Back to Journals » Clinical Ophthalmology » Volume 13
A prospective pilot study using a low power piggy-back toric implantable Collamer lens to correct residual refractive error after multifocal IOL implantation
Authors Duncker GIW, Sasse AC, Duncker T
Received 21 June 2019
Accepted for publication 15 August 2019
Published 3 September 2019 Volume 2019:13 Pages 1689—1702
DOI https://doi.org/10.2147/OPTH.S219738
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Gernot IW Duncker, Anna C Sasse, Tobias Duncker
Institute of Ophthalmology, Halle, Germany
Correspondence: Tobias Duncker
Institute of Ophthalmology, Rathenauplatz 12, 06114 Halle, Germany
Email [email protected]
Purpose: To assess whether residual refractive error after in-the-bag multifocal intraocular lens (mIOL) implantation can efficaciously and safely be corrected with a piggy-back low power Visian Toric Implantable Collamer Lens® (VTICL, STAAR Surgical) placed in the ciliary sulcus.
Patients and methods: Twenty-four eyes of 23 patients (mean age: 57.5 years) with diminished uncorrected distance visual acuity (UDVA) of ≥2 lines due to residual refractive error after mIOL implantation were included in the study. VTICL size was calculated using the standard STAAR Visian ICL calculation software for phakic eyes. Postoperative study visits (1 day, 1 week, 3 months and 6 months after VTICL implantation) included UDVA, corrected distance visual acuity (CDVA), VTICL axis alignment, vault (space between mIOL and VTICL), IOP and documentation of adverse events.
Results: At 6 months, mean UDVA (logMAR) increased from 0.26 preoperatively to −0.01 (P<0.001) while mean CDVA remained unchanged. Mean VTICL misalignment from the preoperative target axis was 5.3° and mean vault was 1385 μm. In the initial phase of the study, 2 VTICL had to be exchanged due to oversizing.
Conclusion: Piggy-back low power VTICL can efficaciously correct residual refractive error after mIOL implantation and significantly increase UDVA. Advantages of this novel surgical approach include: VTICL availability in small diopter steps, no significant surgical-induced astigmatism, atraumatic and reversible procedure.
Keywords: multifocal intraocular lens, ICL, Visian Implantable Collamer Lens®; piggy-back, add-on, residual refractive error
Introduction
Implantation of multifocal intraocular lenses (mIOL) is a successful procedure to achieve spectacle independence by correcting presbyopia and concomitant refractive error. However, postoperative emmetropia is not achieved in all patients. Minor refractive errors remain in 10–25% of cases,1–3 which can significantly diminish uncorrected visual acuity for both near and far vision. Factors that can influence the postoperative refraction include wound healing deficiencies of the tunnel, shrinkage of the capsular bag with shifting of the lens position, late rotation of a toric mIOL, accuracy of the biometry and potentially also floppy or decentered haptics.1 Multifocal IOL patients, who are not emmetropic after surgery, may be disappointed as spectacle independence was the main motivation for undergoing the procedure.4
Different surgical approaches have been established to correct remaining refractive errors after mIOL implantation. One option is a secondary excimer laser correction, either as surface ablation or LASIK.2,5–7 However, these procedures are known to have a negative impact on contrast sensitivity, which is already reduced by the mIOL and further higher-order aberrations may be induced by the laser procedure.8 Another possibility is an exchange of the mIOL, which, however, is more invasive and the postoperative refraction remains somewhat uncertain due to induced astigmatism from the enlarged incision size. In patients who have already undergone a posterior YAG-capsulotomy, an mIOL exchange is even more challenging and an anterior vitrectomy may be necessary. One may also consider correcting residual refractive error after mIOL implantation with secondary arcuate incisions. However, even if the arcuate incisions are performed by femtosecond laser, they are not precise in correcting the spherical refractive error and tend to undercorrect the cylinder. In a retrospective study from Lüdeke et al,9 10% of patients needed additional laser enhancement. Similar to other corneal refractive procedures, long-term regression of the treatment effect may also occur after arcuate incisions.
An alternative approach is the insertion of an additional piggy-back implant designed for pseudophakic eyes into the ciliary sulcus.10–12 Implantable collamer lenses (ICL) are currently only approved for phakic eyes. However, some case reports have indicated that piggy-back implantation of an ICL to correct residual refractive error after IOL implantation may also be a promising option.13–21 There are several theoretical advantages of the ICL approach. Since the incision size can be smaller than 2.0 mm, surgical-induced astigmatism is minimal. The low power Visian Toric Implantable Collamer Lens® (VTICL, STAAR Surgical, Monrovia, CA, USA) is available in small diopter (D) steps, allowing for a precise target refraction. Due to the flexible collamer material, the surgery is atraumatic and easily reversible.
