Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
A Split-Face Study Assessing the Clinical Benefit, Tolerability and Subject Satisfaction of a Dermocosmetic in Subjects with Rosacea Associated with Erythema and Sensitive Skin
Authors Berardesca E , Bonfigli A , Cribier B, Flament F , Vicic M, Kerob D, Tan J
Received 2 July 2020
Accepted for publication 8 September 2020
Published 5 October 2020 Volume 2020:13 Pages 751—758
DOI https://doi.org/10.2147/CCID.S266879
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Jeffrey Weinberg
Enzo Berardesca,1 Adriana Bonfigli,2 Bernard Cribier,3 Frederic Flament,4 Marco Vicic,4 Delphine Kerob,5 Jerry Tan6
1Phillip Frost Department of Dermatology, University of Miami, Miami, FL, USA; 2ISPE, Milano, Italy; 3Clinique Dermatologique, University Hospital, Strasbourg, France; 4L’Oréal Research and Innovation, Chevilly-Larue, France; 5Laboratoires Vichy International, Levallois-Perret, France; 6Western University, Department of Medicine and Windsor Clinical Research Inc, Windsor, ON, Canada
Correspondence: Enzo Berardesca
Phillip Frost Department of Dermatology, University of Miami, Miami, FL, USA
Tel +1 393486962500
Email [email protected]
Objective: This study assessed the efficacy and tolerability of M89 in patients with rosacea associated with erythema and sensitive skin.
Methods: Intra-individual study in a split-face design comparing after 30 days M89 twice daily and usual skin care in 20 adult subjects with rosacea and sensitive skin. M89 contains 89% Vichy volcanic mineralizing water (VVMW) and 0.4% hyaluronic acid. It is hypoallergenic and contains no perfume and this convenes in rosacea. Contained minerals reinforce the natural defences of the skin in restoring the natural skin barrier, stimulating antioxidant activity and reducing inflammation, commonly observed in subjects with rosacea. Clinical evaluations included assessment of erythema, desquamation, papules and pustules, skin tightness, dryness, burning sensation, itching, stinging and stinging test as well as local tolerability. Instrumental evaluations included skin hydration and TEWL. Subject satisfaction was assessed at Days 15 and 30. Demodex density was assessed at Day 30.
Results: A significant superiority of M89 over the standard skin care was observed for erythema, skin tightness and dryness (all P≤ 0.05) as early as Day 15, the skin stinging test was significantly in favour of M89 (P< 0.05 at Day 15 and P< 0.01 at Day 30) and for skin hydration (P< 0.0001) at Day 15 and 30 with no difference in mean Demodex density between M89 and usual skin care after 30 days. Tolerance was excellent and subject satisfaction very high.
Conclusion: Study results concerning M89 are encouraging for its use either alone or as an adjuvant daily skin care to topical medication in patients with persistent centrofacial erythema of rosacea with no more than 3 papules and pustules.
