Back to Journals » Clinical Ophthalmology » Volume 15
A Randomized, Controlled, Prospective Study of the Effectiveness and Safety of an Intracanalicular Dexamethasone Ophthalmic Insert (0.4 Mg) for the Treatment of Post-Operative Inflammation in Patients Undergoing Refractive Lens Exchange (RLE)
Authors Larsen J, Whitt T, Parker B , Swan R
Received 17 March 2021
Accepted for publication 18 May 2021
Published 27 May 2021 Volume 2021:15 Pages 2211—2217
DOI https://doi.org/10.2147/OPTH.S311070
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jacob Larsen,1 Travis Whitt,2 Briana Parker,1 Russell Swan3,4
1Clinical Research, Vance Thompson Vision, Bozeman, MT, USA; 2Department of Optometry, Vance Thompson Vision, Bozeman, MT, USA; 3Department of Ophthalmology, Vance Thompson Vision, Bozeman, MT, USA; 4Ophthalmology Moran Eye Center, University of Utah, Salt Lake City, UT, USA
Correspondence: Jacob Larsen
Clinical Research, Vance Thompson Vision, Bozeman, MT, USA
Tel +1 406-544-2140
Fax +1 406-624-6560
Email [email protected]
Purpose: To determine patient preference and treatment outcomes with an intracanalicular dexamethasone (0.4 mg) insert compared to standard steroid drop regimen in the contralateral eye following bilateral RLE surgery.
Methods: This is a prospective, open-label, interventional, randomized, controlled study in 20 subjects who underwent bilateral RLE. Each patient served as their own control with one eye randomized to the intracanalicular insert (Group A) placed at the time of surgery and the contralateral randomized to topical corticosteroid drops (Group B). All eyes received intracameral moxifloxacin at the time of surgery, and post-operatively, topical moxifloxacin QID for one week and topical NSAID daily for four weeks. Post-operative evaluations were performed on Day 1, Week 1, and Week 4– 8.
Results: Twenty patients participated. At 4– 8 weeks post-operation, 90% of patients evaluated with the COMTOL questionnaire preferred the intracanalicular insert while 10% preferred the topical steroid. Comparative analysis using the visual analog scale showed no difference in pain between the study and control group. No statistical difference was shown in post-operative corneal staining, anterior chamber cell count, anterior chamber flare or intraocular pressure. Mean LogMAR UCVA at 4– 8 weeks post-operation was 0.06 (± 0.230) in the study group and 0.065 (± 0.241) in the control group, which was not statistically or clinically different (p > 0.05).
Conclusion: Patients undergoing bilateral RLE expressed a strong preference towards the use of an intracanalicular insert over a topical steroid for post-operative steroid treatment. There was no clinically or statistically significant difference in outcomes, including rate of cystoid macular edema, visual acuity and elevation of intraocular pressure.
National Clinical Trial Number: 04549935.
Keywords: punctal plug, steroid, visual acuity, cystoid macular edema, patient preference
Introduction
Refractive Lens Exchange (RLE) is a well-studied and common refractive surgery option for patients seeking to improve their vision. RLE offers patients a method for eliminating their refractive error by surgically removing their intraocular lens and replacing it with an appropriate implant lens. It is commonly used when patients do not meet the requirements for other forms of refractive surgery or desired presbyopic correction. The key factors in post-operative evaluation are monitoring the tear film and subsequent corneal staining, evaluating the anterior chamber (AC) for ocular inflammation, ensuring the intraocular pressure (IOP) is within standard ranges, determining if the posterior chamber intraocular lens is centered and properly positioned and evaluating the vitreous and retina for significant signs of inflammation or cystoid macular edema (CME). It is also important to monitor and prevent infection within the eye, better known as endophthalmitis. Post-operatively patients are treated with antibiotic therapy to prevent infection, steroid drops to reduce inflammation and non-steroidal anti-inflammatory drugs (NSAIDs) for pain management and retinal inflammation.