Given these potential advantages compared to other surgical approaches, we decided to investigate in a larger cohort whether an off-label piggy-back implantation of a low power VTICL can be used as an elegant and effective approach to correct remaining refractive error after mIOL implantation. To our knowledge, this is the first prospective study investigating the efficacy and safety of this surgical technique.
Methods
VTICL types used in the study
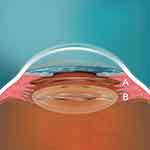
The VTICL is available as a low power version either without (V4b) or with an aquaport (V4c) (Figure 1). The aquaport is a central hole in the VTICL optic with a diameter of 360 µm which facilitates aqueous circulation. The low power VTICL is available in 0.25 D steps from −3 D to +3 D for spherical correction and in 0.50 D steps for up to 6 D astigmatism correction. There are 4 different VTICL sizes (diameter of the haptics) available: 12.1, 12.6, 13.2 and 13.7 mm.
 |
Figure 1 The Visian Toric Implantable Collamer Lens® (VTICL, STAAR Surgical, USA) is available either without (V4b) or with an aquaport (V4c). |
Study subjects
We prospectively recruited patients at a single center who had reduced uncorrected distance visual acuity (UDVA) due to remaining refractive error with astigmatism after mIOL in-the-bag implantation. The mIOL implantation had either been performed at our institute or elsewhere and took place at least 3 months before enrollment in the study. The following refractive errors were included: myopic astigmatism with a spherical equivalent (SE) of at least −0.75 D, hyperopic astigmatism with a SE of at least +0.75 D, and any mixed astigmatism with a spherical error of at least 0.5 D and a cylindrical error of at least 0.5 D. We included only eyes with an expected UDVA increase of at least two lines in a Snellen chart after VTICL implantation.
Eyes with an anterior chamber depth (ACD) of <3 mm were excluded. However, our definition of the ACD changed during the course of the study. Initially, we defined ACD as the distance between endothelium and the anterior mIOL surface. After the first 7 patients, we redefined ACD as the distance from endothelium to the mid-iris plane. We also excluded eyes showing asymmetric mIOL fixation (one haptic in the bag, the other haptic in the sulcus), a mesopic pupil diameter of >7 mm, a history of ocular trauma, additional previous ocular surgery such as corneal transplantation, refractive laser procedures or glaucoma surgery. Further exclusion criteria were a history of glaucoma, uveitis, or any other pathologic eye condition that may affect visual outcome, including diabetic retinopathy, iris neovascularization, any kind of macular edema, corneal dystrophies or ectasia, excessive myopia beyond −20 D or suspected amblyopia in the study eye. Also excluded were patients requiring YAG-capsulotomy due to decreased corrected distance visual acuity (CDVA) secondary to posterior capsule opacification during the course of the study.
All procedures adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all subjects after a full explanation of the study procedures. It was explained to all patients that the VTICL is currently only approved for phakic eyes and that the use of the implant in pseudophakic eyes is an off-label procedure. The study was approved by the local ethics committee of the Medical Association Saxony-Anhalt, Germany.
Study design and endpoints
The primary objective of this study was to assess the efficacy of the surgical approach. Corresponding study endpoints included: change in UDVA and CDVA, predictability (i.e., intended correction vs achieved correction for spherical and cylindrical components), stability of the refractive outcome (i.e., change in sphere and cylindrical outcomes over time) and whether any rotation of the implanted VTICL occurred over time.
The secondary objective of this study was to assess the safety of the VTICL piggy-back sulcus implantation. We therefore measured changes in IOP, ACD, vault (the space between both implants), distance between the corneal endothelium and the VTICL, and the iridocorneal angle (ICA). Any adverse events and necessary secondary interventions were documented.
Surgical procedure
All surgeries were performed by one of the authors (GIWD). For patients with a VTICL without aquaport (V4b, see Figure 1), 2 peripheral iridotomies were performed at the 11 and 1 o’clock position at least 1 week before the surgical procedure using a standard YAG laser to prevent a pupillary block and secondary angle closure after surgery. We did not consider a Pigment Vacuum Iridectomy even though this technique may offer advantages compared to the conventional YAG laser procedure.22 Preoperatively, a reference picture of the limbal blood vessels was taken in upright position with either the SensoMotoric Instruments (SMI) device (Teltow, Germany) or more recently with the Verion device (Alcon, Fort Worth, TX, USA). Based on this reference image, the limbal vessel pattern was automatically recognized intraoperatively and the steep axis was displayed in the ocular of the surgeon’s microscope. This eye-tracking methodology minimizes intraoperative alignment errors that may occur due to cyclorotation of the eye. This technique is superior to marking the axis on the cornea, which on average produces an error of about 5° (R. Zaldivar, personal communication).
