Back to Journals » International Journal of Nanomedicine » Volume 14
A sensitive and rapid chemiluminescence immunoassay for point-of-care testing (POCT) of copeptin in serum based on high-affinity monoclonal antibodies via cytokine-assisted immunization
Authors Wang Y , Dzakah EE, Kang Y, Cai Y, Wu P, Tang B, Li R, He X
Received 5 January 2019
Accepted for publication 3 April 2019
Published 10 June 2019 Volume 2019:14 Pages 4293—4307
DOI https://doi.org/10.2147/IJN.S200556
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Yu Wang,1 Emmanuel Enoch Dzakah,2,3 Ye Kang,1 Yanxue Cai,4 Peidian Wu,5 Bo Tang,5 Run Li,5 Xiaowei He1
1College of Food Science and Engineering, South China University of Technology, Guangzhou 510641, People’s Republic of China; 2Dermatology Hospital, Southern Medical University, Guangzhou 510091, People’s Republic of China; 3Department of Molecular Biology and Biotechnology, School of Biological Sciences, College of Agriculture and Natural Sciences, University of Cape Coast, Cape Coast, Ghana; 4School of Chemical Engineering and Energy Technology, Dongguan University of Technology, Dongguan 523808, People’s Republic of China; 5National & Local United Engineering Lab of Rapid Diagnostic Test, Guangzhou Wondfo Biotech Co., Ltd, Guangzhou 5l0663, People’s Republic of China
Purpose: Antibodies are key reagents in the development of immunoassay. We attempted to develop high-performance CPP immunoassays using high-affinity monoclonal antibodies prepared via cytokine-assisted immunization.
Methods: We used fetal liver tyrosine kinase 3 ligand (Flt3L), CC subtype chemokine ligand 20 (CCL20), and granulocyte-macrophage colony-stimulating factor (GM-CSF) to assist traditional subcutaneous immunization of preparing high-affinity monoclonal antibodies, and further to develop high-performance immunoassay methods for CPP.
Results: This novel immune strategy significantly enhanced immune response against CPP. Six anti-CPP monoclonal antibodies (mAbs) with high affinity were successfully screened and selected for application in a fully automated magnetic chemiluminescence immunoassay (CLIA). This robust and rapid assay can efficiently detect CPP in the range of 1.2–1250 pmol L–1, with a detection limit of 6.25 pmol L–1,. Significantly, the whole incubation process can be completed in 30 min as compared to about 4.5 hr for the control ELISA kit. Furthermore, this assay exhibited high sensitivity and specificity, low intra-assay and inter-assay coefficients of variation (CVs < 15%). The developed assay was applied in the detection of CPP in 115 random serum samples and results showed a high correlation with data obtained using a commercially available ELISA kit (correlation coefficient, 0.9737).
Conclusion: Our assay could be applied in the point-of-care testing of CPP in the serum samples, and also the method developed in this study could be adopted to explore the detection and diagnosis of other biomarkers for various diseases.
Keywords: copeptin, cytokine-assisted immunization, high-affinity antibodies, chemiluminescence immunoassay, point-of-care tests, clinical application
Introduction
The need for a simple, rapid, and more accurate diagnosis and prognostic assessment of various diseases to assist with treatment decision has led to the investigation of new biomarkers. A previous study has indicated that CPP mirrors individual stress levels even more subtly than circulating cortisol.1 This new biomarker can enable early decision making in clinical practice and also provide a reliable degree of correlation with various acute disease states, such as cerebrovascular event,2 myocardial infarction3 or pneumonia,4 kidney disease and hypertension,5 osmotic alterations,6 human obesity,7 bipolar disorder,8 major depression,9 and childhood maltreatment.10 In triage of chest pain patients, determination of CPP, in addition to troponin, improves early diagnostic performance, especially after the onset of chest pain.11 During the onset of an acute myocardial infarction (AMI), CPP is rapidly released from the pituitary gland and starts to return to normal levels within a few hours while troponin T concentrations are still normal.12 In a research on CPP diagnostic cutoff of AMI among 5,000 individuals, the 99th percentile, which is usually suggested as the universal definition of a biomarker, was 18.9 pmol L−1, the 97.5th percentile was 13 pmol L−1, and the 95th percentile was only 9.8 pmol L−1.11 Apparently, the clinical value of CPP can be maximized only by shortening the detection cycle as much as possible while ensuring the sensitivity and specificity of the method. However, till date, only a few techniques have been applied in the determination of CPP concentration, among which are microtubes chemiluminescence immunoassay (tubes CLIA),13 radioimmunoassay (RIA),14 enzyme-linked immunoassay (ELISA),15 and electrochemical assay.16 Although their sensitivity levels could meet the required standards, assay durations are generally too long, making them unsuitable for use in clinical settings and hence their application only in scientific research. To the best of our knowledge, the point-of-care testing (POCT) for CPP has yet not been reported, hence it would be expedient to develop a POCT for the detection of CPP in clinical samples.