The use of drops post-RLE is considered by most to be standard of care, but the treatment regimens vary depending on the surgeon and practice preference. Difficulties with post-operative drop treatments are frequently encountered from both the patient and physician’s perspective. The intracanalicular insert may reduce the challenges often seen with physician offices for obtaining prior authorization for ophthalmic drops. Patients routinely struggle with drop installation, timing, and compliance. With RLE, it is critical to the visual outcome, overall health, and safety of the eye for this post-operative regime to be both convenient and effective.
The intracanalicular insert is a recently approved, sustained release form of dexamethasone. The insert is a polyethylene glycol hydrogel rod containing 0.4 mg of the preservative free steroid dexamethasone and is used for the treatment of pain and post-operative inflammation in patients who have undergone RLE and other forms of ocular surgery. The intracanalicular insert is placed into the canaliculus through the punctum, swells upon hydration and anchors into place.1 As it dissolves, it provides a sustained and tapered release of dexamethasone for up to 30 days without the need for post-treatment removal.1 Due to the presence of fluorescein, the implant is easily visualized with a cobalt blue light and can be placed during the pre-operative period, during the procedure or at a post-operative appointment.
The intracanalicular insert has been evaluated in three Phase 3 trials for the control of postoperative pain and inflammation following phacoemulsification cataract surgery.1,2 Phase 3 trials of the intracanalicular insert versus placebo showed a statistically significant superiority (p < 0.0001) in the absence of anterior chamber cells and lack of ocular pain.2 The insert was also “well-tolerated” and expressed a similar adverse event profile to the placebo.2 When compared to the standard drop regime for RLE surgery, the insert will provide patients with fewer post-operative drops, a preservative free medication and punctual occlusion which will increase tear volume and aid in ocular surface rehabilitation. This will improve compliance and proper drug utilization, reduce the toxic effects of multiple drops placed on the surface of the eye and decrease irritation to the surface of the cornea from preservatives. This prospective contralateral ocular study will compare the outcomes of 20 patients undergoing bilateral RLE surgery. In one eye, the patient will receive a topical antibiotic, NSAID and steroid. The contralateral eye will receive a topical antibiotic and NSAID, as well as the dexamethasone ophthalmic insert.
Materials and Methods
This was a randomized, open-label, single site contralateral eye study. The protocol was reviewed and approved by the ethics committee (ASPIRE IRB) on June 26th, 2020. All participants provided written informed consent and the study was performed in accordance with the Declaration of Helsinki. Each study participant was provided with a fully signed copy of the informed consent. Participants were enrolled from the first initial screening on September 4th, 2020 to the final visit completed on December 21st, 2020.
All participants in the study were above the age of 22, under the age of 75, and voluntarily agreed to undergo bilateral RLE surgery. Pre-operatively, patients included in the trial were required to have the best-corrected visual acuity of 20/30 or better and be willing and able to comply with clinic visits, study-related procedures and sign the IRB approved informed consent form. Exclusion criteria included patients who were pregnant, actively treated with immunosuppression, had history of dexamethasone hypersensitivity, history of concurrent ocular comorbidities (such as ocular inflammatory disease, macular edema, and proliferative diabetic retinopathy), history of corticosteroid implant, inability to receive an intracameral antibiotic, use of systemic NSAIDs > 1200 mg/day, concurrent current corticosteroid therapy (including ocular implants) or manifested corneal pathology that might interfere with surgical outcomes. A total of 20 subjects met all inclusion criteria and none of the exclusion criteria.
All potential subjects underwent a screening/baseline examination visit which included obtaining consent, demographics, concomitant medications, medical/ocular history, and detailed ophthalmological examination to determine if they met the inclusion criteria. The first eye was randomized to either group A or B, while the second eye was selected for the opposite group. Group A received an intracanalicular insert at the time of surgery. Group B eyes received topical dexamethasone 0.1% 4x/day 1 week followed by 3x/day for 1 week, 2x/day for 1 week and then 1x/day for 1 week. All eyes received intracameral moxifloxacin at the time of surgery, and post-operatively, topical moxifloxacin QID for one week and topical bromfenac 0.07% daily for four weeks.
