Back to Journals » Chronic Wound Care Management and Research » Volume 2
A single-center, prospective, randomized, open-label, clinical trial of ceramide 2-containing hydrocolloid dressings versus polyurethane film dressings for pressure ulcer prevention in high-risk surgical patients
Authors Kohta M , Sakamoto K, Kawachi Y, Oh-i T
Received 1 August 2015
Accepted for publication 20 September 2015
Published 6 November 2015 Volume 2015:2 Pages 171—179
DOI https://doi.org/10.2147/CWCMR.S93555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Marco Romanelli
Masushi Kohta,1 Kazumi Sakamoto,2 Yasuhiro Kawachi,3 Tsunao Oh-i4
1Medical Engineering Laboratory, ALCARE Co, Ltd, Tokyo, 2Department of Nursing, 3Department of Dermatology, Tokyo Medical University Ibaraki Medical Center, Ibaraki, 4Department of Dermatology, Tokyo Medical University Hachioji Medical Center, Tokyo, Japan
Purpose: There have been previous clinical studies regarding the impact of dressings on the prevention of pressure ulcer development. However, it remains unclear whether one type of dressing is better than any other type for preventing ulcer development during surgery. Therefore, we compared the effects of ceramide 2-containing hydrocolloid dressing with film dressings in high-risk patients with regard to reducing the incidence of pressure ulcer development during surgery.
Patients and methods: A prospective, randomized, open-label, clinical trial was conducted involving patients who were at a high risk of developing pressure ulcers at a Japanese hospital. The intervention group received ceramide 2-containing hydrocolloid dressings (n=66), and the control group received film dressings (n=64). The primary end point was the incidence rate of pressure ulcer development in both groups; skin damage, such as blanchable erythema, skin discoloration, contact dermatitis, and stripped skin, was recorded as the secondary end point. The relative risk (RR) and 95% confidence interval (CI) were assessed to compare the probability ratios of pressure ulcer development between the groups.
Results: There were significantly fewer patients who developed pressure ulcers in the intervention group than in the control group (RR, 0.37; 95% CI, 0.05–0.99; P=0.04). In the post hoc subgroup analysis, the superiority of the intervention group was more marked when patients had a lower body mass index (P=0.02), lower albumin values (P=0.07), and operation time of 3 hours or more and less than 6 hours (P=0.03). There was no evidence of any statistically significant differences in the types of skin damage reported.
Conclusion: Application of ceramide 2-containing hydrocolloid dressing reduced the risk of pressure ulcer development in patients who were at a high risk during surgery compared with film dressings.
Keywords: operating room, wound dressing, friction, skin protection, shear
Introduction
An increasing number of pressure ulcers have resulted in a wide spectrum of socioeconomic problems.1 This increasing trend has been particularly observed in high-risk surgical patients compared with patients in a general acute care setting, with incidence rates ranging from 16.4% to 30.3%.2–4 A pressure ulcer is defined as any area of the skin or underlying tissue that has been damaged due to unrelieved pressure or pressure in combination with extrinsic factors, including friction and shear.5 Surgical patients are at a distinctive risk due to long periods of immobilization on an operating table and the generation of friction and shearing forces on areas predisposed to pressure ulcers when changing positions.6 These extrinsic factors can also occlude blood vessels and reduce blood flow, resulting in skin damage.7
Prevention is better than treatment in pressure ulcer management. A promising approach for preventive strategies is to disperse excessive body pressure using a pressure redistribution support surface, for example, a viscoelastic polyurethane foam.8,9 In addition, patients should be lifted and not slided when moving them in a specific surgical position, such as prone, lateral, or lithotomy, to avoid friction with shearing forces. However, there are still problems because it is difficult to completely prevent tissue damage in surgical patients at a high risk for ulcer development.
In recent years, there have been numerous studies evaluating either basic or clinical evidence regarding the impact of dressings on the prevention of pressure ulcer development. In a basic science study, researchers reported that shearing forces on both superficial and subcutis layers were significantly reduced when a conventional hydrocolloid dressing was applied to the animal skin.10 In addition, a hydrocolloid dressing with a low frictional outer layer could significantly reduce the friction coefficient on the heel of an elderly person.11 Moreover, Gefen et al clarified that dressings could minimize the compression and shear deformation that may develop in the skin and soft tissue when compression was applied using the finite element modeling technique.12,13 These basic studies substantially supported a review of clinical trials described in the following section.