High-affinity antibodies are important for the establishment of a good performance immunoassay. Effectively improving the immune response may be a viable way to obtain high-affinity antibodies. Several studies have shown that cytokines can effectively improve immune response and successfully break immune tolerance. For example, Flt3L, an important cytokine, has been shown to promote the proliferation, differentiation, and maturation of pre-lymphocytes, dendritic cells (DCs), natural killer cells (NKs), and cytotoxic lymphocytes (CLs) both in vivo and in vitro, and plays an important role in the anti-tumor immune response.17,18 GM-CSF recruits, activates, and enhances the function of antigen presenting cells, making it a useful adjuvant in vaccines. Numerous studies in different animal models have clearly shown that plasmids expressing GM-CSF can enhance immune responses generated against DNA vaccines.19–23 Some researchers have also suggested the potential use of CCL20 chemokine as a vaccine adjuvant to enhance Th1 mediated cellular and humoral immune responses in hepatitis C virus (HCV) core DNA immunization.24 CCL20 chemokine attracts immature DCs, effector/memory T cells, and B cells, thereby increasing the ability of DCs in the capture and presentation of antigens. However, these studies are currently limited to DNA immunization, and the role of this cytokine in the preparation of monoclonal antibodies by improving the immune response of subcutaneous immunization has not been investigated.
Chemiluminescence immunoassays have been widely applied in the routine clinical analysis due to their high sensitivity, wide detection range, methodological reliability, and relative stability.25,26 Recently, magnetic-particles (MPs) have proven to be a suitable tool for highly sensitive detection of various biomarkers due to their higher specific surface area for immobilizing antibody, improved capture efficiency, reduced incubation time, and easy separation.27,28 More importantly, the efficient enrichment of MPs provides an increased amount of captured CPP at extremely low concentrations and thus afford high sensitivity and specificity.
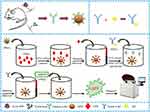
In this work, cytokine-assisted immunization was applied to increase the immunity of the BALB/c mice, and several high-affinity antibodies were successfully screened and purified. For fully automated CLIA constructing, MPs were coated with a high-affinity antibody, alkaline phosphates (AP) labeled another anti-CPP antibody which identifies another site of the CPP and the concentration of the CPP was computed based on the observed luminescence intensity of the substrate. This fully automated CLIA exhibited an excellent specificity, stability, and reproducibility in human serum samples test and has an added advantage of obtaining results in a short period. Furthermore, the result of this CLIA compared favorably with a commercially available ELISA test kit and could serve as an excellent alternative for the detection of CPP in clinical samples.
Experimental
Materials
Peptides related to CPP were chemically synthesized, purified, and quality-controlled by Jill Biochemical Co., Ltd. (Shanghai, China). The peptides were N-CPP (sequence CATQLDGPAGALLLRLV, representing positions 132–147 of pre-provasopressin plus an N-terminal cysteine residue), C-CPP (sequence CLAGAPEPFEPAQPDAY, representing positions 149–164 of pre-provasopressin plus an N-terminal cysteine residue), and CPP (sequence ATQLDGPAGALLLRLVQLAGAPEPFEPAQPDAY, representing positions 132–164 of pre-provasopressin). Peptides were conjugated via mmaleimidobenzoyl-N-hydroxysuccinimide ester to keyhole limpet hemocyanin (KLH) to act as the immunogen (KLH-C-CPP, KLH-N-CPP). Murine CCL20, Flt3L, GM-CSF coding sequence cloned into pCAGGS vector under the control of a CAG promoter using the EcoRI and EcoRI restriction sites were assisted by Atagenix Biotechnology Co., Ltd. (Wuhan, China). The final construct was confirmed by sequencing and transfection of HEK 293 T cells which purchased from the Cell Bank of Chinese Academy of Sciences. Female six-to-eight-week-old BALB/c mice were purchased from the Experimental Animal Center of Nanjing Medical University (Nanjing, China) and were allowed to acclimatize for 1 week prior to immunization. The carboxyl micromagnetic particles (2.8±0.2 µm), alkaline phosphates, and the chemiluminescent substrate (4-methoxy-4-(3-phosphatephenyl)-spiro-(1,2-dioxetane-3,2-adamantane) (AMPPD) were supplied by Guangzhou Wondfo Biotech Co., Ltd. (Guangzhou, China). All buffer reagents and other inorganic chemicals were purchased from Sigma-Aldrich (Shanghai, China). Random clinical serum samples were obtained from The Third Affiliated Hospital of Guangzhou University of Chinese Medicine (Guangzhou, China). All aqueous solutions were prepared using double distilled water.