The pupil was dilated with 1% tropicamide and 2.5% phenylephrine, and surgery was performed under topical anesthesia. The VTICL was loaded into the cartridge and inserted into the STAAR injector. A 3.2 mm-clear cornea incision close to the limbus was performed temporally and one paracentesis was placed in the upper nasal quadrant. After filling 1.6% hydroxypropylmethylcellulose (HPMC) into the anterior chamber, the VTICL was injected through the incision and the foot plates were tucked under the pupillary margin into the ciliary sulcus using a Vukich ICL manipulator (ASICO, Westmont, IL, USA). The VTICL was then rotated in order to match the predefined target axis for implantation which was provided by STAAR based on the preoperative calculations. After checking the proper alignment of the VTICL, HPMC was removed by manually rinsing the anterior chamber with Ringer’s solution. The paracentesis was sealed by hydrating the incision site. At the end of the VTICL implantation, 1 mg intracameral cefuroxime (0.1 mL) was injected as a prophylaxis for endophthalmitis. An eye patch with dexamethasone and gentamicin ointment was applied for 1 night. For the first postoperative week, dexamethasone-gentamicin eye drops were given 5 times per day. During the postoperative weeks 2–4, dexamethasone-gentamicin eye drops were given 3 times per day. The position of the VTICL after surgery is shown in Figure 2.
Examinations
Each patient was examined preoperatively (0–60 days before surgery) and 1 day, 1 week, 3 months and 6 months after surgery. Preoperatively, the mIOL type was documented as well as refraction and biometry data before mIOL implantation. This was important since the VTICL calculation (using the STAAR Visian ICL calculation software for phakic eyes) is different depending on whether the eye was myopic or hyperopic before the mIOL procedure. Furthermore, ocular biometry using the IOL Master 500 (Carl Zeiss, Jena, Germany) and mesopic and scotopic binocular pupillometry (Procyon P3000; Procyon Instruments, London, UK) were performed at the preoperative visit.
At each visit, UDVA and CDVA were obtained using a Snellen chart. Slit lamp examinations of the anterior and posterior segment of the eye were performed in mydriasis and IOP was measured by Goldmann applanation tonometry (Haag-Streit, Köniz, Switzerland). In addition, at each visit, except for postoperative day 1, Scheimpflug images of the anterior segment were taken using the Pentacam® HR (Oculus, Wetzlar, Germany). Pentacam® images were used to obtain multiple measurements. Before VTICL implantation, ACD was measured (undilated pupil) from the endothelium to the mid-iris plane. After VTICL implantation, the central distance between corneal endothelium and VTICL (Figure 3A) and the vault (Figure 3B, space between VTICL and mIOL) was measured at the 0–180° and 90–270° meridian. Distance measurements were done manually using the caliper tool within the Pentacam® software. Furthermore, the ICA was measured based on an automated algorithm within the Pentacam® software. At each visit except for postoperative day 1, a slit lamp photograph of the VTICL position was taken under retroillumination and in upright position with a fully dilated pupil to document the axis alignment of the VTICL.
Power and size calculation for piggy-back VTICL
Two different VTICL types were used in the study, the V4b without aquaport and the V4c with aquaport (Figure 1). As we were initially worried about potential visual disturbances due to the interference of the central aquaport with the mIOL optic, we used the V4b without aquaport for the majority of patients in the study.
The VTICL power and size calculation were performed by using the standard STAAR Visian ICL calculation software for phakic eyes. For VTICL calculation, data of the current refraction, corneal vertex distance, K1/K2 values, corneal thickness, white-to-white measurement, and ACD have to be entered. Particularly the white-to-white measurement and the ACD value are important for the correct lens size calculation. For phakic eyes, the software uses different algorithms depending on whether the patient is hyperopic or myopic. When a VTICL is calculated as a piggy-back lens, regardless of the refraction after mIOL implantation, the algorithm (hyperopic or myopic) should be chosen based on the refraction before mIOL implantation. In cases of mixed astigmatism, the axial length can help to choose either the hyperopic or the myopic algorithm. The piggy-back VTICL power is calculated based on the refraction after mIOL implantation.
In a phakic eye, the ACD value is defined as the distance from the endothelium to the anterior surface of the crystalline lens. In a pseudophakic eye, the distance from the endothelium to the IOL surface is expected to be larger since the IOL is much thinner than the natural crystalline lens. Therefore, an adjusted ACD value seemed necessary to avoid an oversizing of the VTICL. For this reason, we initially decided to always enter an ACD value of 3.2 mm in the calculation software, regardless of the actual ACD value of each individual patient. However, as explained in the “Results” and “Discussion” sections, we realized during the initial course of the study that this approach can result in a hypervault due to an oversized VTICL with flattening of the iridocorneal angle. After the first 7 patients, we therefore decided to adjust the ACD value entered into the calculation software. For the remaining eyes, we used the distance between endothelium and the mid-iris plane as the individual ACD value.
