Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 10 » Issue 1
A randomized multicenter Phase II study of perioperative tiotropium intervention in gastric cancer patients with chronic obstructive pulmonary disease
Authors Fushida S , Oyama K, Kaji M, Hirono Y, Kinoshita J, Tsukada T , Nezuka H, Nakano T, Noto M, Nishijima K, Fujimura T, Ohta T
Received 22 May 2015
Accepted for publication 31 August 2015
Published 12 October 2015 Volume 2015:10(1) Pages 2177—2183
DOI https://doi.org/10.2147/COPD.S89098
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Sachio Fushida,1,2 Katsunobu Oyama,1,2 Masahide Kaji,2 Yasuo Hirono,2 Jun Kinoshita,1,2 Tomoya Tsukada,1,2 Hideaki Nezuka,2 Tatsuo Nakano,2 Masahiro Noto,2 Koji Nishijima,2 Takashi Fujimura,2 Tetsuo Ohta1,2
1Department of Gastroenterological Surgery, Kanazawa University Hospital, 2Digestive Disease Support Organization (DDSO), Kanazawa, Japan
Background: Tiotropium, a long-acting inhaled anticholinergic drug, has been widely used in the treatment of chronic obstructive pulmonary disease (COPD). However, the issue of whether perioperative tiotropium improves postoperative outcomes for gastric cancer patients with COPD remains unclear. Thus, the aim of this study was to determine the efficacy of perioperative tiotropium intervention for gastric cancer patients with COPD.
Patients and methods: Eighty-four gastric cancer patients with mild-to-moderate COPD were randomly assigned to receive perioperative pulmonary rehabilitation alone (control group) or pulmonary rehabilitation with 18 µg of tiotropium once daily (tiotropium group). The patients in the tiotropium group received tiotropium for more than 1 week before surgery and for 2 weeks after surgery. Spirometry was performed prior to group assignment and at 2 weeks after surgery. Postoperative complications, forced expiratory volume in 1 second, forced vital capacity, and the ratio of forced expiratory volume in second to forced vital capacity (%) were compared between the two groups.
Results: There were no significant differences between the two groups in terms of age, body mass index, smoking, gastrectomy incision, operation time, and bleeding volume (all P>0.05). Postoperative complications and pulmonary functions did not differ significantly between the control and tiotropium groups. A subgroup analysis of gastric cancer patients with moderate COPD showed that perioperative tiotropium intervention significantly decreased the rate of postoperative complications compared with the control group (P=0.046). However, even after gastrectomy, many patients with mild COPD in both the control and tiotropium groups showed improved pulmonary function.
Conclusion: Although perioperative tiotropium intervention had no significant effects in gastric cancer patients with mild COPD, it may be beneficial in those with moderate COPD. Therefore, the next prospective study should further evaluate perioperative tiotropium intervention for gastric cancer patients with moderate-to-severe COPD.
Keywords: gastric cancer, COPD, tiotropium
Background
In Japan, a super-aged society, gastric cancer is the second most frequent cause of cancer-related death, because it is largely a disease associated with old age.1 Chronic obstructive pulmonary disease (COPD) is another major age-related public health problem, and its prevalence is increasing worldwide.2,3 COPD is characterized by persistent airflow limitation, resulting in cough, productive sputum, and dyspnea on exertion.4 It is also one of the major risk factors for postoperative pulmonary complications (POPCs). In gastric cancer patients undergoing upper abdominal surgery, including gastrectomy, POPCs are an important cause of postoperative morbidity and mortality because of the impaired wound healing associated with tissue hypoxia.5–7 Jeong et al8 reported that the independent predictor of postoperative morbidity after gastrectomy was abnormal spirometry findings as that of COPD. In the surgical treatment for gastric cancer, lymph node dissection and esophageal transection may lead subphrenic inflammation, resulting in pleural effusion. Wound pain, which suppresses sufficient respiratory movement and early ambulation, often leads to atelectasis. Therefore, in these patients, preoperative evaluation of pulmonary function is an important aspect of patient management.
Preoperative treatment with bronchodilators, antibiotics, and chest physiotherapy, and encouraging patients to quit smoking may decrease the incidence of POPCs in COPD patients.9 To reduce postoperative morbidities, COPD management might be important, and bronchodilators are thought to be a central to disease management. However, it is unknown whether bronchodilators prevent postoperative morbidities. Several reports have shown that tiotropium, a long-acting inhaled anticholinergic drug, improves dyspnea and lung function compared with placebo in patients with COPD.10,11 A significant bronchodilator response was shown within 30 minutes after the first dose of tiotropium and maintained over 24 hours.12 Therefore, tiotropium is the preferred maintenance therapy for patients with moderate-to-very severe COPD.13
The issue of whether preoperative tiotropium improves the postoperative outcome of patients with COPD is unclear. Kobayashi et al14 reported that preoperative use of inhaled tiotropium facilitated the surgical treatment of lung cancer patients with COPD. However, according to a recent report, while tiotropium significantly improved the preoperative global pulmonary function, there were no differences in outcomes, including postoperative complications (POCs) and postoperative stay, compared with the control group.15 Therefore, the aim of the present randomized Phase II study was to determine the efficacy of perioperative intervention with inhaled tiotropium in gastric cancer patients with COPD and to select an appropriate comparison arm for the next Phase III study.
