevalence of osteoporosis in the United States: supplementary presentation. Osteoporos Int. 2017;28(11):3283–3284. doi:10.1007/s00198-017-4207-9
4. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. doi:10.1007/s00198-014-2794-2
5. Lehman RA, Kang DG, Wagner SC. Management of osteoporosis in spine surgery. J Am Acad Orthop Surg. 2015;23(4):253–263. doi:10.5435/JAAOS-D-14-00042
6. Bilezikian JP, Hattersley G, Fitzpatrick LA, et al. Abaloparatide-SC improves trabecular microarchitecture as assessed by trabecular bone score (TBS): a 24-week randomized clinical trial. Osteoporos Int. 2018;29(2):323–328. doi:10.1007/s00198-017-4304-9
7. Marrinan S, Pearce MS, Jiang XY, Waters S, Shanshal Y. Admission for osteoporotic pelvic fractures and predictors of length of hospital stay, mortality and loss of independence. Age Ageing. 2015;44(2):258–261. doi:10.1093/ageing/afu123
8. Cho PG, Ji GY, Shin DA, Ha Y, Yoon DH, Kim KN. An effect comparison of teriparatide and bisphosphonate on posterior lumbar interbody fusion in patients with osteoporosis: a prospective cohort study and preliminary data. Eur Spine J. 2017;26(3):691–697. doi:10.1007/s00586-015-4342-y
9. Cosman F. The evolving role of anabolic therapy in the treatment of osteoporosis. Curr Opin Rheumatol. 2019;31(4):376–380. doi:10.1097/BOR.0000000000000616
10. Ebata S, Takahashi J, Hasegawa T, et al. Role of weekly teriparatide administration in osseous union enhancement within six months after posterior or transforaminal lumbar interbody fusion for osteoporosis-associated lumbar degenerative disorders: a multicenter, prospective randomized study. J Bone Joint Surg Am. 2017;99(5):365–372. doi:10.2106/JBJS.16.00230
11. Hirsch BP, Unnanuntana A, Cunningham ME, Lane JM. The effect of therapies for osteoporosis on spine fusion: a systematic review. Spine J. 2013;13(2):190–199. doi:10.1016/j.spinee.2012.03.035
12. Inoue G, Ueno M, Nakazawa T, et al. Teriparatide increases the insertional torque of pedicle screws during fusion surgery in patients with postmenopausal osteoporosis. J Neurosurg Spine. 2014;21(3):425–431. doi:10.3171/2014.5.SPINE13656
13. Kaliya-Perumal AK, Lu ML, Luo CA, et al. Retrospective radiological outcome analysis following teriparatide use in elderly patients undergoing multilevel instrumented lumbar fusion surgery. Medicine (Baltimore). 2017;96(5):e5996. doi:10.1097/MD.0000000000005996
14. Kim JW, Park SW, Kim YB, Ko MJ. The effect of postoperative use of teriparatide reducing screw loosening in osteoporotic patients. J Korean Neurosurg Soc. 2018;61(4):494–502. doi:10.3340/jkns.2017.0216
15. Ohtori S, Inoue G, Orita S, et al. Teriparatide accelerates lumbar posterolateral fusion in women with postmenopausal osteoporosis: prospective study. Spine (Phila Pa 1976). 2012;37(23):E1464–8. doi:10.1097/BRS.0b013e31826ca2a8
16. Ohtori S, Inoue G, Orita S, et al. Comparison of teriparatide and bisphosphonate treatment to reduce pedicle screw loosening after lumbar spinal fusion surgery in postmenopausal women with osteoporosis from a bone quality perspective. Spine (Phila Pa 1976). 2013;38(8):E487–92. doi:10.1097/BRS.0b013e31828826dd
