Back to Journals » Research and Reports in Urology » Volume 12
A Systematic Review on Rho-Kinase as a Potential Therapeutic Target for the Treatment of Erectile Dysfunction
Authors Zewdie KA , Ayza MA , Tesfaye BA , Wondafrash DZ , Berhe DF
Received 27 March 2020
Accepted for publication 22 June 2020
Published 17 July 2020 Volume 2020:12 Pages 261—272
DOI https://doi.org/10.2147/RRU.S255743
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jan Colli
Kaleab Alemayehu Zewdie, Muluken Altaye Ayza, Bekalu Amare Tesfaye, Dawit Zewdu Wondafrash, Derbew Fikadu Berhe
Department of Pharmacology and Toxicology, School of Pharmacy, Mekelle University, Mekelle, Ethiopia
Correspondence: Kaleab Alemayehu Zewdie
Department of Pharmacology and Toxicology, School of Pharmacy, Mekelle University, P.O. Box: 1871, Mekelle, Ethiopia
Tel +251921546562
Email [email protected]
Background: Erectile dysfunction (ED) is a common clinical condition with limited treatment options. The main aim of the present systematic review was to synthesize information on Rho-kinase as a novel therapeutic approach for the treatment of ED.
Methods: We performed a systematic literature study in PubMed, Google Scholar and Scopus. Included studies were original articles studied the role of Rho-kinase in the pathogenesis and/or new treatment approach for ED in animal models and clinical studies, published between 2014 and 2019. Data derived from each study were study design used, interventions applied and main treatment outcomes. The quality of the selected articles was assessed by CAMARADES criteria and data were analyzed using descriptive statistics.
Results: A total of 1067 original articles were retrieved in the given period and eighteen papers met our inclusion criteria. Five articles explain the role of Rho-kinase in ED pathogenesis using different models such as cavernous nerve crush injury, heart failure-induced ED, vasculogenic and post-radical prostatectomy ED, diabetes-induced ED and age-related ED. Other ten papers explain the role of novel drugs evaluated for ED treatment by targeting Rho-kinase as a new approach for ED therapy. The rest three papers discuss the role of plant extracts used by traditional society for the treatment of ED and assess their potential function in targeting Rho-kinase in animal models. The penile erectile functional index has shown that the ratio of intracavernosal pressure to mean arterial pressure (ICP/MAP) was decreased due to age and various chronic diseases. Whilst, ROCK I and ROCK II expression were increased. Western blot findings have also shown that ROCK II and MYPT-1 phosphorylation rates increased in cavernous tissue after ED induction. Besides, compounds which can inhibit the action of Rho-kinase activity showed relaxation of the corpus cavernosum, decrease in corporal fibrosis, and alleviate increased apoptosis and caspase-3 activity in an NO-independent manner. Moreover, histological and molecular dysregulation have been improved by inhibition of Rho-kinase.
Conclusion: Targeting Rho-kinase may be a possible target for the treatment of ED secondary to specific causes, and Rho-kinase inhibitors may be a new drug family for the treatment of ED. However, this requires further studies for in-depth understanding.