All enrolled patients underwent same-day bilateral RLE. The procedure was performed using a 2.4 mm corneal incision temporally followed by an anterior capsulorhexis. The cortex was separated from the capsule using BSS hydrodissection. This was followed by phacoemulsification of the entire lens material with subsequent polishing of the posterior capsule. Viscoelastic was then placed inside the eye and the implant was inserted. The implant was then properly centered and positioned and the viscoelastic was removed. Finally, intracameral moxifloxacin was administered and proper wound closure was determined. Patients were re-evaluated on post-operative day 1, day 7 and week 4–8.
The goal of the study was to compare the use of a topical steroid to the intracanalicular insert and determine patient preference. This was assessed using a modified Comparison of Ophthalmic Medications for Tolerability (COMTOL) questionnaire, which has been validated for consistency, reliability, and reproducibility.3 The survey was developed to assess the frequency and severity of medication side effects and determine how the side-effects impaired daily routine and effect quality of life.3 The secondary goal of the study was to determine the effect of the dexamethasone intracanalicular insert by comparing the AC cell count, AC flare, ocular pain, IOP and incidence of CME for the study eye to the control eye. An IOP increase > 10 mmHg above baseline was considered abnormal.
Statistical analysis of the COMTOL survey was performed using descriptive statistics and one sample proportion test. Visual acuity, logarithm of the minimum angle of resolution (logMAR), scores for the control and study eye were analyzed using a paired t-test and graphical analysis. The level of significance for all statistical analyses was set at 0.05.
Results
A total of 20 subjects (40 eyes) participated in this study. All were Caucasian, 13 (65%) were male, 7 (35%) were female and their average age was 54.6 years. Twenty subjects successfully completed an initial screening followed by a post-operative visit at day 1, day 7 and week 4–8. The COMTOL questionnaire showed 18 patients (90%) preferred the intracanalicular insert, while 2 patients preferred drops (10%) and zero patients showed no preference (Figure 1). Fourteen (70%) patients reported they were “totally satisfied”, 5 (25%) “very satisfied” and 1 (5%) “somewhat satisfied” with their preferred treatment option (Figure 2). The occurrence of side effects was nearly identical for both the control and study eye (Figure 3). In both eyes, the average reported frequency never reached “a few times” (Figure 3). Most patients cited “I did not have the symptom” and “rarely” for the frequency of side effects (Figure 3).
 |
Figure 1 Patient preference for intracanalicular insert or dexamethasone topical drops assessed using the comparison of ophthalmic medications for tolerability (COMTOL) questionnaire. |
 |
Figure 2 Level of satisfaction with preferred treatment method as reported by the comparison of ophthalmic medications for tolerability (COMTOL) questionnaire. |
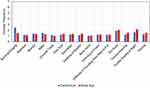 |
Figure 3 Average side effect frequency; 1–7: I did not have the symptom, rarely, a few times, often, usually, almost always, always. |
The mean logMAR uncorrected visual acuity (UCVA) for the study group improved from 0.61 (±.420) pre-operatively to 0.06 (±.230) at 4–8 weeks post-operation (Figure 4). Comparatively, that of the control group improved from 0.521 (± 0.415) pre-operatively to 0.065 (± 0.241) at 4–8 weeks post-operation (Figure 4). There was not a clinically or statistically significant difference between the UCVA of the control and study group (p > 0.05). There was no change in best-corrected visual acuity (BCVA) between pre-operation and 4–8 weeks post operation for either the control or study group. The mean logMAR BCVA for the study group was −.075 (± 0.102) pre-operatively and −.075 (± 0.129) at 4–8 weeks post-operation. Comparatively, that of the control group was −0.1 (± 0.097) pre-operatively and −0.1 (± 0.097) at 4–8 weeks post-operation. There was not a clinically or statistically significant difference between BCVA of the control and study group (p > 0.05).