Patients and methods
Eligibility
The patients enrolled in this study had histologically confirmed gastric adenocarcinoma and mild-to-moderate COPD. Before their enrollment in the study, the patients were diagnosed with COPD based on clinical symptoms or forced expiratory volume after 1 second (FEV1)/forced vital capacity (FVC) ratio of <0.7 after bronchodilator administration as determined by a preoperative pulmonary function test. The COPD stages were evaluated according to the FEV1 ratio as follows: stage I (mild COPD), FEV1 ratio >80% of predicted value; stage II (moderate COPD), FEV1 ratio between 50% and 80%; and stage III (severe COPD), FEV1 ratio between 30% and 50%. Other criteria for inclusion were: 1) Eastern Cooperative Oncology Group performance status of 0–2; 2) no significant cardiac failure or arrhythmia; and 3) preoperative interval for tiotropium use of >1 week.
The criteria for exclusion were: 1) previous history of asthma; 2) moderate-to-severe symptomatic prostatic hypertrophy; and 3) narrow-angle glaucoma.
This study was approved by the institutional review boards of each of the 15 participating institutions, and the procedures were performed in accordance with the Declaration of Helsinki. All patients provided written informed consent to participate in the study, in accordance with the institutional guidelines.
Study design
This was an open-label, randomized, multicenter Phase II study (UMIN 000004496). After checking eligibility, random assignment was carried out at the data center using a minimization method with the following adjustment factors: age (<75 versus ≥75 years), body mass index (<25 versus ≥25 kg/m2), cessation of smoking (yes versus no), COPD stage (1 versus 2), and surgical procedure (laparoscopic versus open). Patients were randomly assigned at a 1:1 ratio to receive perioperative pulmonary rehabilitation alone (control group) or pulmonary rehabilitation with 18 μg of tiotropium (Boehringer Ingelheim Pharma, Ingelheim, Germany) once daily (tiotropium group). Neither investigators nor patients were blinded to the allocated treatment. The patients in the tiotropium group were treated with tiotropium for more than 1 week before surgery and for 2 weeks after surgery. Spirometry was performed prior to group assignment and at 2 weeks after surgery. The operable patients with gastric cancer usually underwent gastrectomy 1–2 weeks after introduction to present participating instructions, and the length of postoperative hospital stay after gastrectomy is 10–14 days. Furthermore, Vincken et al12 showed that the values of both FEV1 and FVC improved just 1 week after tiotropium intervention, and those values were equivalent to the values treated for 1 year. These results support the idea that tiotropium given 1 week before and 2 weeks after may be sufficient for evaluating its efficacy. Preoperative pulmonary rehabilitation was performed according to Pulmonary Rehab Guidelines for COPD as follows: upper-body exercises; simple repetitive lifting of the arms against gravity, and exercises for breathing muscles; breathing through a mouthpiece against resistance.16
The primary endpoint was the rate of change in postoperative FEV1 compared with the preoperative value. The secondary endpoints included the number of days for O2 support after surgery, postoperative morbidity rate, and assessments of postoperative FVC and FEV1/FVC (%) compared with the pretreatment values.
Statistical analysis
This study was designed to assess the efficacy of preoperative pulmonary rehabilitation with and without perioperative tiotropium intervention. To simultaneously estimate the efficacy of these two treatments, and to minimize patient selection bias, we designed the study as a randomized Phase II study, rather than a comparative study. Sample size was calculated with an assumed reduction rate of more than 20% in postoperative FEV1, based on the results from a previous study.17 The tiotropium intervention would be regarded as a promising candidate for further evaluation, if a reduction rate in postoperative FEV1 could be achieved. Assuming a dropout rate of 5%, the target recruitment was 84 patients (42 per arm).
SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) was used for the statistical analyses. Quantitative data were calculated as means and standard deviations, and compared between the groups using Mann–Whitney U test. Other quantitative data were reported as proportion, and analyzed using the χ2 test. Values of P<0.05 were considered to indicate statistical significance.
