Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 9 » Issue 1
A US database study characterizing patients initiating a budesonide–formoterol combination versus tiotropium bromide as initial maintenance therapy for chronic obstructive pulmonary disease
Authors Kern D , Williams S, Tunceli O, Wessman C, Zhou S, Pethick N, Elhefni H, Trudo F
Received 20 March 2014
Accepted for publication 2 May 2014
Published 18 July 2014 Volume 2014:9(1) Pages 775—783
DOI https://doi.org/10.2147/COPD.S64491
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
David M Kern,1 Setareh A Williams,2 Ozgur Tunceli,1 Catrin Wessman,3 Siting Zhou,1 Ned Pethick,2 Hanaa Elhefni,2 Frank Trudo2
1HealthCore Inc., 2AstraZeneca, Wilmington, DE, USA; 3AstraZeneca, Mölndal, Sweden
Objective: To compare clinical and demographic characteristics, resource utilization and costs of chronic obstructive pulmonary disease (COPD) patients prior to initiating budesonide–formoterol combination (BFC) or tiotropium-maintenance therapy.
Materials and methods: This cross-sectional study used claims-based diagnosis to identify COPD patients in the HealthCore Integrated Research Database who initiated BFC or tiotropium therapy between March 1, 2009 and January 31, 2012 (intake period); the index date was defined as the initial prescription fill for either agent. Patients diagnosed with respiratory tract cancer or receiving inhaled corticosteroids/long-acting β2-adrenergic agonists or tiotropium in 12 months prior to index date were excluded. Categorical variables were evaluated with Χ2 tests; mean cost differences were evaluated using γ-regression.
Results: Overall, 6,940 BFC and 10,831 tiotropium patients were identified. The BFC group was younger (mean age 64 versus 67 years), with a greater proportion of females (54% versus 51%). BFC-treated patients had more comorbid respiratory conditions, including asthma (25% versus 13%), but fewer comorbid cardiovascular conditions, including atherosclerosis (7% versus 10%) and myocardial infarction (4% versus 6%). A greater proportion of BFC patients received prior respiratory medication, including oral corticosteroids (46% versus 35%) and short-acting β2-agonists (44% versus 35%). Tiotropium-treated patients had a greater mean number of COPD-related outpatient visits (4.6 versus 4.1). BFC-treated patients had lower total all-cause ($17,259 versus $17,926) and COPD-related ($1,718 versus $1,930) health care costs, driven by lower all-cause and COPD-related inpatient expenditures.
Conclusion: Initiators of BFC or tiotropium showed differences in clinical and demographic characteristics and health care utilization and costs prior to starting COPD maintenance therapy.
Keywords: observational study, retrospective analysis, chronic respiratory condition, outcome research, health care utilization and costs
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality in the US, with an estimated prevalence of 25 million and an annual death rate of 124,477.1,2 Maintenance therapies for COPD recommended by clinical guidelines include long-acting β2-adrenergic agonists (LABAs) alone, inhaled corticosteroid (ICS)–LABA combinations, and anticholinergic agents.3
Budesonide–formoterol combination (BFC) therapy was approved in the US in 2009 for the maintenance treatment of airflow obstruction in COPD patients.4 Tiotropium bromide inhalation powder, approved for COPD treatment in the US in 2004, is indicated for long-term, once-daily maintenance treatment of bronchospasm associated with COPD; it is also indicated to reduce COPD exacerbations.5
To date, no study has compared health care-resource utilization and costs of COPD patients prior to initiating BFC versus tiotropium. The objective of this study was to describe and evaluate demographic and clinical characteristics and health care utilization and costs in COPD patients initiating BFC or tiotropium bromide in the 12 months prior to treatment initiation.
Materials and methods
Study design, population, and data source
This cross-sectional study employed administrative claims data to describe key characteristics of COPD patients initiating BFC or tiotropium therapy between March 1, 2009 and January 31, 2012 (intake period). Patients were identified using Generic Product Identifier codes: BFC (4420990241) and tiotropium (44100080100120). The index date was defined as the processing date of the first pharmacy claim for COPD maintenance medication, and patients were assigned to either the BFC or tiotropium group depending on their index prescription fill. The BFC group consisted of BFC initiators who received no tiotropium or ICS–LABA in the 12 months prior to the index date, and to ensure patients were initiating only one of the study medications, had no tiotropium fills within 15 days of initiating BFC therapy. Similarly, patients in the tiotropium group received neither tiotropium nor ICS–LABA in the 12 months prior to the index date, and did not fill an ICS–LABA prescription within 15 days of starting tiotropium treatment.