In clinical trials, a positive effect on ulcer prevention was typically found for ceramide 2-containing hydrocolloid dressing and film dressings when they were used in elderly patients in Japanese hospitals. A ceramide 2-containing hydrocolloid dressing has skin-protective properties and a relatively low friction coefficient and results in reduced pressure ulcer development in bedridden patients in the general ward.14 Film dressings were believed to provide similar effects in the reduction of friction and shearing force.15 More recently, in a retrospective trial, we have reported that the application of these dressings could reduce the risk of pressure ulcer development in high-risk surgical patients.16
Thus, different dressing types are available for use on the skin in high-risk patients. However, it remains unclear whether one type of dressing is better than any other type for preventing ulcer development during surgery. The purpose of this study was to compare the effects of ceramide 2-containing hydrocolloid dressing with film dressings in reducing the incidence of pressure ulcer development during surgery in patients with a high risk of developing pressure ulcers.
Materials and methods
Study design
We conducted a single-center, prospective, randomized, open-label, clinical trial of patients who were admitted to a 501-bed acute care hospital in Ibaraki, Japan (Trial number: UMIN 000019420). The study period was from February 2014 to July 2014. This study was conducted in accordance with regulations for clinical research established by this hospital. This study was approved by the Institutional Review Board of Tokyo Medical University Ibaraki Medical Center (Approval number: 13–23). Written informed consent was obtained from all the patients. All aspects of this research were in accordance with the principles set forth by the Declaration of Helsinki. There were no major changes in study methods after the trial commencement.
Hypothesis
High-risk patients who received ceramide 2-containing hydrocolloid dressings would have a lower incidence rate of pressure ulcer development during surgery than patients who received film dressings.
Materials
We used two types of adhesive thin-layered dressings: a polyurethane film dressing (Opsite®; Smith and Nephew Wound Management KK, Tokyo, Japan; Tegaderm®; 3M Health Care Ltd, Loughborough, UK) or Multifix® (ALCARE Co, Ltd, Tokyo, Japan) as the control and a ceramide 2-containing hydrocolloid dressing (Remois Pad®; ALCARE Co, Ltd) as the intervention. We will refer to the ceramide 2-containing hydrocolloid dressing as the intervention dressing and the film dressing as the control dressing. We used film dressings as control dressings because they have been widely used as prophylactic dressings for pressure ulcer prevention in Japan. Some brands of hydrocolloid dressing are commercially available, but the positive effects of ulcer prevention have not been proved yet. According to body habitus of the patients, three different dressing sizes (10×12.5 cm, 15×20 cm, and 20×30 cm) were made available for controls. The intervention dressing available in the market was square in shape (20×20 cm), but it was applied to the intervention sites by cutting the dressing to the desired size to fit the shape of each patient’s skin.
Eligible patients
Eligible patients included this study were surgical patients at a high risk of developing pressure ulcers. In Japan, these high-risk patients were defined by the following parameters: 1) those patients placed in a specific position, such as prone, lateral, or lithotomy or 2) those patients for whom the operating time was over 6 hours under general anesthesia.17
Excluded patients
Patients were excluded if they did not give written informed consent to participate. Patients with existing pressure damage and/or with a history of pressure ulcer at the intervention sites at the start of the study were also excluded from this study.
Sample size
According to the incidence of pressure ulcer development from our previous study,16 we needed 64 patients per group to provide a power of 0.08, an alpha of 0.05, and to detect a 17% reduction in the primary end point (from 22% for the control group to 5% for the intervention group). This mathematical process was conducted using a statistical power analysis program, G*Power 3, which was produced by the Institute of Experimental Psychology, Heinrich-Heine University,Düsseldorf, Germany.18
Randomization and blinding
Of the 130 patients, 66 were allocated to the intervention group and 64 to the control group using simple randomization. We produced random numbers that ranged from zero to one using a computer-generated random number table and then assigned these random numbers to patients according to the starting date of the surgery. If the number was less than 0.5, the patients were allocated to the intervention group; the remaining numbers (and patients) were placed in the control group. This trial was not blinded because of the difference in the quality of intervention and control dressings.
End points
The primary end point was the incidence rate of pressure ulcer development in high-risk surgical patients in both groups.
In addition to the primary end point evaluation, instances of skin damage, such as blanchable erythema, skin discoloration, contact dermatitis, and stripped skin, at the intervention sites were recorded to assess patient safety and potential intervention benefits.