Keywords: rosacea, sensitive skin, Vichy volcanic mineralizing water, M89, split face
Introduction
Rosacea is a common chronic inflammatory skin disease characterised by persistent erythema associated with periodic intensification or “flares”. Fixed centrofacial erythema is a characteristic pattern, flushing, papules, pustules and telangiectasia may also be observed.1–3 Its course is irregular, with periods of flares and remission.4–6 Rosacea often remains undiagnosed and inadequately managed.7–11 Patients frequently report, facial flushes skin burning, itching, stinging/tingling and often feel embarrassed, thereby adding psychosocial burden to the visible clinical picture.12–16
Its pathogenesis involves the interplay of genetic factors, immune dysregulation, neurovascular dysregulation, presence of microorganisms, and environmental factors. An increased activation of the immune system occurs through multiple stimuli, including increased levels of cathelicidin and kallikrein 5, Toll-like receptor 2, matrix metalloproteinases, and mast cells within the skin. Their effects are enhanced by the presence of microorganisms and external triggers, such as UV radiation.17
While the elimination of triggers, use of gentle cleansers, moisturizers and photo-protection may often be sufficient to manage milder forms of the disease or as adjuvant to pharmacologically active treatments including topical metronidazole, azelaic acid and ivermectin, oral antibiotics and/or procedures become necessary for the treatment of more severe forms.16
Mineral 89 (M89, Laboratoires Vichy, France) contains 89% Vichy volcanic mineralizing water (VVMW) and 0.4% hyaluronic acid. It is hypoallergenic and contains no perfume, thus being suitable for subjects with rosacea. VVMW originates from the French volcanic region and contains 15 minerals with a total mineral concentration of 5.2g/l. These minerals reinforce the natural defences of the skin in restoring the natural skin barrier, stimulating antioxidant activity and reducing inflammation, commonly observed in subjects with rosacea.18–25
In an unpublished analysis of a global investigation concerning a subgroup of subjects with mild rosacea, M89 applied daily on the face improved clinical signs of rosacea and, according to subjects, improved their symptoms as well as skin hydration, indicating that M89 may strengthen the natural skin barrier and help to protect the skin from environmental and external aggressions.26
The aim of our split-face study was to assess the clinical benefit of M89 compared to standard skin care in subjects with rosacea with erythema and sensitive skin after 30 days of daily use.
Methods
This single centre, split-face, randomised, controlled clinical trial was conducted between September and November 2019 and adhered to the principles of Good Clinical Practices and the declaration of Helsinki. According to local and European regulatory guidelines (Official Journal of EU of March 10th, 2010 paragraph 1.2.9), this type of trial testing marketed cosmetics did not require approval from local ethics committees. Nevertheless, all subjects provided written informed consent prior to participation.
Twenty women aged between 20 and 60 years with a phototype of I to III, with rosacea (defined as persistent centrofacial erythema of rosacea with no more than 3 papules and pustules by Gallo et al), and with a positive reaction to the skin-stinging test were included.1
Investigators assessed at Day 0 (baseline) and after 15 and 30 days, clinical signs such as erythema, desquamation, papules and pustules, tolerability and skin hydration using a corneometer (Corneometer CM825, Courage & Khazaka, Cologne, Germany) and GPSkin Barrier® (GPSB, GPOWER Inc, Seoul, South Korea) as well as transepidermal water loss (TEWL) using a tewameter (Tewameter TM 300 MDD 4, Cologne, Germany) and GPSB. GPSB has been tested and validated as a novel instrumental device for assessing skin hydration and TEWL.27–29 Study evaluations were carried out in a temperature and humidity-controlled room (24 ± 2°C; 50 ± 10% r.h) after an acclimation period of 30 minutes.
Subjects were asked not to wash their face for at least 2 hours before performing the assessments and not to apply any products on the face for 12 hours before the basal visit.
At each time point, a skin-stinging test using a topical solution of 15% of lactic acid applied on the nasolabial folds was performed according to Frosch and Kligman.30 Subjects rated burning/stinging/itching/painful sensations perceived on each nasolabial fold after 2.5 and 5 minutes from the application on a scale from 0=no burning/stinging/itching/painful sensation to 3=severe burning/stinging/itching/painful sensation.
At Day 0, subjects completed a questionnaire containing 14 features about the perception of their sensitive skin.
Clinical evaluations at Day 0, Day 15 and Day 30 included the assessment of the severity of erythema, desquamation and number of papules and pustules, as well as the severity of subject-assessed symptoms including tightness, dryness, burning, itching and stinging sensation; all signs and symptoms were rated on a visual scale from 0=not at all to 10=extremely.