Keywords: ROCK, Rho-kinase inhibitors, novel, therapeutic target, erectile dysfunction
Introduction
Rho Kinase (ROCK)
Rho-associated coil-forming protein kinase (ROCK) is derived from the ACG (cyclic adenosine monophosphate (cAMP)-dependent protein kinase A/protein kinase C/protein kinase G) family of serine/threonine kinases and is activated by the guanosine triphosphate (GTP)-bound form of RhoA (the small monomeric G-protein).1,2 ROCK activity is regulated by its upstream regulators, the Rho-GTPases RhoA and RhoC, which belong to the Ras-superfamily.3 There are two isoforms, ROCK I and II, and are involved in various physiological and pathophysiological signaling pathways.4,5
ROCKs have been studied extensively and known as the major downstream effectors of RhoA.6 It was involved in the generation of actin-myosin contractility and regulation of actin cytoskeleton dynamics.7 It has also crucial roles in cell proliferation, apoptosis, gene expression and multiple other common cellular functions, such as cell shape, motility, gene expression, secretion and proliferation.6,8,9 Moreover, it causes a contractile response in vascular smooth muscle cells by increasing Ca2+ sensitization.10
Rho-binding can modulate the autophosphorylation of the kinase activity of ROCK.11 However, RhoE (the small G protein) negatively control ROCK I activity by binding in N terminus and prevents RhoA binding to Rho-binding domain (RBD).7 Phosphoinositide-dependent kinase-1 can counteract the negative regulation produced by RhoE by preventing its binding to the N terminal. Other small G proteins (Rad and Gem) can bind and inhibit ROCK I and II activity by unknown mechanisms.7,12,13
Various experiments performed on humans, isolated tissues and genetically modified mouse models have shown that ROCKs have been involved in the pathophysiological pathways of different diseases.7,14,16 The two ROCK isoforms have been described as a promising and alternative target of inhibitory molecules for the wide variety of human diseases including erectile dysfunction.17,22
Erectile Dysfunction
Erectile dysfunction (ED) is the most common chronic illness affecting men and often associated with chronic diseases including hypogonadism, cardiovascular diseases and diabetes mellitus (DM).23,24 Age was also considered one of the main risk factors for ED.25
Different mechanisms have been correlated with ED pathology, which involves physical (organic), emotional and psychological components.26,27 The most common reported mechanism were physical causes including neuropathies, neurological disorders, endocrinological disorders (hypogonadism or hyperprolactinaemia), vasculogenic ED (hypertension, atherosclerosis, and pelvic irradiation) and medication side effects due to anti-depressants, non-steroidal anti-inflammatory drugs, neuroleptics and antiepileptic medications.23,28,29 Stressful life events such as daily worries about work, money or other significant occurrences can be associated with emotional and psychological causes of sexual dysfunction.29 Ageing often associated with changes of the endocrine system (especially in elderly’s). At an older age, the testicular Leydig cells do not produce sufficient testosterone and that may lead to ED.25 Moreover, there is also a decreased in blood supply to the penis.30
Different biochemical changes were produced in corporal smooth muscles by local and neuronal neurotransmitters through potassium channels, gap junctions and calcium channels.23,28,30 For example, the neurotransmitter nitric oxide (NO) has a principal role in mediating penile erection. Once synthesized, it diffuses into smooth muscle cells and, binds and stimulates guanyl cyclase, which increases cyclic guanosine monophosphate (cGMP) and activates protein kinase G (PKG). Then after, phosphorylates several intracellular proteins and ion transporters, resulting in hyperpolarization and decreased cytoplasmic Ca,2+ causing smooth muscle relaxation.31 In addition to the reduction of cytosolic Ca2+ resulting from the NO cascade, dephosphorylation of myosin light chain (MLC) by myosin light chain phosphatase (MLCP) facilitates the release of myosin from actin and smooth-muscle relaxation in NO-independent manner.11,31 Any alterations in the ionic channels, gap junctions, cGMP, cAMP and NO pathways affect the corporal smooth muscle contraction and relaxation.23,28,30
The prevalence of ED is estimated to be 50–100% in people over 70 years of age25 and it has been increased in men below 40 years old.28 Besides, there are limited treatment options for ED and even with the available medications, there are concerns regarding their effectiveness and adverse effects. The quest for alternative ED drugs is necessary in order to address these challenges. Therefore, the purpose of the present review was to synthesis data regarding the role of Rho-kinase in the pathogenesis of ED and the efficacy of Rho-kinase inhibitors in the treatment of ED based on original articles published in peer-reviewed journals.
Methods
Search Strategies
A systematic search of the literature was done in PubMed, Google Scholar and Scopus. A manual search was also done. The database search was carried out using different keywords such as Rho-kinase, ROCK, Rho-kinase inhibitors, erectile dysfunction and sexual dysfunction, then search terms were combined using either “AND” or “OR” between two or more terms (Table 1).
 |
Table 1 Databases Employed and Respective Keywords Used |
Inclusion and Exclusion Criteria
Included studies were original articles evaluating the role of Rho-kinase in ED pathogenesis and/or investigating novel ED therapeutic agents by targeting Rho-kinase. Articles were deemed to be eligible for inclusion if the original article was written in the English language and published between 2014 and 2019. Studies that assessed the possible anti-ED effects of Rho-kinase inhibitors using original articles with any kind of study design on animal ED model, ex-vivo model or clinical studies were included. Reviews, opinion pieces, letter to editors, conference abstracts and/or partially accessed (abstract only) articles and commentaries were excluded.
