Back to Journals » Neuropsychiatric Disease and Treatment » Volume 12
A study of patients with aggressive multiple sclerosis at disease onset
Authors Kaunzner U, Kumar G, Askin G, Gauthier S, Nealon N, Vartanian T, Perumal J
Received 4 May 2016
Accepted for publication 25 May 2016
Published 1 August 2016 Volume 2016:12 Pages 1907—1912
DOI https://doi.org/10.2147/NDT.S111885
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Ulrike W Kaunzner,1 Gaurav Kumar,2 Gulce Askin,3 Susan A Gauthier,1 Nancy N Nealon,1 Timothy Vartanian,1 Jai S Perumal1
1Judit Jaffe Multiple Sclerosis Center, Weill-Cornell Medical College, New York City, NY, 2Department of Cell Biology and Neuroscience, Rutgers University, New Brunswick, NJ, 3Institute for Biostatistics and Epidemiology, Weill-Cornell Medical College, New York City, NY, USA
Objective: Identify aggressive onset multiple sclerosis (AOMS) and describe its clinical course.
Methods: AOMS patients were identified from a multiple sclerosis (MS) database based on a set of criteria. The subsequent clinical course of AOMS patients was then reviewed with the goal of potentially identifying the best approaches to manage these patients.
Results: Fifty-eight of 783 (7.4%) patients in the MS database met the criteria for AOMS, and 43 patients who had complete data for the duration of their follow-up were included in the subsequent analysis. The mean duration of the follow-up was 54 months. Thirty-five patients (81%) were started on a conventional first-line agent (injectable therapies for MS). Only two of these 35 patients (5.7%) had no evidence of disease activity. Twenty-two of 35 patients suffering from refractory disease were switched to a more aggressive treatment (natalizumab, rituximab, alemtuzumab, cyclophosphamide). Eight patients were started on aggressive treatment as their initial therapy, and seven of these eight (87.5%) patients showed no evidence of disease activity.
Conclusion: With recognition of the crucial significance of early optimal treatment during the potential window of opportunity for best long-term outcomes, we describe AOMS within 1 year of disease onset and discuss possible treatment considerations for these patients.
Keywords: aggressive multiple sclerosis, algorithm, treatment course, database, retrospective analysis
Introduction
Multiple sclerosis (MS) is the most prevalent autoimmune disease of the central nervous system1 and the most common cause of nontraumatic neurological disability in young adults worldwide.2 The clinical course of MS is highly variable: while at one end of the spectrum there are patients with relatively mild disease, even years into the diagnosis, at the other end there are patients with an aggressive course and rapid accumulation of disability. The latter form of MS has been referred to as “malignant”,3,4 “highly active MS”, or “aggressive MS” (among others).2 Although no biomarker has been discovered that dictates prognosis in patients with aggressive MS, several studies have described potential early characteristics that might be helpful in predicting the subsequent course and disability. One study defining malignant MS as a disease leading to disability within 5 years from symptom onset, based on a Kurtzke Expanded Disability Status Scale (EDSS) score of 6, showed that 8.6% of patients had malignant MS.5 Menon et al defined aggressive MS based on the high EDSS score at 5 years, high disability by age 40 years, and rapid transition to secondary progressive MS.6 Other studies analyzed clinical variables, including age, sex, and recovery rate following relapses, to determine which factors contribute to the onset of irreversible disability. They reported that factors such as residual defects from early relapse, early relapse rate, male sex, and older age at disease onset led to an early EDSS score of 4 and were highly associated with a more aggressive disease.7,8
While several studies have tried to define this aggressive or highly active MS, there is a lack of uniform criteria or guidelines for defining this population at disease onset, which would be most significant. The fact that the concept of a “therapeutic window of opportunity”, where early disease control has the greatest impact on long-term outcome,9–11 has been gaining momentum shows that early recognition of aggressive MS is crucial to start optimal treatment as early as possible. This would have the greatest impact by controlling the disease during the inflammatory phase, thereby delaying the onset and decreasing the severity of the subsequent neurodegenerative phase.12 This approach is especially relevant since our current disease modifying therapies are mostly anti-inflammatory.
We examined studies that described factors which could serve as early predictors of subsequent disease course and disability and studies on natural history of MS, and selected a set of criteria that could be easily applied in clinical practice to identify aggressive onset MS (AOMS). Once we selected this population suffering from AOMS within the institutional review board approved Weill-Cornell Medical College MS database, we analyzed their disease course for the duration of their follow-up. We reviewed treatments, relapses, disability, and magnetic resonance imaging (MRI) activity.