A standardized skin surface biopsy (SSSB) was performed at Day 30. Sampling was performed after clinical and device evaluations at Day 30 only to avoid damaging the stratum corneum at the investigational site thus impacting measurements during the study. The face of the subject was cleaned with ether to remove traces of sebum. Then, a drop (about 0.05 mL) of cyanoacrylate glue was homogeneously applied to an area of 1 cm2 at one end of a microscope slide. (SSSB 1) which was pressed against one cheek. The slide was left in place until the cyanoacrylate changed in consistency and was then gently removed. A second SSSB (SSSB 2) was performed at the same site immediately afterwards. The procedure was repeated on the other cheek in order to collect slides related to both areas (treated with M89 and with the usual skin care product). SSSBs 2 were analysed with a microscope (x40) and the Demodex density (number of Demodex in 1 cm2) was determined for each slide.
During that last visit, subjects also completed a satisfaction questionnaire.
Subjects meeting inclusion criteria were asked to apply M89 twice daily on one half-side of their face and their current commercially available skin cosmetic skin care and moisturisers products on the other half side for 30 days at home respecting indications of use for each product. Application sides were determined randomly using a computer software. Subjects were instructed to wash their face with their current skin cleansing products prior to product applications and to always apply M89 to the same designated half side of the face. Furthermore, subjects were asked to avoid excessive UV exposure and tanning beds. Pharmacologically active rosacea treatments required a 2-week washout period prior to inclusion into the study and no other rosacea care other than that to be used during the study was permitted.
Local tolerance and safety were assessed through the study.
Mean values, standard deviations and variations were calculated for each set of values. Following the results of normality test using the Kolmogorov–Smirnov method, instrumental data registered at the different control times (Day 0, Day 15, Day 30) were compared by means of Repeated Measures ANOVA and the Bonferroni Test. Variations (Day 15-Day 0, Day 30-Day 0) recorded in the treated and in the untreated areas were compared by means of t-test for parametric and dependent data. Scores of the clinical evaluation and the stinging test (Day 0, Day 15, Day 30) were statistically compared by means of Friedman ANOVA and Kendall’s Concordance Coefficient. Variations (Day 15-Day 0, Day 30-Day 0) and the Demodex density recorded in the treated and in the untreated area were compared by means of the Wilcoxon test for non-parametric and dependent data. The significance level was set at 5%. For each subject satisfaction question, the number of the answers given for each level of intensity was calculated and reported as a percentage.
Results
All 20 women recruited conformed to inclusion criteria and all data collected were suitable for statistical analysis purposes. The average age was 48 years, 12 (60%) subjects had photo type II and 8 (40%) phototype III. Demographic and baseline data are provided in Table 1. Results from the sensitive skin questionnaire showed that 40% of subjects considered their sensitive skin when buying clothes and underwear, 35% considered their skin constantly when buying cosmetics, 55% found it sometimes difficult to cope with urban pollution, 45% reported that their face becomes red when doing sport or other physical activities, 35% reported that wearing woollen clothes is unbearable, and 30% reported using their own skin care products when travelling.
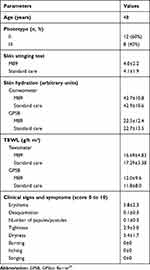 |
Table 1 Demographic and Baseline Data |
A statistically significant improvement of certain clinical signs and symptoms with M89 was observed as early as 15 days for erythema (Day 0: 5.8±2.3, Day 15: 5.4±2.4, Day 30: 5.2±2.2; P<0.05), skin tightness (Day 0: 2.9±3.0, Day 15: 1.8, Day 0: 1.5±2.3; P<0.01) and skin dryness (Day 0: 5.4±1.7, Day 15: 3.9±1.6, Day 30: 3.4±1.7; p<0.001). No statistically significant improvement of any clinical sign or symptom was observed with the standard skin care regimen. Statistically significant differences between M89 and the usual skin care regimen for erythema (P<0.05), skin tightness (P<0.05) and skin dryness (P<0.001) were observed after 15 and 30 days of use. No burning, itching or stinging were observed in any subject, at any point.