All study data were queried from the HealthCore Integrated Research Database, a repository of a broad spectrum of medical, pharmacy, and laboratory information on more than 46 million health plan members from across the US. The service models of the 14 commercial health plans included in the database encompass health maintenance organizations, point of service, preferred provider organizations, and indemnity plans. Throughout this study, researchers’ access was limited to deidentified data to ensure patient privacy and confidentiality. Strict measures were observed to ensure full compliance with the Health Insurance Portability and Accountability Act of 1996.
Inclusion criteria
Patients had to be ≥40 years old at the index date, have COPD and at least one prescription fill for BFC or tiotropium during the intake period. COPD diagnostic criteria included the presence of at least one inpatient claim with a primary diagnosis of COPD and/or at least one emergency department (ER) claim with a COPD diagnosis and/or two other medical claims with a COPD diagnosis, including outpatient visits or inpatient visits with a nonprimary COPD diagnosis (based on the International Classification of Diseases, ninth revision, clinical modification [ICD-9-CM] diagnosis codes 491.xx, 492.xx, and 496.xx). Patients were also required to have at least 12 months of continuous health plan coverage for medical and pharmacy benefits prior to the index date.
Exclusion criteria
Excluded were patients with a diagnosis of respiratory tract cancer (larynx, trachea, pleura, or lung) during the 12-month preindex date, as indicated by the presence of two medical claims, at least 30 days apart (ICD-9-CM codes 161.xx, 162.xx, 163.xx, 231.xx, and 197.0x).
Measures
Important measures in this analysis included indicators of COPD disease activity, such as use of respiratory medications (ICS, LABA, ICS–LABA combination, tiotropium, leukotriene-receptor antagonists, theophylline, roflumilast, short-acting β2-agonists [SABAs], short-acting muscarinic antagonists [SAMAs], SABA–SAMA combination, and oral corticosteroids [OCS]), prior COPD exacerbations, COPD-related health care-resource utilization (inpatient visits, ER visits, outpatient visits, and other places of service with an ICD-9-CM diagnosis code for COPD), and COPD-related procedures (chest X-rays, chest computed tomography scans, pulmonary function tests, and home oxygen use). Costs of COPD-related health care utilization and respiratory medications were also analyzed. COPD exacerbations were analyzed as a single outcome and by each individual component: COPD-related inpatient hospitalizations, ER visits, and OCS prescription fills. Other indicators of overall health status included all-cause health care-resource utilization and costs, presence of chronic comorbid conditions, and total medication use.
Statistical analysis
The frequency and proportion of all categorical and dichotomous variables, along with odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Significant differences between the two treatment groups were analyzed with χ2 tests or Fisher’s exact test when χ2 was not appropriate. Means (± standard deviations), medians, mean differences, and 95% CIs were calculated for each continuous and count variable. Student’s t-test was used to analyze differences in mean age, γ-regression was used to analyze all health care-cost variables, and negative binomial regression was used to analyze the number of all-cause and COPD-related health care visits, prescription fills, and inpatient length of stay for patients having an inpatient visit(s). No adjustment for confounders was performed, and finally, data were not balanced or matched. All statistical analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC, USA), and α was set at 5%.
Results
Preindex demographics and clinical characteristics
A total of 6,940 and 10,831 BFC- and tiotropium-initiated patients, respectively, were identified. There were some differences in demographic characteristics between the two groups of patients. COPD patients initiating BFC relative to those initiating tiotropium were younger (mean ± standard deviation age 64.3±12.1 years versus 66.9±11.8 years) and more likely to be female (54.2% versus 50.6%), respectively (Table 1). Both groups had similar geographic distribution and health plan coverage (59.5% in the BFC and 60.7% in the tiotropium groups were in preferred provider organizations). In both treatment groups, similar proportions of patients were initiated on either medication during each year of the study period – 2009–2012. The index treatment was prescribed by internists (26.0% and 27.1%), family/general practitioners (26.9% and 26.9%), and pulmonologists (23.1% and 26.6%) for BFC and tiotropium, respectively.