 |
Figure 4 Uncorrected visual acuity, logarithm of the minimum angle of resolution (LogMAR), as a function of days after surgery. |
During the study, no evidence of cystoid macular edema was noted via optical coherence tomography at 4–8 weeks post-op. Two subjects did post-operatively manifest an IOP ≥ 10 mmHg from the baseline. The two cases were in different patients and one occurred in a control eye, while the other was in a study eye. In both cases, the subject was started on anti-ocular hypertensive therapy to help lower their IOP. Following rescue therapy, both subjects demonstrated an IOP within the safety limits and were able to stop drop therapy and maintain a normal IOP. In addition, no rebound inflammation was present for any patients in either the control or study eye at 4–8-week post-operation. Furthermore, no patients had an average corneal staining at the 4–8-week post-operation of greater than 1 in either eye. Finally, there was no visually significant Posterior Capsule Opacification (PCO) in either group that required YAG laser capsulotomy.
Discussion
Patients undergoing a bilateral RLE expressed a strong preference for a steroid intracanalicular insert in place of a topical steroid for post-operative healing. The primary reason subjects preferred the insert was due to convenience. Previous research on eyedrop administration following cataract surgery has showed that 31% of patients reported difficulty instilling eye drops and 92.6% showed an improper administration technique.4 In addition, nearly half of individuals prescribed topical ocular hypotensive drops halt therapy within 6 months.5 Thus, it was expected that patients would prefer to use the insert to replace topical drops. Given the challenge often seen with placing drops in the elderly population due to difficulties with manual dexterity, it seems likely they would be more accepting of the treatment regimen with fewer drops. The outcome of this study was also consistent with the results found in the phase 3 trials for the use of an intracanalicular dexamethasone insert following cataract surgery in which most patients (92%) reported the highest level of satisfaction with the insert.6 The biggest advantage of using the intracanalicular insert over a traditional topical regime is the ability to achieve a controlled, sustained, and tapered drug delivery. The insert provides a uniform distribution of medicine which may be more beneficial for healing than pulse doses of topical drops. Other potential benefits of using the intracanalicular insert over a topical drop include increased tear volume, no preservatives, and fewer post-operative drops. These benefits have the potential to increase patient compliance and improve the overall healing process. The use of an intracanalicular insert also has the potential to increase clinic efficiency by decreasing the number of prescriptions that are filled. Approximately 3,000 staff hours annually, or 1.5 full-time equivalents are spent responding to patient and pharmacy phone calls regarding post-cataract surgery drop regime.7 Decreasing the number of prescriptions is beneficial for the practice, physician, and patient. It has the potential to save both time and money for all parties involved.
The primary goal of RLE surgery is to remove the cataract and achieve the best possible uncorrected visual acuity. The results of this study show that visual outcomes for the control and study eye were nearly identical. Both groups demonstrate a UVCA better than 0.07 at 4–8 week visit post-operation and a BCVA better than −0.075. There was no statistically significant difference in visual outcomes between the groups. Additionally, no patients in either group experienced a loss of BCVA from the pre-operative to 4–8-week post-operation visit. Secondary endpoints included corneal staining, inflammation, IOP, pain and overall level of comfort. None of these parameters demonstrated a statistically significant difference between the control and study eye. However, the rate of steroid induced ocular hypertension seen in this study is slightly higher than in previous similar case series (2.4%). Of note, younger patients and those with longer axial lengths had an increased risk for increased steroid response.8,9 In our study, 2/40 eyes (5%) of patients developed steroid induced ocular hypertension, all of which self-resolved without complication. This higher rate could be related to the younger age of our patient population, their relative pre-operative myopia, or small sample size.