Statistical analyses
Data analysis was performed using Microsoft Excel (version 16.0; Microsoft Corporation, Redmond, WA, USA) including the add-in statistics software WinSTAT (version 2012.1.0.96; R. Fitch Software, Bad Krozingen, Germany). For accurate statistical analysis of the visual acuity outcomes, the decimal values were transformed into logMAR notation. Normality of data samples was evaluated by the Shapiro–Wilk test. If parametric analysis was possible, the Student t-test for paired data was used for comparisons between the preoperative and postoperative data, whereas the Wilcoxon rank-sum test was applied to assess the significance of such differences when the parametric analysis was not possible. To detect differences between three or more time points, one-way repeated measures ANOVA or Friedman test was applied. The Spearman's rank correlation coefficient was calculated to assess the correlation between different variables. For all statistical tests, a P<0.05 was considered as statistically significant. To describe the change in refractive astigmatism induced by toric ICL implantation, the astigmatic components of the power vector were analyzed by the two-dimensional vector (J0, J45) in accordance with Thibos and Horner.23 The J0 component expresses the power of a Jackson cross-cylinder with its axes at 180° and 90° and the J45 component expresses the power of a Jackson cross-cylinder with its axes at 45° and 135°. Preoperative and postoperative J0 and J45 refractive cylindrical vectors were calculated as follows:
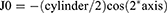
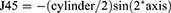
Results
This study comprised 24 eyes of 23 patients. One patient was not available for the 3-month follow-up visit and another patient was not available for the 6-month examination. The demographic data and preoperative clinical information of the patients are summarized in Table 1. Table 2 summarizes the implanted mIOL and VTICL models. The average time between mIOL implantation and VTICL implantation was 1000 days (range: 151–2866 days). In 2 patients, the VTICL had to be replaced by a smaller model and the postoperative data after the exchange was used for data analysis.
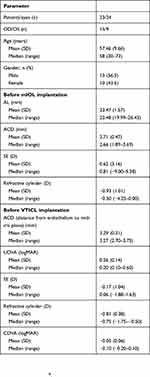 |
Table 1 Patient demographics and preoperative clinical information |
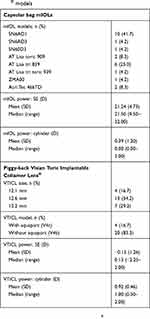 |
Table 2 Implanted mIOL and Visian Toric Implantable Collamer Lens® models |
Visual and refractive results
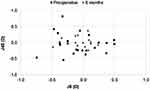
Table 3 summarizes the monocular visual acuity and refractive outcomes before VTICL implantation and over the follow-up period of 6 months. Six months after VTICL implantation, there was a statistically significant increase in UDVA (P<0.001) and a statistically significant reduction in the refractive cylinder (P<0.001) compared to the preoperative data. The 6-month postoperative change in SE and CDVA was not statistically significant (P=0.301 and P=0.308, respectively). Figure 4 shows the distribution of pre- and postoperative monocular UDVA. Preoperatively, no patient had a UDVA of 20/20 (Snellen) and only 4 eyes (17%) achieved a UDVA of 20/25. In comparison, 6-month postoperative UDVA was 20/20 or better in 19 eyes (83%) and 20/25 or better in 22 eyes (96%). Preoperatively, CDVA was clearly better than UDVA (Figure 5). Regarding postoperative UDVA and CDVA, there was no statistically significant difference from 1 week to 6 months after surgery (UDVA: P=0.068; CDVA: P=0.525). The mean preoperative calculated target refraction was −0.02±0.13 D (median: −0.02 D; range −0.27–0.24 D) for the SE and 0.12±0.08 D (median 0.10 D; range 0.01 to 0.33) for the cylindrical component. At 6 months, the mean absolute deviation from target SE and cylinder was 0.15±0.18 D (median 0.11 D; range 0.00 to 0.76 D) and 0.26±0.24 D (median 0.16 D; range 0.01 to 0.84), respectively. Figures 6 and 7 summarize the predictability outcomes after VTICL implantation. After 6 months, about two-thirds of the eyes (n=14 eyes; 61%) showed a clinically insignificant refractive astigmatism of ≤0.25 (Figure 8). The postoperative SE and the refractive cylinder were stable between 1 week and 6 months (SE: P=0.500; cylinder: P=0.903). Figure 9 shows the refractive astigmatic component of the power vectors (J0, J45). The origin in this graph (0.0) represents an eye without any astigmatism. Preoperatively, the data points were relatively spread out, while 6 months after VTICL implantation, the data points appeared more concentrated around the 0.0 position. However, despite this visual difference, there was no statistically significant difference between the preoperative and 6-month postoperative J0 (P=0.627) and J45 (P=0.378) refractive power vectors. At 6 months, preoperative mean power vectors decreased from −0.054±0.327 to −0.035±0.168 (J0) and from −0.046±0.310 to 0.008±0.140 (J45).