Results
Flow diagram and patient demographics
Between December 2010 and May 2013, 84 patients were enrolled in this randomized study carried out in 15 hospitals in Japan. Of these, 42 patients were assigned to the control arm and 42 to the tiotropium arm. A flow diagram of the 84 enrolled patients is shown in Figure 1. One patient refused to undergo surgical treatment and another patient completed treatment with endoscopic submucosal dissection. The demographic characteristics of the remaining 82 patients are presented in Table 1. There were no significant differences in the characteristics of the two groups. After surgical treatment, spirometry could not be performed in two patients because of reoperation and unforced errors. Finally, 80 patients (38 patients in the control arm and 42 patients in the tiotropium arm) were evaluated.
  | Figure 1 Flow diagram of the 84 enrolled patients. |
  | Table 1 Characteristics of the 82 patients enrolled in the study |
Postoperative versus preoperative pulmonary function
Postoperative pulmonary function parameters, including FEV1, FVC, and FEV1/FVC (%), were evaluated in comparison with their preoperative values. Mean decreased rate of FEV1 was 1.12% in the control group and −0.73% in the tiotropium group. A decrease in FEV1 of more than 20% was seen in only three patients (7.9%) in the control group and four patients (9.5%) in the tiotropium group (Figure 2). There was no significant difference in FEV1 between the two groups. Mean rate of FVC compared with preoperative value was 93.1% in the control group and 92.1% in the tiotropium group. A reduction in FVC of more than 20% was seen in three patients (7.9%) in the control group and in nine patients (21.4%) in the tiotropium group (Figure 3). No significant difference was found in FVC between the two groups. Mean changed FEV1/FVC (%) was 4.7% in the control group and 6.7% in the tiotropium group. Although there was no significant difference in FEV1/FVC among two groups (Figure 4), the COPD severity decreased in 18 patients in the control group and in 24 patients in the tiotropium group. None of these patients had spirometry results that indicated COPD (Table 2). The mean length O2 support after surgery was 3 (range: 1–10) days in the control group and 3 (range: 1–16) days in the tiotropium group. There was no significant difference between the two groups.
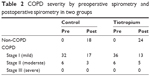  | Table 2 COPD severity by preoperative spirometry and postoperative spirometry in two groups |
Postoperative complications
As shown in Table 3, 19 patients (23.2%) developed POCs according to the Clavien–Dindo classification (grade II and above), including five cases of pneumonia. There were no significant differences between the patients with and without perioperative tiotropium intervention. Table 4 lists the POCs in the two groups according to risk factors for POCs. Perioperative tiotropium intervention significantly decreased the rate of POCs in patients who are smokers (P=0.044) and moderate COPD (P=0.046). Also, there were no significant differences in incidence of POCs among surgical sites. The incidence of POCs in patients who underwent total gastrectomy or proximal gastrectomy was considered to be higher than in those who underwent distal gastrectomy, because left diaphragm inflammation associated with surgical procedures resulted in pleural effusion or atelectasis.
  | Table 3 Postoperative complications after gastrectomy |
Discussion
This study is the first multicenter, prospective, randomized Phase II study to evaluate the efficacy of perioperative tiotropium treatment for gastric cancer patients with COPD. Multiple factors, including advanced age, obesity, smoking, incision site, and operation time, are responsible for the genesis of POPCs.18 In this study, the patients were randomized after stratification for these risk factors, allowing the efficacy of tiotropium intervention to be rigorously evaluated (Table 1).
Following upper abdominal surgery, including gastrectomy, pulmonary function usually decreases because of reduced diaphragmatic activity and microatelectasis, which in turn may result in POPCs. Based on the literature, we expected that postoperative pulmonary function would be better in the tiotropium group than in the control group. Casaburi et al19 found that the combination of tiotropium and pulmonary rehabilitation resulted in greater improvement in exercise tolerance compared with pulmonary rehabilitation alone. However, in our study, there were no significant differences in postoperative pulmonary function between the control and tiotropium groups (Figures 2–4). In a meta-analysis study, the values of FEV1 and FVC were found to be reduced by almost 50% after open cholecystectomy and by 19%–27% after laparoscopic cholecystectomy.17 It is not thought to be necessary to treat patients with mild COPD. However, we expected that tiotropium intervention might be useful for patients who received gastrectomy combined with lymph node dissection, because of its high invasiveness compared with cholecystectomy. Although the postoperative values of FEV1 and FVC were initially expected to decrease by more than 20% in the control group, only a few patients showed the expected values. Spirometry should be performed three times: before randomization, just before surgery, and at 2 weeks postoperatively. However, conduction of three spirometry tests for gastrectomy is not allowed by health insurance schemes. The reduction rate for FEV1 might be large, if the values just before surgery and after surgery are compared. Interestingly, the postoperative spirometry results in many of our patients with mild COPD were the same as those of non-COPD gastric cancer patients (Table 2). This implies that, in gastric cancer patients with mild COPD, pulmonary rehabilitation alone can result in sufficient improvement even within the limited preoperative period.