Screening and Eligibility of Studies
Potential articles have been collected from various databases and duplicates have been removed. Two investigators (KAZ and MAA) evaluated title and abstract separately on the basis of predefined inclusion criteria. This process was accompanied by the assessment and retrieval of the full texts of the relevant citations. A third investigator (BAT) was concerned for potential disputes.
Data Extraction and Quality Assessment
The extraction of data from each study was carried out using a predefined form. The two authors (KAZ and DZW) independently performed data extraction. Variables extracted from the included papers were publication year, study design, study population and/or animal model used, sample size, follow-up duration, outcomes of treatment, and risk estimates with their adjusted covariant. The quality of each paper was assessed by DZW and KAZ using the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) quality assessment tool before data extraction was done.32 The quality of each paper was expressed in mean ± standard error of mean.
Results
Out of 1067 articles, 18 articles fulfil our inclusion criteria (Figure 1). Among these, 16 were animal-based and the rest were clinical and ex-vivo studies conducted on humans and isolated tissues. In this study, we extracted data on the role of Rho-kinase in the pathogenesis and/or novel therapeutic target for the treatment of ED.
 |
Figure 1 Schematic representation of the data extraction process. |
Among the included articles, five articles describe the role of Rho-kinase in ED pathogenesis using various models including cavernous nerve (CN) crush injury, heart failure (HF)-induced ED, Vasculogenic and post-radical prostatectomy (RP) ED, diabetes-induced ED and age-related ED.17,33,36 Ten papers describe the role of novel drugs which have been tested for the treatment of ED by targeting Rho-kinase as a potential therapy for ED.19,20,37,45 The remaining three papers describe the role of traditionally claimed plants for the treatment of ED and evaluated for their potential role in targeting Rho-kinase in animal models.21,46,47 The quality score of the included papers were ranged from 5 to 9 with an average mean articles quality score of 7.39 ± 0.31 (Table 2).
 |
Table 2 The Quality Assessment of Individual Study Obtained According to Modified CAMARADES Checklist Items |
RhoA, ROCK I, and ROCK II expression were increased in different scenarios. ROCK II (Rho-kinase) is majorly expressed in the pathogenesis of ED. According to Alves-Lopes et al (2016), high glucose-induced ED secondary to DM increases levels of nitrotyrosine, protein oxidation/carbonylation, and Rho-kinase activity.34 Ageing has also been reported to increase the risk of developing ED as ICP/MAP ratio decreases with age. A related study showed a marked decline in eNOS protein expression with age, while the Rho-kinase protein level was increased and, in particular, the eNOS/Rho-kinase ratio decreased with age.35 Involvement of Rho-kinase in ED pathogenesis was also evaluated by animal model with HF.17 The results showed that the rats developed ED after ligation of the left anterior descending coronary artery, as demonstrated by decreased ICP/MAP responses. In addition, ROCK II and MYPT-1 phosphorylation rates increased in cavernous tissue (Table 3).17
 |
Table 3 Role of Rho Kinases (ROCK) in the Pathogenesis of Erectile Dysfunction |
Song et al (2015) also suggested that ROCK I has been involved in ED pathogenesis. In this study,33 effects in LIMK2/cofilin pathway (downstream effectors of ROCK I) following bilateral CN injury in male rats were evaluated. It has shown that after one week of CN injury, an upregulation of ROCK I/LIMK II/cofilin pathway was accompanied by impaired erectile response and corporal fibrosis.33
Ten novel drugs have been tested for their Rho-kinase inhibitory activity (Table 4). An ex-vivo study conducted by Uvin et al (2017) showed that the additive effects of the Rho-kinase inhibitor Y-27,632 and vardenafil on the relaxation of the corpus cavernosum (CC) tissue of patients with ED and clinical phosphodiesterase type 5 (PDE-5) inhibitor failure were assessed. According to their study, Y-27,632 induces major relaxation of CC in tissue strips of patients with severe ED. Besides, combination therapy has shown that mutual inhibition of Rho-kinase and PDE-5 can be a successful agent in the treatment of severe ED.37