Methods
We undertook a Medline search using the terms “aggressive onset MS”, “highly active MS”, “malignant MS”, “fulminant MS”, “natural history”, and “early predictors” for relevant publications to decide on the criteria best used to define AOMS within 1 year of diagnosis. Parameters incorporated into this set of criteria were relapses, disability as measured by EDSS score, as well as gadolinium-enhancing lesions and T2 lesion burden on MRIs within 12 months of disease onset. We analyzed the pros and cons of the individual measures that were used. Based on our review, we selected a set of criteria that could be easily used in clinical practice. We selected the following criteria for diagnosing AOMS: 1) two or more relapses in the year after onset and two or more gadolinium-enhancing lesions on brain MRI scan; or 2) one relapse if it results in sustained baseline EDSS score of 3 along with two or more gadolinium-enhancing lesions.
Subjects
The following data are systematically collected in the MS Center database: demographic information including age, sex, and ethnicity; MS history including first symptoms, date of first symptom, and date of diagnosis; detailed history of relapse including date of relapse, symptoms, recovery, and duration. Data with respect to EDSS score, medication history, and MRIs were obtained as part of clinical care and stored in the database. The data were periodically updated during the follow-up visits. The institutional review board of Weill Cornell Medical College did not require that participant consent be obtained, given the retrospective character of the study. Moreover the data was obtained from a consented database.
Statistical analysis
Descriptive statistics on patient characteristics for the entire cohort were provided via mean and standard deviation for continuous variables and proportion for categorical variables. Median and range were provided for non-normally distributed continuous variables. Differences in sex, ethnicity, and annual relapse rate between treatment groups were assessed using the chi-square test and Fisher’s exact test. EDSS score of patients at the onset of treatment was compared to the score at the most recent evaluation to obtain EDSS status (improved, same, or worsened) at the time of analysis.
No evidence of disease activity (NEDA) was expected to be achieved if the following criteria were met: 1) no change in baseline neurological exam, 2) no relapses, and 3) no new or enlarging T2 lesions or gadolinium-enhancing lesions on the MRI scan. The Wilcoxon signed-ranked test was used to assess the median difference in the annual relapse rate between the first-line and aggressive treatment. Fisher’s exact test was used to compare the proportion of patients with NEDA after treatment between patients who started with a first-line agent (injectable treatments including an interferon or glatiramer acetate were counted as first-line treatments) and patients who started directly with the aggressive treatment (natalizumab, rituximab, alemtuzumab, cyclophosphamide). Fisher’s exact test was also used to conservatively compare the proportion of patients with NEDA between patients who started with the first-line agent and patients who ever experienced the aggressive treatment, either at disease onset or when switched from first-line treatment.
A multivariable logistic regression model was used to assess the independent effect of treatment group on the EDSS status, controlling for age and ethnicity. Statistical significance was evaluated at the 0.05 alpha level and all P-values were two sided. Statistical analysis was carried out using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients included in the study were enrolled in the institutional review board approved Weill-Cornell Medical College MS database. A total of 1,000 patients have been enrolled in the database since 2008 and followed longitudinally. Captured data include, among others, time of onset, treatments, relapses, neurological examination, and EDSS and MRI data which are systematically collected and periodically updated. A total of 783 charts in the database were reviewed. Fifty-eight of 783 (7.4%) patients met our criteria for AOMS. Of the 58 patients, 43 patients had complete data set and were included in the subsequent analysis.
The study subjects (n=43) had a median age of 33 years (range 22–59 years), and mean duration of follow-up was 54 months (standard deviation =24). The majority of the samples were female (70%), Caucasian (65%), and had two relapses prior to treatment (84%). Thirty-five of these 43 (81%) patients were started on one of the first-line injectable therapies (Table 1). In eight of 43 patients, the initial treatment was one of the more aggressive options including natalizumab and alemtuzumab (Table 2). A flowchart of the treatment path is shown in Figure 1. Among the 35 patients who were started on an injectable treatment, 22 were switched to more aggressive therapies (natalizumab, rituximab, cyclophosphamide, alemtuzumab) due to continued disease activity. Fifteen of 22 patients were switched because of clinical disease activity, ie, relapses, and seven of 22 patients because of radiologic progression alone. The median time to switch from first-line therapy to a more aggressive therapy in the 22 patients was 9.5 months (range 2–43 months). It is important to note that not all patients were switched at the first sign of refractory disease.
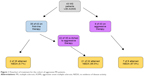  | Figure 1 Flowchart of treatment for the cohort of aggressive MS patients. |
Two of the 35 patients (5.7%) who started with the first-line agent met the criteria for NEDA throughout the study. Out of the eight patients who began directly with aggressive treatment, seven met the criteria for NEDA (87.5%) for the duration of the study (P<0.0001). The total number of patients who received aggressive treatment at onset or after they were switched, was 30. Of the 30 patients who ever experienced the aggressive treatment (P<0.0001), 28 patients showed NEDA. The mean duration of follow-up in patients on aggressive treatment was 32.4 months (range 12–85 months). The annual relapse rate on first-line treatment was 0.45 and that on aggressive treatment was 0.025. Patients who started directly on aggressive treatment had higher odds (odds ratio [OR] 3.5, 95% confidence interval [CI] 0.49–23.97) of having improved EDSS score compared to patients who started with the first-line agent.