Preindex chronic comorbidities
During the 12 months prior to the index date, patients in the BFC group had more comorbid respiratory conditions than the tiotropium group, including asthma (24.7% versus 12.7%, OR 2.26, CI 2.09–2.44), allergic rhinitis (8.0% versus 4.1%, OR 2.04, CI 1.79–2.32), and sinusitis (10.4% versus 6.6%, OR 1.65, CI 1.48–1.84), (Table 2). BFC-treated patients had fewer comorbid cardiovascular conditions, including myocardial infarction (4.3% versus 6.0%, OR 0.71, CI 0.62–0.82) and peripheral vascular disease (7.5% versus 9.8%, OR 0.75, CI 0.67–0.83).
Preindex respiratory medication fills
A greater proportion of BFC-treated patients had prior respiratory medication fills, including at least one fill for OCS (45.8% versus 34.6%, OR 1.60, CI 1.50–1.70), ICS (11.1% versus 8.8%, OR 1.29, CI 1.17–1.43), SABA (43.9% versus 34.8%, OR 1.47, CI 1.38–1.56), leukotriene-receptor antagonists (11.6% versus 6.0%, OR 2.05, CI 1.84–2.29), and SABA–SAMA combinations (16.5% versus 12.6%, OR 1.37, CI 1.26–1.49) (Table 3). The higher respiratory medication use among BFC patients was likely because BFC, unlike tiotropium, is indicated for asthma and more likely to be prescribed to patients with asthma.
Preindex COPD-related health care-resource utilization
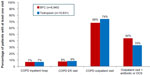
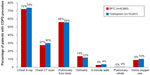
A significantly smaller proportion of BFC-treated patients had COPD-related outpatient visits (68.4% BFC versus 74.4% tiotropium, OR 0.75, CI 0.70–0.80), and the mean number of visits was smaller for BFC patients (4.1 versus 4.6, mean difference 0.52, CI 0.33–0.70). A greater proportion of BFC-treated patients had outpatient visits that were associated with an OCS or antibiotic prescription fill (44.0% BFC versus 33.0% tiotropium, OR 1.59, CI 1.49–1.69). As shown in Figure 1, the proportions of COPD-related inpatient hospitalizations were generally equal between the two groups, while ER visits were slightly more common in tiotropium patients (7.7% BFC versus 8.6% tiotropium, OR 0.89, CI 0.80–0.99). As indicated in Figure 2, there were only small differences between the two groups in the utilization of COPD-related procedures: chest X-rays (71.9% BFC versus 73.7% tiotropium, OR 0.92, CI 0.86–0.98), chest computed tomography scans (27.2% BFC versus 29.5% tiotropium, OR 0.89, CI 0.84–0.96), oximetry (13.4% BFC versus 11.9% tiotropium, OR 1.15, CI 1.05–1.25), and home oxygen use (8.9% BFC versus 10.0% tiotropium, OR 0.87, CI 0.78–0.97), with no difference in pulmonary function tests (55.9% BFC versus 55.4% tiotropium, OR 1.02, CI 0.96–1.08).
Preindex COPD exacerbation rates by age-group
In the year prior to initiation of COPD maintenance medication, patients new to tiotropium were less likely to have a COPD exacerbation compared with those new to BFC (50.2% BFC versus 40.4% tiotropium). The difference in COPD exacerbation rates was driven by differences in OCS use, while COPD-related inpatient hospitalization and ER visits were comparable between the groups. Similar results were observed when exacerbation rates were calculated for those <65 and ≥65 years of age (Table 4). In both treatment groups, patients <65 years old had higher preindex rates of COPD exacerbations compared to those ≥65 years old. Age-adjusted exacerbation-rate differences were more pronounced within BFC patients (53.6% of patients aged <65 years with at least one prior exacerbation compared with 46.3% of patients ≥65) than tiotropium patients (42.8% versus 38.4%).
Preindex COPD and all-cause- related health care costs
In the year prior to initiation of COPD maintenance medication, patients new to BFC had lower total all-cause health care costs compared with patients new to tiotropium ($17,259 versus $17,926, mean difference −$667, CI −$1,254 to −$58). This cost difference was largely due to lower all cause inpatient costs for the BFC group ($7,459 versus $8,599, mean difference −$1,140, CI −$1,712 to −$521) (Figure 3). BFC-treated patients had lower total COPD-related health care costs ($1,718 versus $1,930, mean difference −$213, CI −$302 to −$118), primarily due to lower COPD-related inpatient costs ($627 versus $767, mean difference −$140, CI −$190 to −$85) (Figure 4).
  | Figure 3 All-cause health care costs during 12-month preindex period. |
Discussion
Therapeutic guidelines recommend the treatment and prevention of exacerbations as the main goal of COPD management.3,6 With the availability of different therapeutic options, the decision to initiate COPD maintenance medication is often determined by presenting symptoms, clinical history, and the risk assessment of future COPD exacerbations.7–9 This study is the first to assess differences in characteristics of COPD patients before they initiated BFC or tiotropium using claims data from a large geographically diverse population.