 |
Table 4 Role of Rho Kinases (ROCK) Inhibitors as a Novel Therapeutic Agent for the Treatment of Erectile Dysfunction |
Cui et al (2017a) assessed the function of human tissue kallikrein1 (hKLK1) as an inhibitor of Rho-kinase/LIM-kinase/cofilin pathway in aged transgenic rats. According to this study,41 after ED had tested and validated in older rats, hKLK1 administration has shown to reduce corporal fibrosis by inhibiting Rho-kinase activation and ameliorate age-related ED.41 Another study was also done to assess the role of fasudil (Rho-kinase inhibitor) in the treatment of ED in the CN crush injured rat model.42 Results from this study showed increased phosphorylation of myosin phosphatase (MYPT-1) and increased ROCK II expression in the injured groups. Inhibition of Rho-kinase in the injury plus fasudil group restored erectile responses and dynamic infusion cavernosometry parameters by alleviating increased apoptosis, decreased immune histochemical staining of alpha-smooth muscle actin (α-SMA) and increased caspase-3 activity. Histological and molecular dysregulations have been also alleviated by inhibition of Rho-kinase plus fasudil groups.42
β3-adrenoceptor activator (Mirabegron) was also assessed for the treatment of ED by using clinical and animal models.45 The CC specimens were collected from patients with Peyronie’s disease and ED undergoing penile prosthesis. Besides, erectile responses were evaluated in-vivo after intracavernosal injection (ICI) of mirabegron in anaesthetized rats. Studies have shown that mirabegron induces relaxation of phenylephrine-evoked CC contractions in a concentration-dependent manner by activating β3-adrenoceptors independently of the NO-cGMP pathway and it is hypothesized that there is a near functional link between β3-adrenoceptors and the RhoA/ROCK pathway.45
Various plant extracts have been evaluated for the treatment of ED and their function in inhibiting Rho-kinase in animal models (Table 5). Eupatilin (the main compound present in Artemisia species) was assessed for its relaxant effect in-vitro and protein expression. The finding indicates Eupatilin effectively relaxed the phenylephrine-induced tone in the rabbit CC strips in a concentration-dependent manner. The relaxing effect of Eupatilin on CC smooth muscle cells may be due to activation of BKCa+2 channels and inhibition of RhoA/Rho-kinase.46 Ye et al (2019) have also reported that the effect of HongJing I (HJI), a traditional Chinese herbal remedy, in ED. Once ED was induced by bilateral cavernous nerve injury (BCNI) in rats, ICP was recorded, and histological examination was done. Results showed that RhoA, ROCK I, and ROCK II expression levels were increased with BCNI-ED induction, while HJI successfully inhibited cavernosum fibrosis and RhoA/ROCK II signal activation.47
 |
Table 5 Traditional Plants Used as Rho Kinases (ROCK) Inhibitors as a Therapeutic Agent for the Treatment of Erectile Dysfunction |
Discussion
ED has proved to be a significant health issue for elderly’s and persons with chronic diseases. It is prevalent in people older than 40 years of age and affects their quality of life.23,26 Penile erection is a complex process in which flaccidity/rigidity within it is regulated by a balance between contractile and relaxing effects.48 Erection requires neutrally mediated relaxation of arteriolar smooth muscle and engorgement of cavernosal tissues. Current medical therapies for ED mainly perform by maximizing endogenous NO signaling. However, certain etiologies, including diabetes, are difficult to treat with current modalities.49 In addition, they are also related to increased side effects and cost.24 Therefore, the search for a new target and/or potentially effective new drugs with decreased side effects are important. Recently, research has demonstrated the importance of ROCK signaling in maintaining a flaccid penile state, and inhibition of ROCK signaling potentiates smooth-muscle relaxation in a NO‑independent manner.