A statistically significant (P<0.0001) increase from Day 0 was observed for skin hydration as early as 15 days of use of M89 increasing until Day 30 using the corneometer. Conversely, the difference was statistically significant (P<0.001) with the standard skin care after 30 days of use, only. The difference in skin hydration between the two regimes was significantly (P<0.0001) in favour of M89 at Day 15 and Day 30 (Figure 1). Similar results were observed for M89 using the GPSB; however, with no significant difference from Day 0 for the standard skin care regimen (Figure 1).
When using the tewameter, TEWL had significantly (P<0.05) decreased after 15 and 30 days of use of M89 compared to Day 0. No significant decrease was observed with the standard skin care regimen (Figure 2) as well as between both regimens and when using the GPSB (Figure 2).
The demodex density analysis after 30 days’ use of M89 did not reveal a significant difference between either skin care regimen (1.0±1.2 for M89 compared to 1.4±1.3 for the standard skin care), even though the incidence was slightly lower with M89.
The skin stinging test revealed that M89 had considerably reduced (P<0.01) the stinging effect of lactic acid after 15 days, while there was no significant decrease observed with the standard skin care regimen. The difference between both groups was significant at Day 30 (P<0.01). Results are given in Figure 3.
Figure 4 provides detailed results for subject satisfaction after 15 and 30 days of daily use of M89. According to the subjects, M89 improved facial lesions, redness and flushes and made their skin look better and different. As a result, subjects felt better, more confident and would recommend M89 to friends. Overall, satisfaction increased between Day 15 and Day 30.
 |
Figure 4 Subject satisfaction after 15 and 30 days of continued daily use of M89. |
No tolerance and safety issues were reported with M89.
Discussion and Conclusion
Results from this split-face study confirm the clinical benefit of M89 in subjects with rosacea with erythema and sensitive skin to reinforce the natural defences of the skin in restoring the natural skin barrier, stimulating antioxidant activity and reducing inflammation, commonly observed in subjects with rosacea.18–25,31
Daily use of M89 led to a significant improvement of erythema, skin tightness and skin dryness sensations after 15 and 30 days of treatment compared to usual skin care products. Similarly, statistically significant decreases in the mean basal scores of skin sensitivity, detected by means of a sting-test, were recorded after 15 and 30 days of treatment with M89, although no statistically significant variation was recorded in the same parameter after the treatment with the usual skin care product. A statistically significant difference between M89 and the usual skin care product was evidenced after 30 days of treatment.
M89 significantly reduces TEWL using tewametry and significantly improves skin hydration as shown through corneometry and GPSB, as well as stinging, erythema, tightness and dryness of the skin as early as after 15 days of use with a continued benefit up to 30 days, when compared to standard skin care regimens. Not surprisingly, M89 did not reduce the density of the demodex colony of the tested half-face, confirming that M89 acts on the subjects’ skin and not on its inhabitants, compared to topical pharmacological active treatments such as topical metronidazole or ivermectin.32
During this study, we also used the GPSB, a novel instrumental device to assess skin hydration and TEWL.27,29 Results from our study confirm that GPSB was able to show that skin hydration had improved. However, and even though the device has proven its reliability in the past, the study could not confirm that the device was able to show that TEWL had decreased following the use of M89 as observed with the tewameter.28 The main reason for that may be the small number of subjects recruited in our study.
Another limitation of the study was the non-standardised comparative skin care. Indeed, using a standardised placebo cream would have allowed to assess more objectively the benefit of M89.
However, despite this limitation, overall, M89 provided a better outcome of use. In a recent, large, international observational study of M89, an unpublished subgroup analysis from the data of 64 subjects with rosacea and 134 subjects with sensitive skin and confirmed observed results. After 4 weeks in subjects with rosacea and sensitive skin, respectively, erythema had resolved or improved in 69.5% and 69.3%, desquamation in 91.3% and 79.3% and irritation in 96.4% and 92.7%. Scores for dryness, burning, itching and stinging/tingling had significantly decreased in both groups (all P≤0.0001); 73.0% and 84.3% considered their skin sufficiently hydrated, respectively. There was no significant change from baseline for papule/pustule count in the rosacea group. Almost all subjects reported soothed skin and satisfaction with product texture. Subject and investigator satisfaction were very high. Tolerance was excellent.26
In another yet unpublished in vitro study, M89 did not impact the skin penetration of topical ivermectin, which is recognised as an effective treatment of rosacea.