This study included several parameters to increase the statistical significance of the results. Patient randomization eliminates the possibility of bias and allows for comparability. The use of a contralateral eye design allowed each patient to receive both the traditional treatment (control) and the study treatment (intracanalicular insert). This allowed subjects to directly compare the two and determine their preference based on physical experience rather than perception. The main limitation of this study was the small sample size. Future studies should include a larger sample size to confirm the findings. It would also be beneficial to perform a study in which the antibiotic, steroid and NSAID were injected at the time of surgery followed by placement of the steroid insert versus steroid drop. This method would allow an entirely dropless post-operative experience in the implant eye. Finally, ongoing efforts to combine multiple medications into the insert (steroid and NSAID, or steroid, NSAID and antibiotic) would be useful as well.
Conclusion
Post-operatively, patients undergoing bilateral RLE expressed a strong preference for the intracanalicular insert in comparison to a topical steroid. The intracanalicular insert produced statistically similar outcomes for visual acuity, corneal staining, inflammation, pain, rate of CME and ocular comfort as the traditional topical drop regimen. Two subjects did manifest an IOP ≥ 10 mmHg from baseline, but following rescue therapy, both subjects stopped drop therapy and were able to maintain a normal IOP. Overall, patients preferred the intracanalicular insert due to its simplicity, convenience, and minimally invasive effect. This study proves that the intracanalicular dexamethasone insert is both a safe and effective method for post-operative treatment of inflammation following RLE surgery.
Data Sharing Statement
Able to provide de-identified conglomerate data upon request to Jacob Larsen.
Acknowledgments
Authors acknowledge support for this investigator initiator trial from Ocular Therapeutix.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Walters T, Bafna S, Vold S, et al. Efficacy and safety of sustained release dexamethasone for the treatment of ocular pain and inflammation after cataract surgery: results from two phase 3 studies. J Clin Exp Ophthalmol. 2016;7:1–11. doi:10.4172/2155-9570.1000572
2. Tyson SL, Bafna S, Gira JP, et al. Multicenter randomized phase 3 study of a sustained release intracanalicular dexamethasone insert for treatment of ocular inflammation and pain after cataract surgery. [published correction appears in J Cataract Refract Surg. 2019;45(6):895]. J Cataract Refract Surg. 2019;45(2):204–212. doi:10.1016/j.jcrs.2018.09.023
3. Barber BL, Strahlman ER, Laibovitz R, Guess HA, Reines SA. Validation of a questionnaire for comparing the tolerability of ophthalmic medications. Ophthalmology. 1997;104(2):334–342. doi:10.1016/S0161-6420(97)30314-5
4. An JA, Kasner O, Samek DA, et al. Evaluation of eyedrop administration by inexperienced patients after cataract surgery. J Cataract Refract Surg. 2014;40(11):1857–1861. doi:10.1016/j.jcrs.2014.02.037
5. Nordstrom BL, Friedman DS, Mozaffari E, et al. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4):598–e1. doi:10.1016/j.ajo.2005.04.051
6. Gira JP, Sampson R, Silverstein SM, Walters TR, Metzinger JL, Talamo JH. Evaluating the patient experience after implantation of a 0.4 mg sustained release dexamethasone intracanalicular insert (Dextenza): results of a qualitative survey. Patient Prefer Adherence. 2017;11:487–494. doi:10.2147/PPA.S126283
7. Lindstrom RL, Galloway MS, Grzybowski A, Liegner JT. Dropless cataract surgery: an overview. Curr Pharm Des. 2017;23(4):558–564. doi:10.2174/1381612822666161129150628
8. Chang DF, Tan JJ, Tripodis Y. Risk factors for steroid response among cataract patients. J Cataract Refract Surg. 2011;37(4):675–681. doi:10.1016/j.jcrs.2010.10.051
9. Schallhorn SC, Schallhorn J, Pelouskova M, et al. Refractive lens exchange in younger and older presbyopes: comparison of complication rates, 3 months clinical and patient-reported outcomes. Clin Ophthalmol. 2017;11:1569. doi:10.2147/OPTH.S143201
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