Rho-Kinase Signaling on the Pathophysiology of Erectile Dysfunction
The Rho/Rho-kinase pathway plays an important role in pathophysiology and progression of various diseases.10 Altered RhoA/ROCK activity in the penis is a pathogenic factor contributing to ED development.50 As shown in Figure 2, RhoA and its effector Rho-kinase have been noticed for facilitating vasoconstriction activity in the penis through inhibition of MLCP, thereby increases MLC phosphorylation and increase Ca2+ sensitivity.18,31,33,48 Ca2+ influx into cells increases intracellular Ca2+ that is available to bind to calmodulin. This binding causes a conformational change and enable complex with MLC kinase. After subsequent phosphorylation, the myosin–actin complex resulting smooth muscle contraction and a flaccid penis.31,48 In addition, upregulation of RhoA/ROCK suppresses endothelial nitric oxide synthase (eNOS) activity and it is the expression in the penis (Figure 2). Proteomics analysis on Western blot has shown that the ratio of eNOS/Rho-kinase decreased significantly due to ageing, DM and various chronic diseases.33,35,51
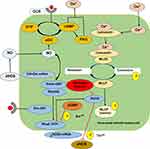 |
Figure 2 RhoA/Rho-kinase signaling pathway for Ca2+ sensitization in cavernosal smooth-muscle cells. |
Effect of Rho-Kinase Inhibition on Erectile Dysfunction
The potential effect of RhoA/Rho-kinase mediated Ca2+ sensitization pathway in ED pathogenesis provided a logical pharmacological target for clinical application.15 Studies have demonstrated that inhibition of tonic contraction of corporal smooth muscle by intracavernosal injection or topical application of Rho-kinase inhibitors to the penis results in increased blood flow into erectile tissue and causing an erection.31 Rho-kinase inhibition restored erectile responses and dynamic infusion cavernosometry parameters by alleviating increased apoptosis, decreased immune histochemical staining of α-SMA and increased caspase-3 activity in a NO-independent manner. Moreover, histological and molecular dysregulation were alleviated by Rho-kinase inhibition,14,19,41,42 which might improve its efficacy as compared with the currently available anti-ED drugs. However, some findings showed that the vasorelaxant effect induced by the administered drugs was not mainly by the involvement of Rho-kinase inhibition, rather, by improving NO-mediated CC relaxations probably by inhibiting NADPH oxidase.43 Likewise, similar changes were noticed in rats with CN injury by involving TGF-β- mediated fibrosis.19
Strengths and Limitations
To the best of our knowledge, this is the first systematic review that gives insight for an alternative treatment option, targeting Rho-kinase as a potential therapeutic option in the treatment of ED. However, our review did not conduct statistical analysis due to the heterogeneous nature of the included studies. Moreover, most of the reviewed papers are animal studies, which makes it difficult to conclude the models have been addressed all the pathogenic mechanisms for ED. So, further in-depth studies will be required to investigate their role in ED pathogenesis and to elucidate mechanisms for Rho-kinase inhibitors.
Conclusion
Overall, our study indicates Rho-kinase can be a potential target for the treatment of ED secondary to different causes. Recently, animal and clinical studies proved that there is an up-regulation of Rho-kinase (especially ROCK II) in ED. Rho-kinase inhibitors improve erectile function in a NO‑independent manner and might be a new drug family for the treatment of ED. However, more information and further detailed understanding are needed to show the ultimate effects on health outcome.