Recently, Thiboutot et al confirmed that mild skin care is important in the management of rosacea, as the skin of these patients is frequently sensitive and irritated, resulting in erythema, stinging and burning. Therefore, preserving and restoring the natural skin barrier using specifically developed adjuvant skin care, in addition to the pharmacologically active treatment of this chronic disease, are important.3
As such, these results concerning M89 are encouraging for its use either alone or as an adjuvant daily skin care to topical medication in patients with persistent centrofacial erythema of rosacea with no more than 3 papules and pustules.
Data Sharing Statement
The authors do not agree to share individual deidentified participant data. However, the authors agree to share raw data, the informed consent, protocol and study report. There are no other documents available. Documents may be requested via e-mail from the corresponding author during 12 months following publication.
Acknowledgments
The study was funded by Laboratories Vichy. The authors acknowledge the participation of the subjects and Karl Patrick Göritz, Scientific and Medical Writing Services, France for the writing assistance.
Disclosure
B. Cribier and J. Tan serve as consultants for Laboratoires Vichy. B. Cribier reports personal fees from Egg au Carre, during the conduct of the study. J. Tan reports personal fees from L’Oreal, during the conduct of the study. F. Flament, M. Vicic are employees of L’Oréal. D. Kerob is an employee of Laboratories Vichy. The authors report no other conflicts of interest in this work.
References
1. Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78(1):148–155.
2. Tan J, Almeida LM, Bewley A, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176(2):431–438. doi:10.1111/bjd.15122
3. Thiboutot D, Anderson R, Cook-Bolden F, et al. Standard management options for rosacea: the 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82(6):1501–1510. doi:10.1016/j.jaad.2020.01.077
4. Fonseca GP, Brenner FM, Muller Cde S, Wojcik AL. Nailfold capillaroscopy as a diagnostic and prognostic method in rosacea. An Bras Dermatol. 2011;86(1):87–90. doi:10.1590/S0365-05962011000100011
5. Crawford GH, Pelle MT, James WD. Rosacea: I. Etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51(3):
6. Powell FC. The histopathology of rosacea: ‘where’s the beef?’ Dermatology. 2004;209(3):173–174. doi:10.1159/000079884
7. Kligman AM. An experimental critique on the state of knowledge of rosacea. J Cosmet Dermatol. 2006;5(1):77–80. doi:10.1111/j.1473-2165.2006.00228.x
8. Quarterman MJ, Johnson DW, Abele DC, Lesher JL
9. Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17(1):70–73. doi:10.1016/S0190-9622(87)70173-X
10. Alexis AF. Rosacea in patients with skin of color: uncommon but not rare. Cutis. 2010;86(2):60–62.
11. Tidman MJ. Improving the management of rosacea in primary care. Practitioner. 2014;258(1775):27–30, 3.
12. Dirschka T, Micali G, Papadopoulos L, Tan J, Layton A, Moore S. Perceptions on the psychological impact of facial erythema associated with rosacea: results of International Survey. Dermatol Ther (Heidelb). 2015;5(2):117–127. doi:10.1007/s13555-015-0077-2
13. Cardwell LA, Nyckowski T, Uwakwe LN, Feldman SR. Coping mechanisms and resources for patients suffering from rosacea. Dermatol Clin. 2018;36(2):171–174. doi:10.1016/j.det.2017.11.013
14. Egeberg A, Hansen PR, Gislason GH, Thyssen JP. Patients with rosacea have increased risk of depression and anxiety disorders: a Danish Nationwide Cohort Study. Dermatology. 2016;232(2):208–213. doi:10.1159/000444082
15. Halioua B, Cribier B, Frey M, Tan J. Feelings of stigmatization in patients with rosacea. J Eur Acad Dermatol Venereol. 2017;31(1):163–168. doi:10.1111/jdv.13748