17. Ohtori S, Orita S, Yamauchi K, et al. Does discontinuing teriparatide treatment and replacing it with bisphosphonate maintain the volume of the bone fusion mass after lumbar posterolateral fusion in women with postmenopausal osteoporosis? Asian Spine J. 2017;11(2):272–277. doi:10.4184/asj.2017.11.2.272
18. Ohtori S, Orita S, Yamauchi K, et al. More than 6 months of teriparatide treatment was more effective for bone union than shorter treatment following lumbar posterolateral fusion surgery. Asian Spine J. 2015;9(4):573–580. doi:10.4184/asj.2015.9.4.573
19. Yagi M, Ohne H, Konomi T, et al. Teriparatide improves volumetric bone mineral density and fine bone structure in the UIV+1 vertebra, and reduces bone failure type PJK after surgery for adult spinal deformity. Osteoporos Int. 2016;27(12):3495–3502. doi:10.1007/s00198-016-3676-6
20. Horwitz MJ, Tedesco MB, Gundberg C, Garcia-Ocana A, Stewart AF. Short-term, high-dose parathyroid hormone-related protein as a skeletal anabolic agent for the treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2003;88(2):569–575. doi:10.1210/jc.2002-021122
21. Uzawa T, Hori M, Ejiri S, Ozawa H. Comparison of the effects of intermittent and continuous administration of human parathyroid hormone(1-34) on rat bone. Bone. 1995;16(4):477–484. doi:10.1016/8756-3282(95)90194-9
22. Bhattacharyya S, Pal S, Chattopadhyay N. Abaloparatide, the second generation osteoanabolic drug: molecular mechanisms underlying its advantages over the first-in-class teriparatide. Biochem Pharmacol. 2019;166:185–191. doi:10.1016/j.bcp.2019.05.024
23. Sleeman A, Clements JN. Abaloparatide: a new pharmacological option for osteoporosis. Am J Health Syst Pharm. 2019;76(3):130–135. doi:10.1093/ajhp/zxy022
24. Varela A, Chouinard L, Lesage E, Smith SY, Hattersley G. One year of abaloparatide, a selective activator of the PTH1 receptor, increased bone formation and bone mass in osteopenic ovariectomized rats without increasing bone resorption. J Bone Miner Res. 2017;32(1):24–33. doi:10.1002/jbmr.3003
25. Bernhardsson M, Aspenberg P. Abaloparatide versus teriparatide: a head to head comparison of effects on fracture healing in mouse models. Acta Orthop. 2018;89(6):674–677. doi:10.1080/17453674.2018.1523771
26. Chandler H, Lanske B, Varela A, et al. Abaloparatide, a novel osteoanabolic PTHrP analog, increases cortical and trabecular bone mass and architecture in orchiectomized rats by increasing bone formation without increasing bone resorption. Bone. 2019;120:148–155. doi:10.1016/j.bone.2018.10.012
27. Doyle N, Varela A, Haile S, et al. Abaloparatide, a novel PTH receptor agonist, increased bone mass and strength in ovariectomized cynomolgus monkeys by increasing bone formation without increasing bone resorption. Osteoporos Int. 2018;29(3):685–697. doi:10.1007/s00198-017-4323-6
28. Sahbani K, Cardozo CP, Bauman WA, Tawfeek HA. Abaloparatide exhibits greater osteoanabolic response and higher cAMP stimulation and β-arrestin recruitment than teriparatide. Physiol Rep. 2019;7(19):e14225. doi:10.14814/phy2.14225
29. Leder BZ, O’Dea LS, Zanchetta JR, et al. Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2015;100(2):697–706. doi:10.1210/jc.2014-3718
30. Hans D, Shevroja E, Lamy O. Use of trabecular bone score as a complementary approach to DXA for fracture risk assessment in clinical practice. Rev Med Suisse. 2017;13(559):844–850.