Apparatus
Automatic chemiluminescence immunoassay instrument (Limiray1200) was supplied by Rayto Life Science Co., Ltd. (Shenzhen, China). The concentration of antibody was detected by NanoDrop (2000c) spectrophotometer from Thermo fisher scientific (Shanghai, China). The affinity of antibodies was evaluated by the BLItz® System from PALL ForteBio LLC (Menlo Park, USA). Commercial enzyme-linked immunosorbent assay (ELISA) kits obtained from Cusabio Biotech Co. Ltd. (Wuhan, China) were used in the determination of CPP concentration in serum samples following the manufacturer’s instructions.
Production and characterization of anti-CPP monoclonal antibody
Female BALB/c mice were injected subcutaneously with 100 µg immunogen emulsified with the identical volume of complete Freund’s adjuvant for the first immunization. Subsequent booster immunizations with 50 µg immunogen emulsified with an equal volume of incomplete Freund’s adjuvant were injected at two-week intervals. Seven days after each immunization, cytokine plasmids (10 µg) were injected through the tail vein. Seven days after the second and third injection, antibody titers in mouse serum samples collected from the tail vein were checked by ELISA. The hybridoma development and monoclonal antibody characterization were carried out as previously reported.29,30 Meanwhile, the affinity of antibodies was evaluated according to the protocol provided by the manufacturer. All animal experiments and welfare of the animals were performed under ethical approval from and in agreement with the guidelines of the Institutional Animal Care and Use Ethics Committee of South China University of Technology and also in accordance with the policy of the National Ministry of Health.
Coating MPs with CPP antibody
High-affinity CPP monoclonal antibody was covalently coupled to MPs in the binding buffer via the terminal amine. Firstly, 20 mg mL−1 MPs were placed in a 2 mL Eppendorf tube. The MPs were then washed five times with binding buffer. During the washing, the tube was placed on a magnetic concentrator and the supernatant was removed. Afterward, the MPs were resuspended in 2 mL binding buffer. Antibody solution was added to the above suspension for the conjugation of CPP antibodies and MPs by incubation at 37°C with shaking overnight. After incubation, the tube was placed in a magnetic concentrator to separate the supernatant. The residual binding sites on the MPs were blocked with 3% bovine serum albumin (BSA) incubated at 37°C with slight shaking for 2 hrs. After washing 5 times, the coated MPs (mAb-MPs) were dispersed in 2 mL buffer and stored at 4°C.
Preparation of AP-conjugated CPP antibody
The AP and anti-CPP antibody were coupled with glutaraldehyde. First, the AP and anti-CPP antibody were suspended in ultrapure water and diluted to 4 and 8 mg mL−1, respectively. A 250 µL aliquot of the 4 mg mL−1 AP solution was then transferred to a 1.5 mL Eppendorf tube and gently mixed with 250 µL of 8 mg mL−1 anti-CPP antibody solution. Second, 0.5 mL of 1% glutaraldehyde in 0.1 mol L−1 phosphate buffer (pH 7.4) was added to the solution. The resulting mixture was incubated at 37°C with gentle shaking for 4 hrs in the dark. Third, 0.1 mL of 1 mol L−1 monoethanolamine solution was added to the mixture, which was subsequently incubated with shaking for 2 hrs at room temperature. The mixture was dialyzed against PBS solution at 4°C overnight. After dialysis, CPP antibody-AP conjugate was then transferred to an Eppendorf tube and mixed with an equal volume of glycerin and 1% BSA. Finally, the AP-conjugated CPP antibody (AP-mAbs) was stored at −20°C for use in further experiments.
MPs-based fully automated CLIA
CPP was detected with the automatic chemiluminescence immunoassay instrument (Limiray1200) which is based on the sandwich immunoassay using the anti-CPP antibody coated MPs and AP-labeled anti-CPP antibody. These two monoclonal antibodies can recognize different epitopes of the CPP. The reaction procedure for the MPs-based CLIA was slightly different from the conventional microtubes CLIA. In the MPs-based CLIA, MPs coated antibodies served as the solid phase instead of the microtubes, and hence the whole immunoassay process can be performed using the automated chemiluminescence immunoassay instrument. A schematic diagram of the methodology is shown in Figure 1. About 50 µL of mAb-MPs and CPP samples (30 µL) of different concentrations were pipetted into the corresponding tubes and incubated at 37°C for 20 mins (capture time) with gentle shaking. The tubes then were passed through washing stations with magnets to wash with washing buffer (0.01 mol L−1 PBS containing 0.05% Tween 20) for three times to remove non-specific bindings. Then, the AP-mAbs was added and incubated at 37°C for 10 min with gentle shaking. At this point, the sandwich immunocomplex MPs-CPP-AP had formed. The sandwich immunocomplex was magnetically separated, and the excess AP-mAbs were removed by washing. Subsequently, the solution containing AMPPD (200 µL) was added to the sandwich complex. The resulting mixture was placed in the immunoassay instrument, and the value of relative light units (RLU) was measured.