 |
Table 3 Monocular visual acuity and subjective refraction data before and after VTICL implantation |
 |
Figure 4 Cumulative Snellen visual acuity (20/X or better) of pre- and postoperative UDVA.Abbreviation: UDVA, uncorrected distance visual acuity. |
 |
Figure 5 Pre- and postoperative UDVA and CDVA.Abbreviations: CDVA, corrected distance visual acuity; UDVA, uncorrected distance visual acuity. |
 |
Figure 6 Percentage of eyes achieving the stated accuracy in spherical equivalent. |
 |
Figure 7 Percentage of eyes achieving the stated accuracy in refractive cylinder. |
 |
Figure 8 Distribution of pre-and postoperative refractive astigmatism. |
Stability of VTICL axis alignment over time
Postoperatively, the difference between the preoperative target axis and the measured VTICL axis was recorded at each study visit except for postoperative day 1. The VTICL misalignment is demonstrated in Table 4 and Figure 10. No statistically significant difference in the deviation from the target axis was observed over the follow-up period (P=0.444). There was no correlation between the postoperative VTICL misalignment and the postoperative UDVA (3 months: rs=0.174, P=0.245; 6 months: rs=−0.004, P=0.494). No eye needed secondary surgery to reposition the VTICL axis.
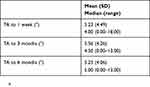 |
Table 4 Postoperative VTICL misalignment data |
 |
Figure 10 Cumulative VTICL misalignment from the target axis over the follow-up period.Abbreviation: VTICL, Visian Toric Implantable Collamer Lens®. |
Iridocorneal angle, vault, and endothelium-VTICL-distance
Table 5 summarizes the postoperative change of ICA data measured with the Pentacam®. After implantation of the piggy-back ICL, the iridocorneal angle narrowed on average about 50% compared to the preoperative value and remained stable over the follow-up period of 6 months. The postoperative outcomes of the vault and the distance between endothelium and VTICL are shown in Table 6.
 |
Table 5 Pre- and postoperative ICA data |
 |
Table 6 Postoperative clinical outcomes regarding vault (distance between the VTICL and the mIOL) and the distance between the endothelium and the VTICL |
Statistically, there was no significant difference of vault and VTICL-endothel-distance from 1 week to 6 months (vault: P=0.061; VTICL-endothel-distance: P=0.116).
Safety and IOP
For the first 7 patients, the VTICL size was calculated using an ACD value of 3.2 mm for each patient regardless of the actual individual ACD value (see “Methods” section). This resulted in an overly large vault, causing angle narrowing with episodes of IOP increase and/or corneal edema in 4 of these 7 patients. Patient 1 developed an acute angle closure 4 days after VTICL implantation with an IOP of up to 54 mmHg. After enlarging the iridotomies with a YAG laser, the IOP normalized without further IOP peaks during a follow-up period of 5 years. Patient 2 developed an acute angle closure 3 months after VTICL implantation with an IOP of up to 46 mmHg. After enlarging the iridotomies with a YAG laser, the IOP normalized. Patients 3 and 4 had a large vault after VTICL implantation resulting in an anterior bulging of the iris with angle narrowing and intermittent corneal edema. Therefore, for both patients, the initially implanted VTICL with a diameter of 12.6 mm was exchanged by a smaller VTICL with a diameter of 12.1 mm after 3 months (Patient 3) and 6 months (Patient 4). After the VTICL exchange, the corneal edema disappeared. After optimization of the VTICL size calculation using the individual ACD for each patient (distance between endothelium and the mid-iris plane), no further size-related complications such as IOP increase or corneal edema occurred anymore.
Another patient (Patient 15) developed a retinal detachment 4 months after VTICL implantation which was treated by pars plana vitrectomy with C3F8 gas tamponade. Nine months after VTICL implantation, the UDVA was 1.6 (decimal). After the vitrectomy, the IOP was controlled using dorzolamide and timolol 0.5% eye drops.
Except for a subset of the first 7 patients, where the VTICL size calculation was suboptimal, the IOP before and 6 months after VTICL implantation did not change significantly (P=0.759). No episodes of IOP rise were noted in patients that underwent implantation of an VTICL with aquaport (V4c). Table 7 shows the outcomes regarding the pre- and postoperative IOP.
 |
Table 7 Preoperative versus 6-month postoperative IOP |
Discussion
Patients that undergo mIOL implantation expect emmetropia after the procedure, in order to achieve spectacle independence. Despite significant improvements in biometry and calculation formulas and the introduction of toric mIOLs, remaining refractive error after mIOL implantation can occur, which may leave patients unsatisfied. For these patients, surgical strategies to correct the remaining refractive error are needed.