31. McCloskey EV, Oden A, Harvey NC, et al. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res. 2016;31(5):940–948. doi:10.1002/jbmr.2734
32. Miller PD, Hattersley G, Lau E, et al. Bone mineral density response rates are greater in patients treated with abaloparatide compared with those treated with placebo or teriparatide: results from the ACTIVE Phase 3 trial. Bone. 2019;120:137–140. doi:10.1016/j.bone.2018.10.015
33. Miller PD, Hattersley G, Riis BJ, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316(7):722–733. doi:10.1001/jama.2016.11136
34. McClung MR, Harvey NC, Fitzpatrick LA, et al. Effects of abaloparatide on bone mineral density and risk of fracture in postmenopausal women aged 80 years or older with osteoporosis. Menopause. 2018;25(7):767–771. doi:10.1097/GME.0000000000001080
35. Cosman F, Hattersley G, Hu MY, Williams GC, Fitzpatrick LA, Black DM. Effects of abaloparatide-SC on fractures and bone mineral density in subgroups of postmenopausal women with osteoporosis and varying baseline risk factors. J Bone Miner Res. 2017;32(1):17–23. doi:10.1002/jbmr.2991
36. Reginster JY, Hattersley G, Williams GC, Hu MY, Fitzpatrick LA, Lewiecki EM. Abaloparatide is an effective treatment option for postmenopausal osteoporosis: review of the number needed to treat compared with teriparatide. Calcif Tissue Int. 2018;103(5):540–545. doi:10.1007/s00223-018-0450-0
37. Leder BZ, Tsai JN, Jiang LA, Lee H. Importance of prompt antiresorptive therapy in postmenopausal women discontinuing teriparatide or denosumab: the Denosumab and Teriparatide Follow-up study (DATA-Follow-up). Bone. 2017;98:54–58. doi:10.1016/j.bone.2017.03.006
38. Bone HG, Cosman F, Miller PD, et al. ACTIVExtend: 24 months of alendronate after 18 months of abaloparatide or placebo for postmenopausal osteoporosis. J Clin Endocrinol Metab. 2018;103(8):2949–2957. doi:10.1210/jc.2018-00163
39. Tymlos (R) [Prescirbing Information]. Radius Health, Inc. Waltham, MA; 2018.
40. Le QA, Hay JW, Becker R, Wang Y. Cost-effectiveness analysis of sequential treatment of abaloparatide followed by alendronate versus teriparatide followed by alendronate in postmenopausal women with osteoporosis in the United States. Ann Pharmacother. 2019;53(2):134–143. doi:10.1177/1060028018798034
41. Hiligsmann M, Williams SA, Fitzpatrick LA, Silverman SS, Weiss R, Reginster JY. Cost-effectiveness of sequential treatment with abaloparatide vs. teriparatide for United States women at increased risk of fracture. Semin Arthritis Rheum. 2019;49(2):184–196. doi:10.1016/j.semarthrit.2019.01.006
42. Liu Y, Dash A, Krez A, et al. Low volumetric bone density is a risk factor for early complications after spine fusion surgery. Osteoporos Int. 2020;31(4):647–654. doi:10.1007/s00198-019-05245-7
43. McCoy S, Tundo F, Chidambaram S, Baaj AA. Clinical considerations for spinal surgery in the osteoporotic patient: a comprehensive review. Clin Neurol Neurosurg. 2019;180:40–47. doi:10.1016/j.clineuro.2019.03.010
44. Kong M, Zhou C, Zhu K, et al. 12-month teriparatide treatment reduces new vertebral compression fractures incidence and back pain and improves quality of life after percutaneous kyphoplasty in osteoporotic women. Clin Interv Aging. 2019;14:1693–1703. doi:10.2147/CIA.S224663
45. Leder BZ, Mitlak B, Hu MY, Hattersley G, Bockman RS. Effect of abaloparatide versus alendronate on fracture risk reduction in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2019;105(3):938–43.
46. Leder BZ, Zapalowski C, Hu MY, et al. Fracture and bone mineral density response by baseline risk in patients treated with abaloparatide followed by alendronate: results from the phase 3 ACTIVExtend trial. J Bone Miner Res. 2019;34(12):2213–2219. doi:10.1002/jbmr.3848
47. McCloskey EV, Fitzpatrick LA, Hu MY, Williams G, Kanis JA. Effect of abaloparatide on vertebral, nonvertebral, major osteoporotic, and clinical fractures in a subset of postmenopausal women at increased risk of fracture by FRAX probability. Arch Osteoporos. 2019;14(1):15. doi:10.1007/s11657-019-0564-7
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