Abbreviations
α-SMA, alpha-smooth muscle actin; CC, corpus cavernosum; CN, cavernous nerve; DM, diabetes mellitus; ED, erectile dysfunction; eNOS, endothelial nitric oxide synthase; hKLK1, human tissue kallikrein1; HF, heart failure; ICP, intracavernosal pressure; NO, nitric oxide; MAP, mean arterial pressure; MLC, myosin light chain; MLCK, myosin light chain kinase; MLCP, myosin light chain phosphatase; MYPT, myosin phosphatase regulatory; ROCK, Rho-associated coiled-coil-forming protein kinase; STZ, streptozotocin.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. LoGrasso PV, Feng Y Rho kinase (ROCK) inhibitors and their application to inflammatory disorders. Cur Top Med Chem. 2009;9:704–723. doi:10.2174/156802609789044452
2. Nakagawa O, Fujisawa K, Ishizaki T et al. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189–193. doi:10.1016/0014-5793(96)00811-3
3. Roser AE, Tönges L, Lingor P Modulation of microglial activity by rho-kinase (rock) inhibition as therapeutic strategy in parkinson’s disease and amyotrophic lateral sclerosis. Front Aging Neurosci. 2017;9:1–8. doi:10.3389/fnagi.2017.00094
4. Chen -H-H, Namil A, Severns B et al. In vivo optimization of 2,3-diaminopyrazine Rho Kinase inhibitors for the treatment of glaucoma Bioorg Med Chem Lett. 2014;24:1875–1879. doi:10.1016/j.bmcl.2014.03.017
5. Fukata Y, Kaibuchi K, Amano M, Kaibuchi K Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci. 2001;22:32–39. doi:10.1016/S0165-6147(00)01596-0
6. Schofield AV, Steels R, Bernard O Rho-associated coiled-coil kinase (ROCK) protein controls microtubule dynamics in a novel signaling pathway that regulates cell migration. J Biol Chem. 2016;145:43620–43629. 10.1074/jbc.M112.394965
7. Loirand G, Touyz RM, Touyz RM Rho kinases in health and disease. Pharmacol Rev. 2015;67:1074–1095. doi:10.1124/pr.115.010595
8. Pan P, Shen M, Yu H et al. Advances in the development of Rho-associated protein kinase (ROCK) inhibitors. Drug Discov Today. 2013;18:1323–1333. doi:10.1016/j.drudis.2013.09.010
9. Dong M, Yan BP, Liao JK et al. Rho-kinase inhibition: a novel therapeutic target for the treatment of cardiovascular diseases. Drug Discov Today. 2010;15:622–629. doi:10.1016/j.drudis.2010.06.011
10. Hirooka Y, Shimokawa H Therapeutic potential of Rho-kinase inhibitors in cardiovascular diseases. Am J Cardiovasc Drugs. 2005;5:31–39.
11. Lu G, Hein TW, Kuo L Rho kinase-mediated coronary arteriolar constriction to endothelin-1: mechanistic implications for cardiac syndrome X. Transl Biomed. 2010;1:13–15.
12. Amin E, Dubey BN, Zhang S-C et al. Rho-kinase: regulation, (dys)function, and inhibition. Biol Chem. 2013;394:1399–1410. doi:10.1515/hsz-2013-0181
13. Amano M, Nakayama M, Kaibuchi K Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton. 2010;67:545–554. doi:10.1002/cm.20472
14. Yin H, Ru H, Yu L et al. Targeting of Rho kinase ameliorates impairment of diabetic endothelial function in intrarenal artery.Int J Mol Sci. 2013;14:20282–20298. doi:10.3390/ijms141020282
15. Jin L, Burnett AL RhoA/Rho-kinase in erectile tissue: mechanisms of disease and therapeutic insights. Clin Sci. 2006;110:153–165. doi:10.1042/CS20050255
16. Doe C, Bentley R, Behm DJ et al. Novel Rho kinase inhibitors with anti-inflammatory and vasodilatory activities J Pharmacol Exp Ther. 2007;320:89–98. doi:10.1124/jpet.106.110635
17. Rodrigues FL, Lopes RAM, Fais RS et al. Erectile dysfunction in heart failure rats is associated with increased neurogenic contractions in cavernous tissue and internal pudendal artery. Life Sci. 2016;145:9–18. doi:10.1016/j.lfs.2015.12.005
18. Rodrigues FL, Fais RS, Pereira MGAG et al. Erectile dysfunction in wistar audiogenic rats is associated with increased cavernosal contraction and decreased neuronal nitric oxide synthase protein expression. Urology. 2017;106:
19. Liu K, Cui K, Feng H, et al. JTE-013 supplementation improves erectile dysfunction in rats with streptozotocin-induced type Ⅰ diabetes through the inhibition of the rho-kinase pathway, fibrosis, and apoptosis. Andrology. 2019;0–2. doi:10.1111/andr.12716
20. Zhang Y, Jia L, Ji W, Li H MicroRNA-141 inhibits the proliferation of penile cavernous smooth muscle cells associated with down-regulation of the Rhoa/Rho kinase signaling pathway. Cell Physiol Biochem. 2018;48:348–360. doi:10.1159/000491741
21. Ezzat SM, Okba MM, Ezzat MI, Aborehab NM, Mohamed SO Rho-kinase II inhibitory potential of Eurycoma longifolia new isolate for the management of erectile dysfunction. Evid Based Complement Alternat Med. 2019;2019:1–8. doi:10.1155/2019/4341592
22. Silva DF, Wenceslau CF, Mccarthy CG et al. TRPM8 channel activation triggers relaxation of pudendal artery with increased sensitivity in the hypertensive rats Pharmacol Res. 2019;147:104329. doi:10.1016/j.phrs.2019.104329
23. Burnett AL, Rodney AN, Daniel HB, et al. Erectile dysfunction – AUA guideline – unabridged. Am Urol Assoc Guidel. 2018;633–641.