As a result, there is a dearth of comparable study data in the literature with which to evaluate our results. While studies have compared patients who initiated COPD-controller medications, they have not studied BFC relative to tiotropium, nor have they focused on the baseline period, before patients are indexed on maintenance therapy.10,11 While there are studies that have incorporated one of the agents we assessed,12 it would appear that no published study to date has compared preindex demographics, clinical characteristics, chronic comorbidities, respiratory medication fill, COPD-related health care utilization, exacerbation rates, and COPD and all-cause health care costs during the preindex period for patients initiating BFC and tiotropium as maintenance therapy.
Current study results indicate that COPD patients who initiate maintenance therapy with BFC may be different than those who initiate tiotropium. Patients who initiated BFC in this study had a higher prevalence of chronic respiratory conditions and higher utilization of respiratory medication at baseline. BFC initiators had a higher percentage of COPD exacerbations in the 12 months preindex for both the <65- and ≥65-year age-groups compared with patients indexing on tiotropium. The majority of COPD exacerbations were captured by OCS prescription fills, which contributed most to the differences observed between the groups.
BFC is also indicated for asthma, which likely contributes to the higher proportion of patients in the BFC group with a prior asthma diagnosis compared to tiotropium. Greater respiratory disease comorbidities in the BFC group also likely accounts for the increased utilization of respiratory medications preindex, including but not limited to OCS. Such comorbid conditions as allergic rhinitis, sinusitis, and gastroesophageal reflux disease were also more prevalent in the BFC-treated population. Conversely, the observed higher overall health care costs among tiotropium initiators may be due to their older age and the higher prevalence of comorbid cardiovascular disease.
Among the proxy measures for exacerbations in this study were COPD-related inpatient hospitalizations and ER visits and OCS fills. While antibiotic usage may also be used as a proxy for COPD exacerbations, it is not specific to COPD. In this analysis, COPD-related visits with OCS or antibiotic prescription fills occurring within 10 days of the visit were also captured. This broadened definition of an exacerbation did not result in the inclusion of additional patients who were not already captured by the initial definition. Prior exacerbations are predictive of future exacerbations; as a consequence,13,14 it would be informative to control for prior exacerbations in analyses of postindex exacerbation rates in future comparative effectiveness studies.15
Limitations
This study adds new insights on the clinical characteristics of COPD patients prior to initiation of COPD maintenance medication. The interpretation of findings should be within the context of limitations inherent to the use of administrative claims databases.16–22 Primarily, COPD-related health care-resource utilization is based on claims diagnosis; however, the actual visit may be due to routine follow-up and monitoring.
Claims data do not include clinical indicators of COPD disease severity (ie, symptoms or lung-function data), and thus only measures of disease activity were analyzed (ie, COPD-related health care utilization and costs). Smoking status, a primary cause of COPD, is also unknown in this data source. Because administrative claims data may be subject to incorrect or rule-out diagnoses, the diagnostic criteria for COPD required more than one claim containing a diagnosis of COPD for most cases, the exceptions being an inpatient hospitalization with a primary COPD diagnosis or ER visit with a COPD diagnosis.
For the pharmacy claims, a prescription-fill date was recorded as the beginning date of treatment, which might not have been the actual date a patient started therapy. Patients aged 65 years and older were underrepresented in the database used for this study. Overall, these findings may have limited applicability beyond similar managed care patient populations.
Conclusion
Patients initiating BFC or tiotropium therapy had important differences prior to the start of treatment. COPD patients initiating BFC had a higher proportion of COPD exacerbations before starting controller therapy compared with tiotropium initiators, which may be indicative of more severe disease or attributable to a higher prevalence of comorbid respiratory disorders in this group. The higher overall health care costs among tiotropium-treated patients could be a reflection of a greater degree of cardiovascular disease within this group. A much higher prevalence of asthma in the BFC population was observed, which may have influenced such characteristics as other comorbid respiratory conditions and concomitant respiratory medication use. Such differences among patient groups must be considered in outcome studies evaluating the comparative effectiveness of different therapies, as they may influence patients’ probability of initiating and benefiting from a particular treatment.