Unfortunately, our results did not provide any evidence for differences in the frequency of POCs, including POPCs, between the control and tiotropium-treated patients. However, this study had important limitations: the number of patients with moderate COPD was small and patients with severe COPD were not included. Nonetheless, by stratifying patients according to risk factors for POPCs, the effects of tiotropium intervention could be evaluated accurately. Thus, a subgroup analysis showed that perioperative tiotropium intervention significantly decreased the rate of POCs in patients who are smokers and with moderate COPD. Kobayashi et al14 also reported that the response to tiotropium was more effective in lung cancer patients in accordance with the severity of COPD.
In conclusion, although patients undergoing upper abdominal surgery are prone to respiratory complications, among those with mild COPD, simple pulmonary rehabilitation alone may be sufficient to avoid such complications. Despite the absence of differences in postoperative outcomes between our control and tiotropium-treated patients, perioperative tiotropium intervention may still be beneficial for gastric cancer patients with moderate-to-severe COPD. A study based on a larger sample size of gastric cancer patients with moderate-to-severe COPD is needed to further evaluate the efficacy of perioperative tiotropium intervention.
Acknowledgment
This study was financially supported by the Digestive Disease Support Organization (DDSO).
Disclosure
The authors report no conflicts of interest in this work.
References
Maehara Y, Emi Y, Tomisaki S, et al. Age-related characteristics of gastric carcinoma in young and elderly patients. Cancer. 1996;77(9):1774–1780. | ||
Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–773. | ||
De Marco R, Pesce G, Marcon A, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS One. 2013;8(5):e62985. | ||
Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. | ||
Arozullah AM, Khuri SF, Henderson WG, Daley J; Participants in the National Veterans Affairs Surgical Quality Improvement Program. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med. 2001;135(10):847–857. | ||
Brooks-Burnn JA. Predictors of postoperative pulmonary complications following abdominal surgery. Chest. 1997;111(3):564–571. | ||
Kanat F, Golcuk A, Teke T, Golcuk M. Risk factors for postoperative pulmonary complications in upper abdominal surgery. ANZ J Surg. 2007;77(3):135–141. | ||
Jeong O, Ryu SY, Park YK. The value of preoperative lung spirometry test for predicting the operative risk in patients undergoing gastric cancer surgery. J Korean Surg Soc. 2013;84(1):18–26. | ||
Gracey DR, Divertie MB, Didier EP. Preoperative pulmonary preparation of patients with chronic obstructive pulmonary disease: a prospective study. Chest. 1979;76(2):123–129. | ||
Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19(2):217–224. | ||
Van Noord JA, Bantje TA, Eland ME, Korducki L, Cornelissen PJ. A randomized controlled comparison of tiotropium and ipratropium in the treatment of chronic obstructive pulmonary disease. Thorax. 2000;55(4):289–294. | ||
Vincken W, van Noord JA, Greefhorst APM, et al. Improved health outcomes in patients with COPD during 1 yr’s treatment with tiotropium. Eur Respir J. 2002;19(2):209–216. | ||
Global initiative for chronic Obstructive Lung Disease (GOLD). Global Strategy for Diagnosis, Management, and Prevention of COPD. Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed December 25, 2014. | ||
Kobayashi S, Suzuki S, Niikawa H, Sugawara T, Yanai M. Preoperative use of inhaled tiotropium in lung cancer patients with untreated COPD. Respirology. 2009;14(5):675–679. | ||
Ueda K, Tanaka T, Hayashi M, Hamano K. Role of inhaled tiotropium on the perioperative outcomes of patients with lung cancer and chronic obstructive pulmonary disease. Thorac Cardiovasc Surg. 2010;58:38–42. | ||
Nici L. Adherence to a pulmonary rehabilitation program: start by understanding the patient. COPD. 2012;9(5):445–446. | ||
Ravimohan SM, Kaman L, Jindal R, Singh R, Jindal SK. Postoperative pulmonary function in laparoscopic versus open cholecystectomy: prospective, comparative study. Indian J Gastroenterol. 2005;24(1):6–8. | ||
Kocabaş A, Kara K, Özgür G, Sönmez H, Burgut R. Value of preoperative spirometry to predict postoperative pulmonary complications. Respir Med. 1996;90(1):25–33. | ||
Casaburi R, Kukafka D, Cooper CB, Witek TJ Jr, Kesten S. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest. 2005;127(3):809–817. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