Procedure
The predisposition to pressure ulcer development depended on the type of surgery and the body type of patients, according to a previous report.19 In this study, the intervention and control dressings were applied to the breast itself and iliac crests in the prone position, to the sacral area and scapulae in the lithotomy position, and to the axillae and greater trochanter in the lateral position. Moreover, these dressings were applied to the sacral area and scapulae in the supine position when the operating time was over 6 hours under general anesthesia. The sites that were predisposed to pressure ulcer development in each patient were determined by a certified expert nurse (in wound, ostomy, and continence [WOC]) along with floor and surgical nurses during preoperative periods. The surgical nurse applied dressings to the skin in the operating room before the induction of general anesthesia. One day after surgery, these dressings were carefully removed by the floor nurse. A WOC nurse lectured the surgery nurses and the floor nurses on characterization, application, and removal of dressings before the trial. Furthermore, the pressure ulcer prevention strategy was documented in a manual, which was easily accessible to all the nurses. When a pressure ulcer or other skin damage was present, appropriate treatment was performed by the WOC nurse and dermatologist during postoperative periods.
Additional preventive strategies
Some additional preventative strategies used in our hospital have been described in a previous report.16 In brief, during the preoperative period, floor nurses performed skin assessments in each patient to check for any skin complications. When these skin complications developed, appropriate skin care was provided by a WOC nurse. Each patient was laid on a pressure redistribution support surface (Soft-nurse®; LAC Healthcare Ltd, Osaka, Japan), and the surgical team was required to lift and not slide the patient to minimize any friction or shearing forces. Pressure redistribution pads were also placed under the arm and iliac crest to disperse local pressures. The lower limbs were fixed using a few pillows to prevent knees from overlapping, when the patients underwent surgery on lateral position. In the intraoperative period, water-absorbing pads were placed between the body and the pressure redistribution support surface to protect the skin from the antiseptic solution. No other additional preventive actions were performed in this study.
Data collection
Baseline data were obtained from medical records. The records of the intervention- and control-allocated patients were also reviewed for the preoperative medical data on age, sex, body mass index (BMI), and albumin levels, and for the intraoperative data on operation time, type of position, and type of surgery.
The pressure ulcer assessment was performed in accordance with the diagnosis and treatment guidelines, which were created by the Japanese Society of Pressure Ulcers, and on the basis of widely accepted international diagnosis and treatment guidelines.20 The final decisions about the patient’s skin condition were assessed by these guidelines. The development of a pressure ulcer was visually observed by one WOC nurse within 24 hours postsurgery, as noted in a previous report.15,16
The data collection for both the side effect and pressure ulcer assessments was conducted within 24 hours after surgery. Blanchable erythema is a sign of capillary occlusion and tissue damage-related pressure.21,22 Blanchable erythema turns white when pressed with a finger and immediately turns red again when the pressure is removed.23 We chose this method to determine whether the tissue damage that was present should be categorized as a pressure ulcer or blanchable erythema. Besides blanchable erythema, we also evaluated for other skin damage, such as skin discoloration, contact dermatitis, and stripped skin.
Data analysis
The analysis was based on intention to treat where all patients randomized to the intervention group were analyzed regarding protocol violations. The results for continuous variables were expressed as means ± standard deviations (SDs) and those for categorical variables were expressed as numbers and percentages. A nonparametric Mann–Whitney U test was used to compare patient group results for continuous variables, and a chi-square test was used to compare results for categorical variables. A P-value of <0.05 was considered significant. To compare the ratio of probability of the pressure ulcer development between two groups, relative risk (RR) with 95% confidence interval (CI) was also reported, according to Nakagami et al.14 We performed an additional analysis to explore the possibility that the effects of the intervention dressing differed for subgroups of patients in the post hoc subgroup analysis. We focused on the BMI, albumin values, operation time, and prone position. The previous reports had indicated that these parameters were important risk factors.16,24–27 Furthermore, we investigated a comparison between various types of surgeries in two age groups (≤65 and >65) in the subgroup analysis. All statistical analyses were conducted using Statistical Package for the Social Sciences version 20.0 software (IBM Corporation, Armonk NY, USA).
Results
Study flow and patient characteristics
The flowchart of the experiment is shown in Figure 1. Among the 150 eligible patients, 20 were excluded from this study because they did not wish to participate in this trial. No patients in this study had a pressure ulcer and/or a history of pressure ulcers at the start of the study.