24. Steers WD Pharmacologic treatment of erectile dysfunction. Rev Urol. 2002;4(Suppl 3):S17–25.
25. Gareri P, Castagna A, Francomano D, Cerminara G, De Fazio P Erectile dysfunction in the elderly: an old widespread issue with novel treatment perspectives. Int J Endocrinol 2014;2014:1–15. doi:10.1155/2014/878670
26. Shamloul R, Ghanem H Erectile dysfunction. Lancet. 2013;381:153–165. doi:10.1016/S0140-6736(12)60520-0
27. Persu C, Cauni V, Gutue S et al. Diagnosis and treatment of erectile dysfunction – a practical update. J Med Life. 2009;2:394–400.
28. Minh H, Nguyen T, Gabrielson AT, Hellstrom WJG Erectile dysfunction in young men: a review of the prevalence and risk factors. Sex Med Rev. 2017;5:508–520. doi:10.1016/j.sxmr.2017.05.004
29. Giuliano F, Droupy S Erectile dysfunction. Prog Urol. 2013;23:629–637. doi:10.1016/j.purol.2013.01.010
30. Mobley DF, Khera M, Baum N Recent advances in the treatment of erectile dysfunction. Postgr Med J. 2017;93:679–685. doi:10.1136/postgradmedj-2016-134073
31. Sopko NA, Hannan JL, Bivalacqua TJ Understanding and targeting the Rho kinase pathway in erectile dysfunction. Nat Public Gr. 2014;1–7. doi:10.1038/nrurol.2014.278
32. Macleod MR, O’Collins T, Howells DW, Donnan GA Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35:1203–1208. doi:10.1161/01.STR.0000125719.25853.20
33. Song SH, Park K, Kim SW, Paick JS, Cho MC Involvement of Rho-kinase/LIM kinase/cofilin signaling pathway in corporal fibrosis after cavernous nerve injury in male rats. J Sex Med. 2015;12:1522–1532. doi:10.1111/jsm.12903
34. Alves-Lopes R, Neves KB, Montezano AC et al. Internal pudental artery dysfunction in diabetes mellitus is mediated by NOX1-derived ROS-, Nrf2-, and Rho kinase-dependent mechanisms. Hypertension. 2016;68:1056–1064. doi:10.1161/HYPERTENSIONAHA.116.07518
35. Ding Z, Shen X, Li Y Association of nNOS and Rho-kinase with age-associated erectile dysfunction in Sprague-Dawley rats. Exp Ther Med. 2017;13:1133–1136. doi:10.3892/etm.2017.4064
36. Karakus S, Musicki B, Burnett AL Phosphodiesterase type 5 in men with vasculogenic and post-radical prostatectomy erectile dysfunction: is there a molecular difference? BJU Int. 2018;122:1066–1074. doi:10.1111/bju.14433
37. Uvin P, Albersen M, Bollen I et al. Additive effects of the Rho kinase inhibitor Y-27632 and vardenafil on relaxation of the corpus cavernosum tissue of patients with erectile dysfunction and clinical phosphodiesterase type 5 inhibitor failure. BJU Int. 2017;119:325–332. doi:10.1111/bju.13691
38. Cui K, Ruan Y, Wang T et al. FTY720 supplementation partially improves erectile dysfunction in rats with streptozotocin-induced type 1 diabetes through inhibition of endothelial dysfunction and corporal fibrosis J Sex Med. 2017;14:323–335. doi:10.1016/j.jsxm.2017.01.006