When we analyzed the influence of age, sex, and ethnicity, we observed that Caucasian ethnicity was associated with lower odds of having improved EDSS score compared to other ethnicities (OR 0.31, 95% CI 0.06–1.6) and that increase in age was associated with lower odds of having improved EDSS score (OR 0.90, 95% CI 0.79–1.12). Sex had no effect on the outcome.
Discussion
Currently, there are no guidelines or consensus criteria for defining highly active MS at onset or at any point during the disease course. Increasing acceptance of the potential “window of opportunity”9,10 to treat the disease early, to prevent or delay long-term disability, makes it imperative to identify aggressive disease early, in order not to potentially lose this opportunity. Based on the existing literature on natural history and early predictors of disease course, we selected a set of criteria utilizing early clinical features and MRI findings that can be easily applied in clinical practice to identify AOMS. The proposed criteria were created with the intention of identifying this population within 1 year of symptom onset, thereby facilitating early decision making with regard to most appropriate treatment.
Using these criteria, we found that 58 of 783 patients (7.4%) within the database had AOMS, correlating with the observation that ~4%–14% of all MS patients suffer from the aggressive form of MS.5,6 Of these 58 patients, 43 patients were included in the final analysis. Traditional first-line treatment was used initially in 35 of these patients (81%), and ultimately only two of the 35 patients (5.7%) maintained a NEDA status during the duration of follow-up. The majority continued to be treatment refractory, necessitating a change toward a more aggressive therapy. This is compared to seven of eight patients (19%) who were started on more aggressive therapy at disease onset and continued to demonstrate NEDA (87.5%) up to their last follow-up. An early aggressive approach appears to be warranted in this group to control the inflammation and stabilize the disease. If indeed, our findings are reflective of what it takes to achieve disease control in this subgroup of patients, then we would be losing valuable time and potentially missing the “therapeutic window of opportunity”9,10 by following a conventional escalation therapy approach versus an early aggressive or perhaps even an induction followed by maintenance approach.
The limitations of our study include the retrospective nature of the analysis and the relatively small number of patients with AOMS. We realize that the criteria we chose are arbitrary, but given the lack of a uniform set of criteria or widely accepted guidelines, we used what we felt would be the most useful in clinical practice to help identify this population at disease onset. Even though our mean follow-up was 54 months, a much longer follow-up would be needed to gauge the true long-term impact of therapies.
Conclusion
We identified patients with AOMS within the first year of their diagnosis. In our MS database, 7.4% of patients presented with AOMS. Patients presenting with AOMS benefited most from early treatment with aggressive therapies and appeared to need more aggressive immune suppression to control their disease. Given the potential limited window of opportunity in limiting long-term disability, this might serve the goal of identifying this population as early as possible and initiate or modify treatment to slow down the disease process and improve outcomes.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The study was supported by a grant from Genzyme Corporation (Cambridge, MA, USA). The authors report no other conflicts of interest in this work.
References
Noonan CW, Williamson DM, Henry JP, et al. The prevalence of multiple sclerosis in 3 US communities. Prev Chronic Dis. 2010;7(1):A12. | ||
Rush CA, MacLean HJ, Freedman MS. Aggressive multiple sclerosis: proposed definition and treatment algorithm. Nat Rev Neurol. 2015;11(7):379–389. | ||
Mancardi G, Saccardi R. Autologous haematopoietic stem-cell transplantation in multiple sclerosis. Lancet Neurol. 2008;7(7): 626–636. | ||
Piechiecchio A, Tavazzi E, Maccabelli G, et al. What insights have new imaging techniques given into aggressive forms of MS – different forms of MS or different from MS? Mult Scler. 2009;15(3): 285–293. | ||
Gholipour T, Healy B, Baruch NF, Weiner HL, Chitnis T. Demographic and clinical characteristics of malignant multiple sclerosis. Neurology. 2011;76(23):1996–2001. | ||
Menon S, Shirani A, Zhao X, et al. Characterizing aggressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 2013;84(11):1192–1198. | ||
Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain. 2003;126(Pt 4):770–782. | ||
Finisku LK, Brex PA, Altmann DR, et al. Disability and T2 MRI lesions: a 20 year follow-up of patients with relapse onset of multiple sclerosis. Brain. 2008;131:808–817. | ||
Le Page E, Leray E, Taurin G, et al. Mitoxantrone as induction treatment in aggressive relapsing remitting multiple sclerosis: treatment response factors in a 5 year follow-up observational study of 100 consecutive patients. J Neurol Neurosurg Psychiatry. 2008;79(1):52–56. | ||
Coles AJ, Cox A, Le Page E, et al. The window of therapeutic opportunity in multiple sclerosis. Evidence from monoclonal antibody therapy. J Neurol. 2006;253(1):98–108. | ||
Freedman MS. Introduction versus escalation of therapy for relapsing multiple sclerosis: the evidence. Neurol Sci. 2008;29(Suppl 2):S250–S252. | ||
Lassman H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012;8(11):647–656. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