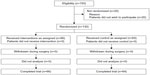  | Figure 1 Patient disposition. |
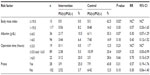
The demographic and clinical characteristics of patients in the two groups are shown in Table 1. The mean ± SD age was 65.2±13.3 (range: 32–89 years) and 66.1±13.2 years (range: 25–88 years) in the intervention group and control group, respectively. The preoperative and intraoperative characteristics were similar, with no significant differences between the groups.
  | Table 1 Characteristics of patients in the intervention and control groups |
Primary outcome evaluation
Table 2 shows that five patients (7.6%) developed a pressure ulcer in the intervention group during the study period, whereas 13 patients (20.3%) developed ulcers in the control group, which was significant (P=0.04). The RR was 0.37 (95% CI, 0.14–0.99), which indicated that patients who received the interventional care had 0.37 times the risk of developing a pressure ulcer compared with patients who received the control care. The most common location for pressure ulcer formation was the breast in both the groups (Table 2). All pressure ulcers that developed in the intervention group were judged to have non-blanchable erythema. Of the 17 pressure ulcers in the control group, 15 were non-blanchable erythema and two were blistered. We could not confirm the brands of film dressings for each patient who developed pressure ulcer in the control group, because the types of film dressings were randomly used for the patients.
  | Table 2 Pressure ulcer development in each patient group |
There were characteristic differences among the patients in pressure ulcers within and between groups based on units that the patients were cared for postoperatively. Of 66 patients in the intervention group, 16 were transferred to an intensive care unit (ICU) and the remaining were transferred to general wards in the postoperative period. The number of the patients who developed pressure ulcer in the intervention group was three (19%) in the ICU and two (4%) in the general wards. Of 64 patients in the control group, eleven were transferred to an ICU and the remaining were transferred to general wards in the postoperative period. The number of the patients who developed pressure ulcer in the control group was five (45%) in the ICU and eight (15%) in the general wards. In the intervention group, the type of surgery that led to the development of pressure ulcer in patients included neurosurgery (n=3) in the ICU and orthopedic (n=2) in the general ward. In the control group, the type of surgery that induced the development of pressure ulcer included neurosurgery (n=2), gastrointestinal (n=2), and respiratory (n=1) in the ICU, and orthopedic (n=5) and gastrointestinal (n=3) in the general wards.
Subgroup analysis
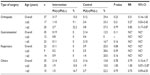
The RR, 95% CI, and statistically significant differences in each subgroup are shown in Table 3. The superiority of the intervention dressing was more marked when patients were at a high risk of ulcer development. When BMI value was ≤19.0 kg/m2, there were fewer patients who developed ulcers during surgery with the intervention dressing than with the control dressing (P=0.02). A similar result was shown in the subgroup with lower albumin values, although this difference was not significant (P=0.07). When the operation time was less than 3 hours, the number of patients who developed an ulcer was zero and one in the intervention and control groups, respectively. In contrast, in patients with an operation time of 3 hours or more and less than 6 hours, fewer patients developed pressure ulcers in the intervention group than in the control group, with a RR of 0.25 (range: 0.05–0.99) in favor of the intervention (P=0.03).
The results of pressure ulcer development were categorized into types of surgery and ages (≤65 and >65) (Table 4). In the intervention group, the patients who underwent orthopedic surgery showed the highest risk of pressure ulcer development. None of the patients who underwent gastrointestinal and respiratory surgery in the intervention group developed pressure ulcer. The control groups tended to develop pressure ulcer compared to the intervention group in all the surgery types and age groups. In the control groups, the patients who underwent orthopedic and respiratory surgery tended to develop pressure ulcer, although there was no significant difference among surgery types.
Side effects
There was no evidence of a statistically significant difference in the number of side effects between the groups (Table 5). Blanchable erythema occurred in 4.5% of the patients in the intervention group and in 12.5% of the patients in the control group (P=0.1). The number of patients who had stripped skin or skin discoloration ranged from one to two in both groups. Other types of skin damage, such as contact dermatitis, were not present in either of the groups.