Some case studies have reported that anisometropia and refractive error in monofocal pseudophakic patients can be corrected by a piggy-back ICL implantation.13–21 After the introduction of low power VTICLs, we became interested in whether low power VTICLs could also be used to correct minor refractive error after mIOL implantation. To our knowledge, the implantation of a piggy-back ICL has not been performed in mIOL patients so far and has not been studied systematically yet. In this paper, we have prospectively investigated for a larger group of patients over a follow-up period of 6 months whether low power VTICLs are a reliable and safe option to correct minor remaining refractive error after mIOL implantation.
The ICL was developed for the refractive correction of phakic eyes and is currently only approved for this purpose. Consequently, there is only a calculation software available from STAAR for phakic eyes. While some case reports suggested that ICLs could also be used to correct the refractive error in pseudophakic eyes,13–21 it was unclear prior to this study how well the calculation algorithm based on phakic eyes works in pseudophakic eyes and how well the refraction can be targeted.20
In the algorithm of the STAAR calculation software, there are 3 major components that determine ICL size: the white-to-white distance (not influenced by the lens status and therefore not of any concern here), the ACD, and whether the patient was hyperopic or myopic before mIOL surgery.
In a pseudophakic eye, the distance between the endothelium and the anterior surface of the IOL is generally larger than in a phakic eye since the natural crystalline lens is much thicker than the IOL. If the ACD value used for ICL calculation is relatively too large, the VTICL size (diameter of the haptics) will be oversized, resulting in an excessive vault. To avoid ICL oversizing, we realized after the initial phase of the study that the distance between the endothelium and mid-iris plane is more appropriate to define the pseudophakic ACD as opposed to the distance between the endothelium and the anterior surface of the mIOL.
The refraction before mIOL implantation is important to differentiate whether the hyperopic or myopic calculation algorithm should be used to calculate VTICL size. As a rule of thumb: In patients with identical white-to-white distance and ACD, the STAAR software algorithm would calculate a VTICL one size smaller in an originally hyperopic eye compared to a myopic eye. If a hyperopic patient becomes slightly myopic after mIOL implantation, and the myopic calculation algorithm is used erroneously, the patient would receive a VTICL that is one size too large as a consequence, which may result in hypervaulting and could potentially lead to secondary angle closure and corneal edema.
The only significant VTICL-induced complications occurred in the beginning of the study and were related to VTICL oversizing. Two patients experienced an IOP increase which was managed by enlarging the 2 iridotomy sites using a YAG-laser. In 2 other patients, the VTICL had to be exchanged for a smaller-sized VTICL because of intermittent corneal edema. After a critical review of the initial results, we realized that an arbitrarily chosen ACD value of 3.2 mm can result in an oversized VTICL in some patients. No further sizing issues and significant VTICL-induced complications were observed after using an individual ACD value (defined as the distance between the endothelium and the mid-iris plane) for the ICL size calculation for each patient.
The refractive results achieved by VTICL piggy-back implantation in this cohort of “unhappy” mIOL patients were excellent. As shown in Figure 4, preoperatively, none of the eyes had an UDVA of 20/20 and only 17% of eyes achieved 20/25. In comparison, 6 months after VTICL implantation, 83% and 96% of eyes had a UDVA of 20/20 or 20/25, respectively. There was no statistically significant difference between the preoperative and 6-month postoperative CDVA. The VTICL axis alignment was stable over the follow-up period. One week after VTICL implantation, the mean difference between the intended and the achieved VTICL axis was 5° and no changes were noted up to the 6-month visit.
The postoperative vault did not seem to have an impact on the refractive results. In phakic eyes, the vault between the anterior surface of the crystalline lens and the ICL should ideally be between 250 and 750 µm. While the anterior surface of the crystalline lens is convex, the anterior surface of an IOL is much flatter in comparison to the crystalline lens. Therefore, a higher vault in pseudophakic eyes compared to phakic eyes is expected. In this study, at the 6-month visit, the mean pseudophakic vault was 1385 µm and the mean distance between the corneal endothelium and the VTICL surface was 2205 µm. The VTICL was therefore located in the lower third of the anterior chamber. Given the higher vault, an IOL/ICL touch is not expected, reducing the risk of interface opacifications, which have been reported for other sulcus-fixated piggy-back IOLs.24,25 To further reduce the vault in pseudophakic eyes, one may consider to routinely select a VTICL one size smaller than proposed by the STAAR calculation algorithm. However, a smaller VTICL size may incur the risk of an increased rotational instability.