Optimization of immunoassay reagents
Serial dilutions of AP-mAbs (1:50, 1:100, 1:200, 1:500) and mAb-MPs (1:20, 1:50, 1:100, 1:200, 1:500) were allowed to react with a standard positive sample S3 and negative sample S0 of CPP as described earlier. The optimal dilution was determined from the maximum RLU ratio of the positive vs negative samples (RLUS3/RLUS0). Other experimental parameters, such as the pH, incubation time, accuracy, precision, sensitivity, and stability were also investigated. To monitor the feasibility of the newly developed immunoassay, 115 clinical serum specimens were analyzed using our newly developed immunoassay test kit and a commercially available ELISA kit.
Statistical analysis
Bland–Altman plot and Passing–Bablok regression analysis were performed with MedCalc Software (MedCalc, Mariakerke, Belgium). Statistical analysis on a completely randomized design was conducted using the one-way ANOVA procedure, with SPSS 19.0 software, at a level of significance set at p=0.05. All data were presented as mean±standard deviation (mean±SD). Differences were accepted as significant when p<0.05.
Results and discussion
Production and characterization of anti-CPP mAbs
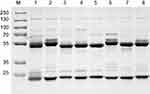
In antibody affinity maturation, the average affinity of the antibodies produced in the secondary immune response is usually higher than the primary immune response.31 Longer duration of antigen presentation is likely to generate higher levels of immunity. For example, a simple extension of the interval between influenza vaccine doses resulted in higher levels of neutralizing antibodies.32 Likewise, a modeling study predicted that sequential immunogens would be more effective than mixed immunogens to target the HIV-1 Env CD4bs epitope.33 Hence, antibodies preparation for research, diagnostic, and therapeutic applications frequently relied on a series of immunization of animals with antigen over the process of several weeks to enhance the proliferation of mature cells producing antigen-specific antibodies. Adjuvants are often used for promotion of affinity maturation through increases the persistence of antigen.34 The use of CCL20 as a vaccine adjuvant could enhance Th1-mediated cellular and humoral immune responses.24 CCL20 chemokine attracts immature antigen presenting cells, and Flt3L could promote the proliferation, differentiation, and maturation. Similarly, GM-CSF is also able to recruit, activate, and enhance the function of antigen presenting cells. Subcutaneous and cytokine-assisted immunization approaches were taken to produce monoclonal antibodies. For the cytokine-assisted immunization, we first subcutaneously immunized mice and after a few days, cytokines were delivered via the tail vein to further enhance the presentation of the antigen. In the second and third vaccinations, we observed that serum titers were more increased with cytokine-assisted immunization. The rate of positive hybridoma and the amount of stable hybridoma was greatly increased following the application of this novel immunization strategy (Table S1). Eight hybridoma cell lines with a stable secretion of mAb against C-CPP were successfully selected after a series of screening. It is worth noting that the affinity of monoclonal antibodies prepared via cytokine-assisted immunization was significantly improved (Figure 2A and 2B). As shown in Figure 2C, the 1/kD value of cytokine-assisted antibody (6-18G10) was approximately 16.7 times higher than that of antibodies produced through the normal subcutaneous immunization (4-30E2). The smaller the kD value, the higher the affinity, hence Li et al recommended that KD values in the range of 5.3–14.0 nM or less are high-affinity antibodies.35 Six of antibodies produced through the cytokine-assisted method had KD values less than 5.3 nM with high-affinity for antigen detection (Table S2). The purity of all mAbs was above 90% as analyzed on SDS-PAGE (Figure S1). The titer values of the antibodies against CPP standards were up to 1:106 using CPP concentration of 1 µg mL−1 and absorbance above 0.3 at 450 nm (Figure S2). These properties are basic experimental requirements for any good antibody and hence these antibodies could be used in further investigation.
Optimization of immunoassay
Selection of the optimal antibody combination
Two C-CPP antibodies (6-20G5 and 6-18G10) with high affinity and one N-CPP antibody (4-30E2) were used for coating and labeling, respectively. In pairs 1 and 2, 4-30E2 was used as the coating antibody with MPs while 6-18G10 and 6-20G5 were labeled with AP. Conversely, in pairs 3 and 4, 6-20G5 and 6-18G10 were used for coating MPs and 4-30E2 for labeling. Since there were four possible combinations, the first development step was the selection of the optimal antibody combination. A standard positive sample S3 and negative sample S0 of CPP were analyzed under all combinations. Then, the signal ratio of RLUS3/RLUS0 was calculated and compared for the determination of the best antibody combinations. As shown in Figure 3, the RLUS3/RLUS0 of pairs 1 and 2 were lower than the other two pairs because of the lower affinity for 4-30E2 (only 5.18 nM), showing relatively weak capture capacity for the antigen. It can be observed that the high-affinity antibodies were more suitable for use as capture antibodies. Ultimately, pair 3 was selected as the most suitable antibody pair with the highest RLUS3/RLUS0 ratio of all (p<0.05).