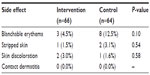  | Table 5 Skin damage at the intervention sites |
Discussion
The clinical guidelines have recommended that high-quality studies are needed to confirm the comparative benefits of technologies for pressure ulcer prevention to assist clinical specialists worldwide.27 However, there have been no prospective studies on pressure ulcer prevention using adhesive dressing in the operating room. To the best of our knowledge, this is the first randomized, controlled, clinical trial that compared adhesive dressings for surgical patients at a high risk of developing pressure ulcers, although it was impossible to blind both caregivers and analyzers. The most significant finding of this study was that patients in the intervention group had significantly fewer pressure ulcers than those in the control group (Table 2). In this trial, the positive effects of the dressing applications contained the effects of the preventive actions. However, the comparison between the intervention and control groups enables us to determine which dressing is more effective to reduce pressure ulcer development under general preventive actions.
The intervention dressing was sized prior to use, while the control dressing was selected from three different sizes according to the body habitus of patients. The sizing of the intervention dressing could have little impact on the outcomes in this study. Generally, pressure ulcer occurs in locations that contact a pressure redistribution support surface. The sizes of the dressings were decided to cover such locations completely; therefore, it is reasonable that the difference of shapes between the dressings was not considered in the trial. Also, the use of three types of film dressings could have little impact on the outcomes in this study. The most important characteristic of the control dressing is the ability to transmit water vapor from the dressing to the external environment. It is reasonable that the three types of control dressings used in this study were equally used as a vapor-permeable dressing because all the dressings were made of polyurethane film and had the same thickness (~0.03 mm). A previous study showed that the water vapor transmission rate of Tegaderm® and Opsite® was similar, although the rate of Multifix® has not been investigated yet.28
The intervention dressing was effective for reducing pressure ulcer development in malnutrition population with lower BMI and albumin values. The BMI and albumin values are known to be nutrition-related risk factors associated with increased pressure ulcer: patients with lower BMI and albumin values tend to develop pressure ulcer. A long-term consequence of malnutrition was loss of fat, muscle, and tissue, resulting in the formation of bone prominence sites. It has been reported that an intervention dressing reduced shearing force on bone prominence sites (such as heel) of elderly patients.11 Additionally, our previous study had already reported that the intervention and control dressings could reduce the tissue damage due to a reduction in the friction coefficient and the associated shearing force between a patient’s body and any object.16 It is likely that the intervention dressing was effective in malnutrition older population with high comorbidities by reducing shearing force on bone prominence sites.
Our results in this study also suggested that the intervention dressing may have a positive effect due to high water-holding ability of the hydrocolloid layer in contact with the skin, which results in creating an occlusive environment at the intervention sites during surgery. When the hydrocolloid dressing was applied to bone prominence sites, which were predisposed to pressure ulcerations, the maintenance of skin hydration reportedly showed some positive effect in controlling skin pH, improving the water-holding capacity, and protecting the skin barrier function.29–31 In addition, excess perspiration increased the risk of pressure ulcer development for patients in specific positions (prone and lateral) during surgery in Japanese hospitals, and this led to miceration.4,32 Thus, intervention dressing may be successful in reducing the friction and shear and in keeping the water-holding capacity of the skin surface; this resulted in a reduction in pressure ulcer formation during the intraoperative period.
Patients in the subgroups that had a lower BMI, lower albumin values, and a prolonged operation time had a lower risk of pressure ulcer development when using the intervention dressing (Table 3). The RR ranged from 0.25 to 0.70 in this study and indicated that the intervention dressing had a positive effect in almost all the subgroups during surgery. Therefore, we recommend using the intervention dressing to prevent tissue damage, particularly in patients at a high risk for ulcer development and who have a lower BMI, lower albumin values, and a longer operation time.
In addition, patients in the subgroup that had orthopedic surgery tended to develop pressure ulcer in the intervention and control groups (Table 4). Surgical positioning is a risk factor in the formation of pressure ulcer in the operating room. The most common location for the development of pressure ulcer formation in this study was breast in both the groups. The patients who developed pressure ulcer on the breast had undergone surgery in the prone position, which was the most frequently used position in orthopedic surgery for spine procedures. Excess pressure occurred on the breast when the patient was positioned prone for the surgery. It is obvious that this type of surgery influenced the risk of developing higher number of pressure ulcers on the breast.
During the comparison of side effects, there were concerns that skin problems would occur with the intervention dressing, although no significant differences with the two types of dressings were identified. However, the surgical staff at our hospital was not quite satisfied with this result because according to them, the dressing may cause associated skin problems (skin discoloration and stripped skin). The surgical staff require an improvement in the quality of the dressings to ensure patient safety in the operation room; therefore, they are responsible for conducting the preventative strategy. To resolve these skin problems, we will investigate into the causes of skin damage associated with both dressings in future research.