Initially, we were worried that the central aquaport opening in the V4c model could interfere with the visual performance of the mIOL. For the majority of cases, we therefore implanted the V4b model without aquaport. However, in the 4 eyes in which the V4c model with aquaport was implanted, no increase of halos or a subjective decrease in contrast sensitivity were noted and patient satisfaction was high. Even though no iridotomy was performed in the 4 eyes, no angle closure or IOP peaks were observed. Therefore, going forward, we would prefer to use the V4c model with aquaport in pseudophakic eyes, since no iridotomies are needed.
There are several limitations to our study. Given that this was a pilot study, the optimal algorithm to calculate the correct VTICL size for pseudophakic patients was not known. In retrospect, our initial approach to calculate the VTICL size was not ideal, leading to complications in the early phase of the study. After optimizing the ACD measurement for the VTICL size calculation, no further complications with regard to sizing were observed. The incidence of complications associated with a relatively too large VTICL size in this study (e.g., secondary angle closure in 17% of patients) is therefore likely overestimated. Another limitation is that the study period was only 6 months. It is therefore unclear how stable the refractive correction is long term and whether late rotations of the VTICL or long-term interface opacifications can occur. In our study, the ICA was reduced by the VTICL on average from 44.79° preoperatively to 23.55° at the 6-month postoperative visit. Whether narrowing of the ICA may result in a long-term IOP increase in some patients is not known. However, VTICL implantation of the first patients in the study already dates back more than 6 years by now. In all patients that presented to our clinic for routine visits after the study period, the refractive correction appeared to be stable and we have not observed any changes in IOP or any interface opacifications. An additional limitation is that we did not monitor endothelial cell density during the course of the study. However, to our knowledge, there are no reports of significant endothelial cell loss after ICL implantation. Another limitation is that low power VTICLs are currently not available in some countries. As this was a pilot study, no comparative group with secondary excimer laser correction or secondary arcuate incisions was included. In the future, a prospective study comparing the VTICL approach with alternative approaches to correct residual refractive error after mIOL implantation would be interesting. Such studies could investigate differences with regards to quality of vision (contrast sensitivity, induction of higher-order aberrations) and long-term stability of the refractive correction.
There are different surgical approaches to correct residual refractive error after mIOL implantation. In general, we believe that each surgeon should choose the surgical approach she or he feels most comfortable with. However, compared to already established surgical approaches, the piggy-back VTICL has some advantages: Effective and stable refractive correction without inducing any significant surgical astigmatism, easy correction of axis misalignments, atraumatic reversibility, fast visual recovery, no known negative impact on contrast sensitivity, and no known risk for interface opacification. Nevertheless, using a piggy-back VTICL to correct residual refractive error after mIOL or monofocal IOL implantation is currently an off-label procedure. Given the promising results presented here, we hope that an approval of the VTICL also for pseudophakic patients will be considered.
Acknowledgment
We would like to thank our optometrist Cindy Döring for her technical assistance. The authors did not receive any financial compensation for the study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lundström M, Dickman M, Henry Y, et al. Risk factors for refractive error after cataract surgery: analysis of 282 811 cataract extractions reported to the European registry of quality outcomes for cataract and refractive surgery. J Cataract Refract Surg. 2018;44(4):447–452. doi:10.1016/j.jcrs.2018.01.031
2. Seiler TG, Wegner A, Senfft T, Seiler T. Dissatisfaction after trifocal IOL implantation and its improvement by selective wavefront-guided LASIK. J Refract Surg. 2019;35:346–352. doi:10.3928/1081597X-20190510-02
3. Toygar B, Yabas Kiziloglu O, Toygar O, Hacimustafaoglu AM. Clinical outcomes of a new diffractive multifocal intraocular lens. Int J Ophthalmol. 2017;10(12):1844–1850. doi:10.18240/ijo.2017.12.09