Optimization of AP-mAbs and mAb-MPs concentration
The amount of AP-mAbs antibody and mAb-MPs are key parameters that affect the sensitivity and accuracy of immunoassay, especially in a sandwich immunoassay. In this study, the dilution ratios of AP-mAbs and mAb-MPs were optimized by comparing with serial dilutions of standard positive sample (S3) and negative sample (S0). Similarly, the signal ratio of RLUS3/RLUS0 served as the parameter for the determination of the best assay. As shown in Figure 4, the RLUS3/RLUS0 increased when the dilution ratios of AP-mAbs increased from 1:500 to 1: 50 at all dilution ratios of the examined mAb-MPs (p<0.05). When the dilution ratios of AP-mAbs were 1:50 and 1:100, the difference in RLUS3/RLUS0 was insignificant. In the case of the mAb-MPs, the RLUS3/RLUS0 showed a peak corresponding to the dilution ratio of 1:50 (p<0.05), the RLUS3/RLUS0 increased when the concentration of mAb-MPs decreased from 1:50 to 1:500, indicating that not all of the available CPP was captured. When 1:50 of mAb-MPs were added, there was sufficient binding of the antigen to mAb-MPs resulting in the highest RLUS3/RLUS0 ratio. By increasing the concentration of antibodies above 1:50 of mAb-MPs, that is, 1:20 dilution, the excess mAb-MPs led to lower sensitivity because of the close association between mAb-MPs, thereby absorbing the emitted light and thereby blocking luminescence.36 Hence, considering both the sensitivity and the assay cost, we selected the dilution ratios of 1:100 and 1:50 for AP-mAbs and mAb-MPs, respectively.
Optimization of substrate buffer pH
The pH value and incubation time are also two important parameters since the acidity of the solution greatly affects the activity of the immobilized protein and extended incubation time probably results in dissociation of the antigen-antibody complex. These two parameters were assessed by detecting higher maximal RLU values for the immunoassay detection of CPP standard sample S9. AP-mAbs solution (1:100, 50 µL), mAb-MPs solution (1:50, 50 µL), and standard sample (S9, 30 µL) were incubated at 37°C for 0–60 mins. The MPs-CPP-AP complex was washed at 5-min interval, and the chemiluminescent substrates (200 µL) with different pH values were added to measure the RLU. Substrate buffers of different pH were prepared by adding 1 mol L−1 NaOH solution. The influence of pH and incubation time on the overall kinetics of light emission is shown in Figure 5. The RLU intensity increased gradually until it reached a steady state. The observed biphasic behavior may be due to the overall dynamics of light emission, which was a two-step process that causes emission delays before steady state chemiluminescence.37 When the pH was 7.0, the RLU value increased from 0 to 20 mins and reached an unstable plateau. Furthermore, the maximum RLU at this pH was lower than others. The RLU apparently reached a maximum at pH of 9.0, thus making it the best suitable for diagnostic testing, similar to the results reported in a literature.36 Concerning the incubation time of the assay, the maximal RLU increased with incubation time from 0 to 30 mins. However, there was no change from 30 to 50 mins, indicating that the antigen–antibody complex formation had reached equilibrium. Hence, 30 mins were selected as the most suitable incubation time period.
 | Figure 5 Optimization of substrate buffer pH and incubation time. Detection methods: RLUS9 obtained for all tests, which 1,250 pmol L−1 of the CPP standards.Abbreviation: RLU, relative light units. |
Method evaluation
Calibration curve for the determination of CPP
Under the optimal condition, a series of concentrations of standard CPP solutions (Table 1) were measured. The calibration curve for CPP standards was constructed, as shown in Figure 6. The limit of detection (LOD) was determined from the average absorbance by adding twice the mean of the standard deviation from 10 S0 wells. The standard curve exhibited a detection range of 1.2–1250 pmol L−1 with a LOD of 6.25 pmol L−1 and a correlation coefficient of 0.9993. These features of our newly developed assay are within the required parameters for use in clinical diagnostic (below 18.9 pmol L−1).11 We also compared this assay with previously reported assays used in the detection of CPP to evaluate the competitive advantage of this assay. As shown in Table 2, the incubation time of our assay was much lower than that of previously reported assays, including Tubes CLIA, RIA, and ELISA. Although the assay time for the electrochemical assay method was only 5 min, the LOD was 37 pmol L−1, which is far beyond the clinical requirement limit for use in clinical applications. Taken together, these results indicated that our fully automated MPs based-CLIA developed displayed better performance.