There were several limitations in this study. First, the open-label design presented a possibility of bias in the outcome reporting; however, we believe that our data may provide important information – about prophylactic care in the high-risk patients – for wound management specialists who deal with ulcer prevention. The second limitation is the absence of a WOC nurse at the time when the dressings were removed, although the outcome was measured within 24 hours of dressing removal. The busy schedule made it difficult for investigators and WOC nurse to attend the dressing removal. Finally, our subgroup analysis was underpowered because this trial was powered to determine the overall effect of treatment. Our subgroup analysis showed that the risk of pressure ulcer development was reduced in favor of the intervention group. However, several subgroups did not show statistically significant differences and the upper limit of the 95% CI crossed 1.0, suggesting that the CI in the subgroups was too wide because of the smaller numbers in each analysis.33,34 This subgroup analysis may answer practical questions, such as how the treatment should be used more effectively and which patients would get the most benefit. Therefore, more prospective clinical studies with a larger patient sample size, including more high-risk surgical patients, will be needed to determine the effectiveness of each subgroup. We will continue to perform similar research for a longer period of time, which could eliminate the influence of surgery type and surgical positioning in future.
Conclusion
In this randomized controlled trial, we concluded that applying ceramide 2-containing hydrocolloid dressings reduced the risk of pressure ulcer development in surgical patients who were at a high risk compared with film dressings.
Acknowledgments
We would like to thank the nursing staff, including the head nurse, chief nurse, all members of the pressure ulcer committee, and the surgical nurses of our hospital for their valuable cooperation. We would also like to thank Enago (http://www.enago.jp) for its review of English language.
Author contributions
All authors substantially participated in the conception, study design, data acquisition, and data analysis. All authors also contributed to drafting the manuscript and critical revisions for important intellectual content and gave their final approval for the version to be published. Finally, all authors agreed to be accountable for all aspects of this work to ensure that questions related to the accuracy or integrity of any part of this work would be appropriately investigated and resolved.
Disclosure
KS, YK, and TO were supported by the funds from ALCARE Co, Ltd (Tokyo, Japan) for this trial. MK is an employee of ALCARE Co, Ltd (Tokyo, Japan). The authors have no other conflicts of interest to disclose related to this work.
References
Cushing CA, Phillips LG. Evidence-based medicine: pressure sores. Plast Reconstr Surg. 2013;132(6):1720–1732. | |
Schultz A, Bien M, Dumond K, Brown K, Myers A. Etiology and incidence of pressure ulcers in surgical patients. AORN J. 1999;70(3):434, 437–440, 443–449. | |
Chen HL, Shen WQ, Xu YH, Zhang Q, Wu J. Perioperative corticosteroids administration as a risk factor for pressure ulcers in cardiovascular surgical patients: a retrospective study. Int Wound J. 2015; 12(5):581–585. | |
Yoshimura M, Nagata O, Kohno M, Yamasaki T, Mae T, Ohashi S. [Perioperative risk factors associated with pressure ulcer in the park bench position]. J Jpn Soc Clin Anesth. 2013;33(1):75–83. Japanese. | |
National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention of pressure ulcers. In: Haesler E, editor. Prevention and treatment of pressure ulcers: clinical practice guideline. Washington (DC): National Pressure Ulcer Advisory Panel; 2014. | |
Lindgren M, Unosson M, Krantz AM, Ek AC. Pressure ulcer risk factors in patients undergoing surgery. J Adv Nurs. 2005;50(6):605–612. | |
Linder-Ganz E, Gefen A. The effects of pressure and shear on capillary closure in the microstructure of skeletal muscles. Ann Biomed Eng. 2007;35(12):2095–2107. | |
Aronovitch SA, Wilber M, Slezak S, Martin T, Utter D. A comparative study of an alternating air mattress for the prevention of pressure ulcers in surgical patients. Ostomy Wound Manage. 1999;45(3):34–40, 42–44. | |