16. Schaller M, Almeida LMC, Bewley A, et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182(5):1269–1276.
17. Ahn CS, Huang WW. Rosacea pathogenesis. Dermatol Clin. 2018;36(2):81–86. doi:10.1016/j.det.2017.11.001
18. Nusgens BV. Hyaluronic acid and extracellular matrix: a primitive molecule? Ann Dermatol Venereol. 2010;137(Suppl 1):S3–S8. doi:10.1016/S0151-9638(10)70002-8
19. Burke KE. Mechanisms of aging and development-a new understanding of environmental damage to the skin and prevention with topical antioxidants. Mech Ageing Dev. 2018;172:123–130. doi:10.1016/j.mad.2017.12.003
20. Hughes MC, Williams GM, Baker P, Green AC. Sunscreen and prevention of skin aging: a randomized trial. Ann Intern Med. 2013;158(11):781–790. doi:10.7326/0003-4819-158-11-201306040-00002
21. Nonotte I, Montastier C, Boisnic S, Branchet-Gumila MC, Breton L. Inhibitory effect of Lucas spring water on substance P induced inflammation in organ culture of human skin. NouvDermatol. 1998;17:2–11.
22. Moyal D, Tricaud C, Pham DM, Ngyen QL. P2619 efficacy of a spa water in preventing UVA-induced catalase degradation. J Am Acad Dermatol. 2006;54(3).
23. Mascarenhas NL, Wang Z, Chang YL, Di Nardo A. TRPV4 mediates mast cell activation in cathelicidin-induced rosacea inflammation. J Invest Dermatol. 2017;137(4):972–975. doi:10.1016/j.jid.2016.10.046
24. Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:1885. doi:10.12688/f1000research.16537.1
25. Tacheau C, Weisgerber F, Fagot D, et al. Vichy Thermal Spring Water (VTSW), a cosmetic ingredient of potential interest in the frame of skin ageing exposome: an in vitro study. Int J Cosmet Sci. 2018;40(4):377–387. doi:10.1111/ics.12470
26. Tan J, Spada J, Orlandi C, et al. Vichy mineralizing water with hyaluronic acid is effective and well tolerated as an adjunct to the management of various dermatoses and after esthetic procedures. J Cosmet Dermatol. 2020;19(3):682–688. doi:10.1111/jocd.13229
27. Caberlotto E, Cornillon C, Njikeu S, Monot M, Vicic M, Flament F. Synchronized in vivo measurements of skin hydration and trans-epidermal water loss. Exploring their mutual influences. Int J Cosmet Sci. 2019;41(5):437–442. doi:10.1111/ics.12556
28. Ye L, Wang Z, Li Z, Lv C, Man MQ. Validation of GPSkin barrier® for assessing epidermal permeability barrier function and stratum corneum hydration in humans. Skin Res Technol. 2019;25(1):25–29. doi:10.1111/srt.12590
29. Cointereau-Chardon S, Caberlotto E, Vicic M, Flament F. Self-recording the skin hydration and trans-epidermal water loss parameters: a pilot study. Skin Res Technol. 2020. doi:10.1111/srt.12862
30. Frosch PJ, Frosch PJ, Kligman AM. A method for appraising the stinging capacity of topically applied substances. J Soc Cosmet Chem. 1977;28:197–209.
31. Schlesinger TE, Powell CR. Efficacy and tolerability of low molecular weight hyaluronic acid sodium salt 0.2% cream in rosacea. J Drugs Dermatol. 2013;12(6):664–667.
32. Sahni DR, Feldman SR, Taylor SL. Ivermectin 1% (CD5024) for the treatment of rosacea. Expert Opin Pharmacother. 2018;19(5):511–516. doi:10.1080/14656566.2018.1447562
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