 | Table 1 The concentration of standard CPP solutions (pmol L−1) |
 | Table 2 Comparison of performance CPP detection methods |
Accuracy, precision, and stability analysis
The recovery rate of dilution is often used to assess the accuracy of assays.13 In this study, five serum samples were tested after linear dilutions (up to 1:32) (Figure 7). The obtained values were multiplied by the dilution factor and compared with the original undiluted concentrations. None of the 5 samples showed a deviation >15% of the original concentrations. To confirm the precision of this method, intra-assay and inter-assay calibrations were performed using three different concentrations of CPP standards with six duplicates for both intra- and inter-assays conducted in 7 days. As shown in Table 3, both CVs were below 15%. Furthermore, the stability of the fully automated CLIA was also investigated. Upon storage of the AP-mAbs at 4°C, and CPP standards and AP-mAbs at −20°C for 30 days, there was no significant change in the readings obtained (Table S3). These results point to the establishment of a high performance and accurate CPP detection method in clinical samples.
 | Table 3 Intra-assay and inter-assay tests |
 | Figure 7 Linearity on dilution (up to 1:32) of 6 representatives of 5 tested samples. |
Cross-reactivity studies
The specificity of the CLIA for CPP was evaluated using other structural or functional analogs such as B-type natriuretic peptides (BNP), N-terminal pro-B-type natriuretic peptide (NT-proBNP), troponin T (cTnT), myoglobin, creatine kinase-myocardial band (CK-MB) that are often detected in combination with CPP in the diagnosis of heart failure or AMI.11,39 The RLU value was measured for each analyte and the cross-reactivity (CR) for each analog was calculated according to the following equation:
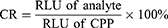
As indicated in Table S4, none of these analytes showed significant reactivity with CPP antibody (<0.2%) even at a higher concentration than that of CPP. These observations demonstrated that the antibody obtained by cytokine-assisted immunization were of high affinity and excellent sensitivity for the detection of CPP.
Method comparison
To further assess the effectiveness of this analytical method, 115 serum specimens consisting of 53 low-level concentration samples (<18.9 pmol L−1), 46 median value samples (18.9–50 pmol L−1), and 16 high-value samples (>50 pmol L−1) were analyzed and compared with a commercially available ELISA kit. It is worth mentioning that the incubation process of the ELISA method for the diagnosis of CPP takes over 4.5 hrs, while this proposed method was 30 mins. The results of both methods were compared based on the Passing–Bablok regression analysis (Figure 8A) and Bland–Altman plot (Figure 8B). The correlation coefficient (R2) of the regression line of the Passing–Bablok regression analysis was 0.9737, indicating a good linear relationship between the two measurements. The Bland–Altman plot between the two methods was performed with serum samples, and the mean relative difference (95% limits of agreement) was 3.5% of the Bland-Altman plot results, revealing that there was no significant bias between these two methods.
Conclusions
The production of high-quality antibodies and the application of advanced analytical technologies would guarantee the development of high-performance immunoassay methods. In this study, we have successfully produced ultra-high affinity antibodies against the CPP and have applied them in a chemiluminescence immunoassay for the rapid and sensitive determination of CPP in human serum. The affinity of the monoclonal antibody prepared via cytokine-assisted immunization was significantly improved approximately 16.7 times that of the traditional immunization method. Using these high-affinity antibodies for the detection of CPP, the sensitivity and specificity of our novel assay were significantly improved. Ultra-high affinity antibody weakens the dissociation of the antigen-antibody complex and MPs provide a large surface-to-area ratio,hence the enhanced detection of CPP at low concentration. The utilization of these antibodies in the detection of CPP provides a suitable detection range between 1.2-1250 pmol L−1 and a detection limit of 6.25 pmol L−1 which is far below the standard requirement for the detection of CPP (<18.9 pmol L−1). In addition, the application of the automatic chemiluminescence immunoassay instrument greatly shortened the incubation time (within 30 mins), whereas a minimum of 2-hr incubation time is required for other commercially available methods. This method was also applied in the detection of CPP in clinical serum specimens, and the results were significantly consistent as compared with other commercially available CPP detection ELISA kit. Hence, our assay could provide an alternative platform for the rapid and accurate detection of CPP in clinical samples. The method developed in this study could be adopted to explore the detection and diagnosis of other biomarkers for various diseases.