Huang HY, Chen HL, Xu XJ. Pressure-redistribution surfaces for prevention of surgery-related pressure ulcers: a meta-analysis? Ostomy Wound Manage. 2013;59(4):36–38, 42, 44, 46, 48. | |
Ohura T, Takahashi M, Ohura N Jr. Influence of external forces (pressure and shear force) on superficial layer and subcutis of porcine skin and effects of dressing materials: are dressing materials beneficial for reducing pressure and shear force in tissues? Wound Repair Regen. 2008;16(1):102–107. | |
Nakagami G, Sanada H, Konya C, Kitagawa A, Tadaka E, Tabata K. Comparison of two pressure ulcer preventive dressings for reducing shear force on the heel. J Wound Ostomy Continence Nurs. 2006; 33(3):267–272. | |
Gefen A. The biomechanics of heel ulcers. J Tissue Viability. 2010; 19(4):124–131. | |
Levy A, Frank MB, Gefen A. The biomechanical efficacy of dressings in preventing heel ulcers. J Tissue Viability. 2015;24(1):1–11. | |
Nakagami G, Sanada H, Konya C, Kitagawa A, Tadaka E, Matsuyama Y. Evaluation of a new pressure ulcer preventive dressing containing ceramide 2 with low frictional outer layer. J Adv Nurs. 2007; 59(5):520–529. | |
Imanishi K, Morita K, Matsuoka M, et al. Prevention of postoperative pressure ulcers by a polyurethane film patch. J Dermatol. 2006;33(3):236–237. | |
Kohta M, Sakamoto K, Oh-i T. Polyurethane film dressings and ceramide 2-containing hydrocolloid dressing reduce the risk of pressure ulcer development in high-risk patients undergoing surgery: a matched case-control study. Chronic Wound Care Manage Res. 2015;2:23–30. | |
Sanada H, Nakagami G, Mizokami Y, et al. Evaluating the effect of the new incentive system for high-risk pressure ulcer patients on wound healing and cost-effectiveness: a cohort study. Int J Nurs Stud. 2009;47(3):279–286. | |
Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. | |
Walton-Geer PS. Prevention of pressure ulcers in the surgical patient. AORN J. 2009;89(3):538–551. | |
The Japanese Society of Pressure Ulcers Revision Committee. [JSPU guidelines for the prevention and management of pressure ulcers (3rd ed)]. Jpn J PU. 2014;16(1):12–90. Japanese. | |
Bethell E. Controversies in classifying and assessing grade 1 pressure ulcers. Nurs Times. 2003;99(13):73–75. | |
Lyder CH. Conceptualization of the stage 1 pressure ulcer. J ET Nurs. 1991;18(5):162–165. | |
Evans J, Stephen-Haynes J. Identification of superficial pressure ulcers. J Wound Care. 2007;16(2):54–56. | |
Lewicki LJ, Mion L, Splane KG, Samstag D, Secic M. Patient risk factors for pressure ulcers during cardiac surgery. AORN J. 1997; 65(5):933–942. | |
Tschannen D, Bates O, Talsma A, Guo Y. Patient-specific and surgical characteristics in the development of pressure ulcers. Am J Crit Care. 2012;21(2):116–125. | |
Hyun S, Li X, Vermillion B, et al. Body mass index and pressure ulcers: improved predictability of pressure ulcers in intensive care patients. Am J Crit Care. 2014;23(6):494–500; quiz 501. | |
Qaseem A, Mir TP, Starkey M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians. Risk assessment and prevention of pressure ulcers: a clinical practice guideline from the American college of physicians. Ann Intern Med. 2015; 162(5):359–369. | |
Sussman G. Technology update: understanding film dressings. Wounds Int. 2010;1(4):23–25. | |
Park KH. The effect of a ceramide-containing dressing in preventing pressure ulcers. J Wound Care. 2014;23(7):347–353. | |
Hao DF, Feng G, Chu WL, Chen ZQ, Li SY. Evaluation of effectiveness of hydrocolloid dressing vs ceramide containing dressing against pressure ulcers. Eur Rev Med Pharmacol Sci. 2015;19(6):936–941. | |
Kohta M, Iwasaki T. The effect of concentration of tackifying agent on adhesive and skin-protective properties of ceramide 2-containing hydrocolloid dressings. J Wound Care. 2015;24(1):41–48. | |
Yoshimura M, Nagata O, Kohno M, Toyoda M, Ohashi S, Akizuki T. [Effectiveness of using a new perioperative fixation method in the Park-bench position, combining a decompression posture fixation device with a support surface, for the prevention of pressure ulcers and influence of perspiration]. Jpn J PU. 2014;16(2):135–143. Japanese. | |
Cuzick J. Forest plots and the interpretation of subgroups. Lancet. 2005;365(9467):1308. | |
Rothwell PM. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005; 365(9454):176–186. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.