4. Gibbons A, Ali TK, Waren DP, Donaldson KE. Causes and correction of dissatisfaction after implantation of presbyopia-correcting intraocular lenses. Clin Ophthalmol. 2016;10:1965–1970. doi:10.2147/OPTH.S114890
5. Alfonso JF, Fernández-Vega L, Montés-Micó R, Valcárcel B. Femtosecond laser for residual refractive error correction after refractive lens exchange with multifocal intraocular lens implantation. Am J Ophthalmol. 2008;146(2):244–250. doi:10.1016/j.ajo.2008.03.022
6. Leccisotti A. Secondary procedures after presbyopic lens exchange. J Cataract Refract Surg. 2004;30(7):1461–1465. doi:10.1016/j.jcrs.2003.11.056
7. Muftuoglu O, Prasher P, Chu C, et al. Laser in situ keratomileusis for residual refractive errors after apodized diffractive multifocal intraocular lens implantation. J Cataract Refract Surg. 2009;35(6):1063–1071. doi:10.1016/j.jcrs.2009.01.028
8. Kamiya K, Igarashi A, Shimizu K, Matsumura K, Komatsu M. Visual performance after posterior chamber phakic intraocular lens implantation and wavefront-guided laser in situ keratomileusis for low to moderate myopia. Am J Ophthalmol. 2012;153(6):1178–1186. doi:10.1016/j.ajo.2011.12.005
9. Lüdeke I, Gonnermann J, Jørgensen J, et al. Refractive outcomes of femtosecond laser-assisted secondary arcuate incisions in patients with residual refractive astigmatism after trifocal intraocular lens implantations. J Cataract Refract Surg. 2019;45:28–34. doi:10.1016/j.jcrs.2018.08.024
10. El Awady HE, Ghanem AA. Secondary piggyback implantation versus IOL exchange for symptomatic pseudophakic residual ametropia. Graefes Arch Clin Exp Ophthalmol. 2013;251(7):1861–1866. doi:10.1007/s00417-013-2283-x
11. Gayton JL, Sanders V, Van Der Karr M, Raanan MG. Piggybacking intraocular implants to correct pseudophakic refractive error. Ophthalmology. 1999;106(1):56–59. doi:10.1016/S0161-6420(99)90005-2
12. Habot-Wilner Z, Sachs D, Cahane M, et al. Refractive results with secondary piggyback implantation to correct pseudophakic refractive errors. J Cataract Refract Surg. 2005;31(11):2101–2103. doi:10.1016/j.jcrs.2005.05.023
13. Kothari KJ, Nayak PR, Mehta BK. Pseudophakic hyperopia in nanophthalmic eyes managed by a posterior chamber implantable collamer lens. Indian J Ophthalmol. 2011;59(2):165–166. doi:10.4103/0301-4738.77014
14. Chen X, Wang X, Zhou X. Pseudophakic ametropia management with toric implantable collamer lens with a central hole (case report). BMC Ophthalmol. 2017;17:17. doi:10.1186/s12886-017-0414-6
15. Chiou AGY, Bovet J, de Courten C. Pseudophakic ametropia managed with a phakic posterior chamber intraocular lens. J Cataract Refract Surg. 2001;27(9):1516–1518.
16. Eissa SA. Management of pseudophakic myopic anisometropic amblyopia with piggyback visian® implantable collamer lens. Acta Ophthalmol. 2017;95(2):188–193. doi:10.1111/aos.13203
17. Eissa SA, Khafagy MM, Sidky MK. Implantable collamer lens in the management of pseudophakic ametropia. J Refract Surg. 2017;33(8):532–537. doi:10.3928/1081597X-20170606-02
18. Hsuan JD, Caesar RH, Rosen PH, Rosen ES, Gore CL. Correction of pseudophakic anisometropia with the staar collamer implantable contact lens. J Cataract Refract Surg. 2002;28(1):44–49.
19. Kojima T, Horai R, Hara S, et al. Correction of residual refractive error in pseudophakic eyes with the use of a secondary piggyback toricImplantable collamer lens. J Refract Surg. 2010;26(10):766–769. doi:10.3928/1081597X-20100512-02
20. Ashraff NN, Kumar BV, Das A, Moriarty AP. Correction of pseudophakic anisometropia in a patient with pseudoexfoliation using an implantable contact lens. Br J Ophthalmol. 2004;88(2):309. doi:10.1136/bjo.2003.021915
21. Emara KE, Al Abdulsalam O, Al Habash A. Secondary implantation of implantable collamer lens (ICL) for correction of anisometropic hyperopia in a 3-year-old pseudophakic child. Saudi J Ophthalmol. 2016;30(1):75–77. doi:10.1016/j.sjopt.2015.12.002
22. Hoffer KJ. Pigment vacuum iridectomy for phakic refractive lens implantation. J Cataract Refract Surg. 2001;27(8):1166–1168.
23. Thibos LN, Horner D. Power vector analysis of the optical outcome of refractive surgery. J Cataract Refract Surg. 2001;27(1):80–85.
24. Werner L, Shugar JK, Apple DJ, et al. Opacification of piggyback IOLs associated with an amorphous material attached to interlenticular surfaces. J Cataract Refract Surg. 2000;26(11):1612–1619.
25. Eleftheriadis H, Marcantonio J, Duncan G, Liu C. Interlenticular opacification in piggyback AcrySof intraocular lenses: explantation technique and laboratory investigations. Br J Ophthalmol. 2001;85(7):830–836. doi:10.1136/bjo.85.7.830
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.