39. Wang J, Zhou X, Lu H et al. Fluoxetine induces vascular endothelial growth factor/Netrin over-expression via the mediation of hypoxia-inducible factor 1-alpha in SH-SY5Y cells. J Neurochem. 2016;136(6):1186–1195. doi:10.1111/jnc.13521
40. Bastaskın T, Kaya E, Ozakca I et al. Effects of silodosin, a selective alpha-1A adrenoceptor antagonist, on erectile function in a rat model of partial bladder outlet obstruction. Neurourol Urodyn. 2017;36(3):597–603. doi:10.1002/nau.23015
41. Cui K, Luan Y, Wang T et al. Reduced corporal fibrosis to protect erectile function by inhibiting the Rho-kinase/LIM-kinase/cofilin pathway in the aged transgenic rat harboring human tissue kallikrein 1. Asian J Androl. 2017;19(1):67–72. doi:10.4103/1008-682X.189209
42. Cho MC, Park K, Kim SW, Paick JS Restoration of erectile function by suppression of corporal apoptosis, fibrosis and corporal veno-occlusive dysfunction with Rho-kinase inhibitors in a rat model of cavernous nerve injury. J Urol. 2015;193(5):1716–1723. doi:10.1016/j.juro.2014.10.099
43. Dalaklioglu S, Tasatargil A, Kuscu N et al. Protective effect of exendin-4 treatment on erectile dysfunction induced by chronic methylglyoxal administration in rats. Peptides. 2018;106:1–8. doi:10.1016/j.peptides.2018.05.005
44. Li H, Jia L, Li H, Li H, Li H Angiotensin II silencing alleviates erectile dysfunction through down-regulating the Rhoa/Rho kinase signaling pathway in rats with diabetes mellitus Cell Physiol Biochem. 2018;45(1):419–427. doi:10.1159/000486919
45. Gur S, Peak T, Yafi FA et al. Mirabegron causes relaxation of human and rat corpus cavernosum: could it be a potential therapy for erectile dysfunction? BJU Int. 2016;118(3):464–474. doi:10.1111/bju.13515
46. Choo SH, Lee SW, Chae MR et al. Effects of eupatilin on the contractility of corpus cavernosal smooth muscle through nitric oxide-independent pathways. Andrology. 2017;5(5):1016–1022. doi:10.1111/andr.12397
47. Ye MY, Zhao F, Ma K et al. Effect of HongJing i in treating erectile function and regulating RhoA pathway in a rat model of bilateral cavernous nerve injury. Evid Based Complement Alternat Med. 2019;2019:1–11 doi:10.1155/2019/1083737
48. Linder AE, Webb RC, Mills TM, Ying Z, Lewis RW, Teixeira CE. Rho-kinase and RGS-containing RhoGEFs as molecular targets for the treatment of erectile dysfunction. Curr Pharm Des. 2005;4029–4040.
49. Lasker GF, Maley JH, Kadowitz PJ A review of the pathophysiology and novel treatments for erectile dysfunction. Adv Pharmacol Sci. 2010; 2010.
50. Zhou Q, Mei Y, Shoji T et al. Rho-associated coiled-coil-containing kinase 2 deficiency in bone marrow-derived cells leads to increased cholesterol efflux and decreased atherosclerosis. Circulation. 2012;126(18):2236–2247. doi:10.1161/CIRCULATIONAHA.111.086041
51. Li R, Park S-Y, Nishio T et al. Metabolic syndrome in rats is associated with erectile dysfunction by impairing PI3K/Akt/eNOS activity. Sci Rep. 2017;7(1):1–10. doi:10.1038/s41598-016-0028-x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