Acknowledgments
This work is supported by the Program of Guangdong Provincial Science & Technology (2017A020208014) and the Program of Guangzhou Science & Technology (201802010030).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Widmer IE, Puder JJ, König C, et al. Cortisol response in relation to the severity of stress and illness. J Clin Endocrinol Metab. 2005;90(8):4579–4586. doi:10.1210/jc.2005-0354
2. Czupryna P, Moniuszko-Malinowska A, Garkowski A, et al. Usefulness of copeptin as a potential biomarker in TBE. Biomarkers. 2016;22(3–4):321–325. doi:10.1080/1354750X.2016.1264999
3. Katan M, Müller B, Christ-Crain M. Copeptin: a new and promising diagnostic and prognostic marker. Crit Care. 2008;12(2):1–2. doi:10.1186/cc6996
4. Mohamed GB, Saed MA, Salah K, Saed AM. Predictive value of copeptin as a severity marker of community-acquired pneumonia. Electron Physician. 2017;9(7):4880–4885. doi:10.19082/4880
5. Afsar B. Pathophysiology of copeptin in kidney disease and hypertension. Clin Hypertens. 2017;23(1):13. doi:10.1186/s40885-017-0068-y
6. Fenske WK, Schnyder I, Koch G, et al. Release and decay kinetics of copeptin versus AVP in response to osmotic alterations in healthy volunteers. J Clin Endocrinol Metab. 2017;103(2):505–513. doi:10.1210/jc.2017-01891
7. Schinke C, Hesse S, Stoppe M, et al. Post-dexamethasone serum copeptin corresponds to HPA axis responsiveness in human obesity. Psychoneuroendocrinology. 2017;78:39–47. doi:10.1016/j.psyneuen.2017.01.004
8. Mansur RB, Rizzo LB, Santos CM, et al. Plasma copeptin and metabolic dysfunction in individuals with bipolar disorder. Psychiatry Clin Neurosci. 2017;71(9):624–636. doi:10.1111/pcn.12535
9. Krogh J, Gøtze JP, Jørgensen MB, Lø K, Kistorp C, Nordentoft M. Copeptin during rest and exercise in major depression. J Affect Disord. 2013;151(1):284–290. doi:10.1016/j.jad.2013.08.002
10. Coelho R, Levandowski ML, Mansur RB, et al. Serum copeptin in children exposed to maltreatment. Psychiatry Clin Neurosci. 2016;70(10):434–441. doi:10.1111/pcn.12412
11. Keller T, Tzikas S, Zeller T, et al. Copeptin improves early diagnosis of acute myocardial infarction. J Am Coll Cardiol. 2010;55(19):2096–2106. doi:10.1016/j.jacc.2010.01.029
12. Itoi K, Jiang YQ, Iwasaki Y, Watson SJ. Regulatory mechanisms of corticotropin‐releasing hormone and vasopressin gene expression in the hypothalamus. J Neuroendocrinol. 2004;16(4):348. doi:10.1111/j.0953-8194.2004.01172.x
13. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52(1):112. doi:10.1373/clinchem.2006.073882
14. Xiao Y, Zhang ZG, Chai SB. etermination of plasma copeptin levels in some cardiacand pulmonary disorders. J Radioimmunology. 2008;21(02):97–100.
15. Zhu XD, Chen JS, Zhou F, Liu QC, Chen G, Zhang JM. Detection of copeptin in peripheral blood of patients with aneurysmal subarachnoid hemorrhage. Crit Care. 2011;15(6):1–13.
16. Yang Y. Development of an ultrasensitive electrochemical method for copeptin content determination. Int J Electrochem Sci. 2017;6694–6704. doi:10.20964/2017.07.36
17. Lyman SD. Biologic effects and potential clinical applications of Flt3 ligand. Curr Opin Hematol. 1998;5(3):192. doi:10.1097/00062752-199805000-00008
18. Ali S, Curtin JF, Zirger JM, et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther J Am Soc Gene Ther. 2004;10(6):1071. doi:10.1016/j.ymthe.2004.08.025
19. Pu OY, Hwang LH, Tao MH, Chiang BL, Chen DS. Co‐delivery of GM‐CSF gene enhances the immune responses of hepatitis C viral core protein‐expressing DNA vaccine: role of dendritic cells. J Med Virol. 2002;66(3):320.