Acknowledgments
This study was funded by AstraZeneca. Bernard B Tulsi, MSc provided writing and other editorial support for this manuscript.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work. This submission complies with all ICMJE conditions.
Disclosure
This manuscript represents original research by the eight listed authors. Three of the authors (DMK, OT, and SZ) and the medical writer (BBT) are employees of HealthCore Inc., a wholly owned research and consulting subsidiary of WellPoint, a national health insurance company. Five of the authors (SAW, NP, HE, CW, and FT) are employees of AstraZeneca, which provided funding for this study. The authors report no other conflicts of interest in this work.
References
American Lung Association. Chronic obstructive pulmonary disease (COPD) fact sheet. 2014. Available from: http://www.lung.org/lung-disease/copd/resources/facts-figures/COPD-Fact-Sheet.html#note_12. Accessed May 4, 2014. | |
Xu JQ, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: Final Data for 2007. Natl Vital Stat Rep. 2010;58:1–136. | |
Global initiative for chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Bethesda (MD): GOLD; 2011. | |
AstraZeneca. Symbicort inhalation aerosol [package insert]. London: AstraZeneca; 2009. | |
Boehringer Ingelheim. Spiriva HandiHaler [package insert]. Ingelheim, Germany: Boehringer Ingelheim; 2012. | |
Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance – United States, 1971–2000. Respir Care. 2002;47:1184–1199. | |
Clark BT, Garcia-Tsao G, Fraenkel L. Patterns and predictors of treatment initiation and completion in patients with chronic hepatitis C virus infection. Patient Prefer Adherence. 2012;6:285–295. | |
Halava H, Helin-Salmivaara A, Junnila J, Huupponen R. Selective prescribing of simvastatin and atorvastatin by patient characteristics at treatment initiation over a 7-year period in Finland. Eur J Clin Pharmacol. 2009;65:927–933. | |
Mitra D, Trask PC, Iyer S, Candrilli SD, Kaye JA. Patient characteristics and treatment patterns in chronic myeloid leukemia: evidence from a multi-country retrospective medical record chart review study. Int J Hematol. 2012;95:263–273. | |
Abudagga A, Sun SX, Tan H, Solem CT. Exacerbations among chronic bronchitis patients treated with maintenance medications from a US managed care population: an administrative claims data analysis. Int J Chron Obstruct Pulmon Dis. 2013;8:175–185. | |
Abudagga A, Sun SX, Tan H, Solem CT. Healthcare utilization and costs among chronic bronchitis patients treated with maintenance medications from a US managed care population. J Med Econ. 2013;16:421–429. | |
Dalal AA, Roberts MH, Petersen HV, Blanchette CM, Mapel DW. Comparative cost-effectiveness of a fluticasone-propionate/salmeterol combination versus anticholinergics as initial maintenance therapy for chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:13–22. | |
Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. | |
Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309:2353–2361. | |
Blais L, Forget A, Ramachandran S. Relative effectiveness of budesonide/formoterol and fluticasone propionate/salmeterol in a 1-year, population-based, matched cohort study of patients with chronic obstructive pulmonary disease (COPD): effect on COPD-related exacerbations, emergency department visits and hospitalizations, medication utilization, and treatment adherence. Clin Ther. 2010;32:1320–1328. | |
Baron JA, Barrett J. Utility and limitations of claims data. Ann Intern Med. 1994;120:1049. | |
Green J. Utility and limitations of claims data. Ann Intern Med. 1994;120:1050–1051. | |
Huff ED. Utility and limitations of claims data. Ann Intern Med. 1994;120:1049. | |
Prochazka AV. Utility and limitations of claims data. Ann Intern Med. 1994;120:1050. | |
Strom BL. Data validity issues in using claims data. Pharmacoepidemiol Drug Saf. 2001;10:389–392. | |
Ramsey SD, Scoggins JF, Blough DK, McDermott CL, Reyes CM. Sensitivity of administrative claims to identify incident cases of lung cancer: a comparison of 3 health plans. J Manag Care Pharm. 2009;15:659–668. | |
Riley GF. Administrative and claims records as sources of health care cost data. Med Care. 2009;47:S51–S55. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.