20. Kusakabe K, Xin KQ, Katoh H, et al. The timing of GM-CSF expression plasmid administration influences the Th1/Th2 response induced by an HIV-1-specific DNA vaccine. J Immunol. 2000;164(6):3102. doi:10.4049/jimmunol.164.6.3102
21. Sin JI, Kim JJ, Ugen KE, Ciccarelli RB, Higgins TJ, Weiner DB. Enhancement of protective humoral (Th2) and cell‐mediated (Th1) immune responses against herpes simplex virus‐2 through co‐delivery of granulocyte‐macrophage colony‐stimulating factor expression cassettes. Eur J Immunol. 1998;28(11):3530. doi:10.1002/(SICI)1521-4141(199811)28:11<3530::AID-IMMU3530>3.0.CO;2-C
22. Yen HH, Scheerlinck JP. Co-delivery of plasmid-encoded cytokines modulates the immune response to a DNA vaccine delivered by in vivo electroporation. Vaccine. 2007;25(14):2575–2582. doi:10.1016/j.vaccine.2006.12.025
23. Lena P, Villinger F, Giavedoni L, Miller CJ, Rhodes G, Luciw P. Co-immunization of rhesus macaques with plasmid vectors expressing IFN-gamma, GM-CSF, and SIV antigens enhances anti-viral humoral immunity but does not affect viremia after challenge with highly pathogenic virus. Vaccine. 2002;20(Suppl 4):A69. doi:10.1016/S0264-410X(02)00391-2
24. Hartoonian C, Sepehrizadeh Z, Mahdavi M, et al. Modulation of hepatitis C virus core DNA vaccine immune responses by co-immunization with CC-chemokine ligand 20 (CCL20) gene as immunoadjuvant. Mol Biol Rep. 2014;41(9):5943–5952. doi:10.1007/s11033-014-3470-5
25. Dong J, Zhao H, Zhou F, Li B. Rapid and sensitive detection of potassium ion based on K(+)-induced G-quadruplex and guanine chemiluminescence. Anal Bioanal Chem. 2016;408(7):1–7. doi:10.1007/s00216-015-9285-y
26. Hou C, Zhao L, Geng F, Wang D, Guo LH. Donor/acceptor nanoparticle pair-based singlet oxygen channeling homogenous chemiluminescence immunoassay for quantitative determination of bisphenol A. Anal Bioanal Chem. 2016;408(30):1–10. doi:10.1007/s00216-016-9584-y
27. Fu HJ, Yuan LP, Shen YD, et al. A full-automated magnetic particle-based chemiluminescence immunoassay for rapid detection of cortisol in milk. Anal Chim Acta. 2018;1035(1):129–135. doi:10.1016/j.aca.2018.06.015
28. Xie X, Ohnishi N, Takahashi Y, Kondo A. Application of magnetic nanoparticles in full-automated chemiluminescent enzyme immunoassay. J Magn Magn Mater. 2009;321(10):1686–1688. doi:10.1016/j.jmmm.2009.02.115
29. Hongo JA, Tsai SP, Moffat B, et al. Characterization of novel neutralizing monoclonal antibodies specific to human neurturin. Hybridoma. 2000;19(4):303. doi:10.1089/027245700429855
30. Dzakah EE, Kang K, Chao N, et al. Plasmodium vivax aldolase-specific monoclonal antibodies and its application in clinical diagnosis of malaria infections in China. Malar J. 2013;12(1):199. doi:10.1186/1475-2875-12-166
31. Chodorge M, Fourage L, Ravot G, Jermutus L, Minter R. In vitro DNA recombination by L-Shuffling during ribosome display affinity maturation of an anti-Fas antibody increases the population of improved variants. Protein Eng Des Sel. 2008;21(5):343–351. doi:10.1093/protein/gzn013
32. Belshe RB, Frey SE, Irene G, et al. Safety and immunogenicity of influenza A H5 subunit vaccines: effect of vaccine schedule and antigenic variant. J Infect Dis. 2011;203(5):666–673. doi:10.1093/infdis/jiq093
33. Wang S, Mata-Fink J, Kriegsman B, et al. Manipulating the selection forces during affinity maturation to generate cross-reactive HIV antibodies. Cell. 2015;160(4):785–797. doi:10.1016/j.cell.2015.01.027
34. Lambrecht BN, Mirjam K, Willart MAM, Hamida H. Mechanism of action of clinically approved adjuvants. Curr Opin Immunol. 2009;21(1):23–29. doi:10.1016/j.coi.2009.01.004
35. Li J, Schantz A, Schwegler M, Shankar G. Detection of low-affinity anti-drug antibodies and improved drug tolerance in immunogenicity testing by Octet(®) biolayer interferometry. J Pharm Biomed Anal. 2011;54(2):286–294. doi:10.1016/j.jpba.2010.08.022
36. Liu R, Wang C, Jiang Q, Zhang W, Yue Z, Liu G. Magnetic-particle-based, ultrasensitive chemiluminescence enzyme immunoassay for free prostate-specific antigen. Anal Chim Acta. 2013;801(4):91–96. doi:10.1016/j.aca.2013.09.050
37. Beck S, Koster H. Applications of dioxetane chemiluminescent probes to molecular biology. Anal Chem. 1990;62(21):2258. doi:10.1021/ac00220a003
38. Lui CT, Lam H, Cheung KH, et al. Role of copeptin in dual-cardiac marker strategy for patients with chest pain presented to ED. Am J Emergency Med. 2015;33(12):1732–1736. doi:10.1016/j.ajem.2015.08.011
39. Voors AA, Stephan VH, Anker SD, et al. C-terminal provasopressin (copeptin) is a strong prognostic marker in patients with heart failure after an acute myocardial infarction: results from the OPTIMAAL study. Eur Heart J. 2009;30(10):1187. doi:10.1093/eurheartj/ehp098
Supplementary materials
 | Table S1 The comparison of subcutaneous and cytokine-assisted immunization |
 | Table S2 The affinity of the antibodies prepared via cytokine-assisted immunization |
 | Table S3 Stability of the reagents at store (n=3) |
 | Table S4 Cross-reactivity of CLIA to related compounds |
 | Figure S2 The titer of the mAbs against CPP.Abbreviation: mAbs, monoclonal antibodies. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.