Back to Journals » Risk Management and Healthcare Policy » Volume 16
A Prediction Nomogram for Recurrent Retinal Detachment
Authors Zhou Y , Lu Q, Chen Z, Lu P
Received 11 January 2023
Accepted for publication 10 March 2023
Published 28 March 2023 Volume 2023:16 Pages 479—488
DOI https://doi.org/10.2147/RMHP.S403136
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Yongying Zhou,1,2 Qianyi Lu,1 Zhigang Chen,1 Peirong Lu1
1Department of Ophthalmology, The First Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China; 2Department of Ophthalmology, Children’s Hospital of Wujiang District, Suzhou, People’s Republic of China
Correspondence: Peirong Lu, Department of Ophthalmology, the First Affiliated Hospital of Soochow University, Shizi Street 188, Suzhou, Jiangsu Province, 215006, People’s Republic of China, Email [email protected]
Purpose: Recurrent retinal detachment (re-RD) is one of the complications in rhegmatogenous retinal detachment patients who underwent surgical treatment. We investigated the risk factors for re-RD and developed a nomogram for estimating clinical risk.
Methods: Univariate and multivariable logistic regression models were performed to determine the association between variables and re-RD, and a nomogram was then developed for re-RD. The nomogram performance was assessed based on its discrimination, calibration, and clinical usefulness.
Results: This study analyzed 15 potential variables of re-RD in 403 rhegmatogenous retinal detachment patients who underwent initial surgical treatment. Axial length, inferior breaks, retinal break diameter, and surgical methods were independent risk factors for re-RD. A clinical nomogram incorporating these four independent risk factors was constructed. The diagnostic performance of the nomogram was excellent (area under the curve = 0.892, 95% CI: 0.831– 0.953). Our study further validated this nomogram by bootstrapping for 500 repetitions. The area under the curve of the bootstrap model was 0.797 (95% CI: 0.712– 0.881). This model showed good calibration curve fitting and a positive net benefit in decision curve analysis.
Conclusion: Axial length, inferior breaks, retinal break diameter, and surgical methods could be risk factors for re-RD. We have developed a prediction nomogram of re-RD for rhegmatogenous retinal detachment following initial surgical treatment.
Keywords: recurrent retinal detachment, nomogram, risk factors
Introduction
Rhegmatogenous retinal detachment (RRD) is the most common form of retinal detachment. In the treatment of RRD, the most commonly used techniques are scleral buckling (SB), pars plana vitrectomy (PPV), and pneumatic retinopexy (PR). Some surgeons prefer a combination of these interventions to treat RRD. Previous studies on the safety and efficacy of these interventions found that the primary reattachment rate and final visual acuity were similar between SB and PPV.1,2 Compared with standalone PPV, PPV with supplemental SB did not improve primary reattachment rates or vision.3 Likewise, in the comparison of PR to SB, there was no difference between the initial and final reattachment rates.2
Although substantial progress has been made in surgical techniques and devices to reduce recurrent retinal detachment (re-RD), up to 10–40% of cases require more than one intervention to repair retinal detachment; even as many as 5% of eyes may sustain permanent anatomic failure.4,5 In addition, the reported incidence of RRD ranges from 6.3 to 17.9 per 100,000 population,6 and the increasing number of patients have brought with it a greater number of recurrences. Therefore, re-RD remains a significant challenge for vitreoretinal surgeons and patients, considering the economic and emotional burden of undergoing multiple interventions.
Comprehensive analysis of risk factors for re-RD in RRD patients who underwent surgical treatment was reported previously in several articles, including the development of proliferative vitreoretinopathy (PVR),7 retinal break diameters,8 subretinal fluid (SRF),9 high myopia,10 posterior staphyloma,11 multiple surgeries,12 and silicone oil removal before silicone oil emulsification.13 However, thus far, few reports have combined these factors to establish a risk prediction model to predict re-RD occurrence in RRD patients following surgical treatment.
Prediction models are tools that combine multiple predictors by assigning relative weights to each predictor to obtain a risk or probability.14 Prediction model studies can broadly be divided into model development, model validation, or both.15 Studies developing new prediction models should always include some form of internal validation or external validation to quantify the predictive performance of the developed model.16 Besides, nomograms are commonly used to visualize prediction models. As a simple statistical visual tool, the nomogram has been widely used to predict disease occurrence, development, prognosis, and survival.17,18
This study sought to investigate the risk factors and establish a prediction nomogram for re-RD in RRD patients. The model mainly focuses on the early prediction of re-RD caused by preoperative risk factors and surgical methods.
Materials and Methods
Patients
The Institutional Ethics Committee of the First Affiliated Hospital of Soochow University approved this retrospective study in compliance with the principles of the Declaration of Helsinki and obtained informed consent from the patients. Seven hundred sixty-five patients with retinal detachment underwent their first surgery intervention at the First Affiliated Hospital of Soochow University (Suzhou, Jiangsu, China) between October 2020 and April 2022. Patients were excluded from the final analysis for the following reasons: traction retinal detachment, exudative retinal detachment, macular hole retinal detachment, open globe injury-induced RD, PVR grade > B, combined with other fundus diseases: diabetic retinopathy, retinal vein occlusion, and history of any intraocular vitreoretinal procedure. Finally, 403 RRD patients were included in the final analysis.
Baseline characteristics of the patients included age, sex, hypertension, diabetes, laterality, trauma history, contralateral eyes, duration of central vision loss, preceding surgeries, lens status, axial length, location of retinal breaks, the diameter of breaks, macular status, and surgical methods.
Surgical Procedures
The retinal breaks were identified and treated for SB surgery by transscleral cryotherapy. Mattress sutures were placed 7.0 to 7.5 mm apart with 4–0 supramid for the circumferential segmental buckle, and a silicone sponge was sutured as an explant in all cases. The SRF was drained if necessary. For the PPV surgery, a 3-port 23-gauge pars plana vitrectomy was performed, and subretinal fluid was internally aspirated. Fluid-air exchange was followed by retinopexy using endophotocoagulation and cryotherapy. Then, the sterile gas or silicone oil (SO) was injected into the vitreous completion of the PPV. Cataract surgery was implemented during PPV if the cataract was visually significant. None of the patients had any PPV supplements with SB. Only patients who achieved anatomical success in primary surgery were included in the study. PPV or SB was selected according to individual condition assessment and surgeons’ clinical experience.
Developing and Validating the Model
Univariate and multivariable logistic regression models were used to detect the relationship between variables and re-RD. A crude analysis was conducted to identify possible risk factors in the univariate analysis. All variables having a bivariate association with re-RD with P < 0.05 were included in the multivariable model. Backward stepwise selection using the Akaike information criterion was applied to select the independent predictors for constructing the prediction model in the multivariate logistic regression. We developed a nomogram based on the multivariate logistic regression results. The area under the curve (AUC) of the receiver operator characteristic (ROC) was used to evaluate the model’s discrimination. Calibration was assessed with a calibration plot to evaluate the goodness-of-fit of the nomogram. A bootstrap validation was also performed using simple random sampling with the replacement for 500 repetitions to verify the accuracy of our model. Finally, the clinical usefulness of our model was evaluated using the decision curve analysis (DCA) by calculating the net benefits at different threshold probabilities.
Statistical Analysis
The statistical analyses were performed using R statistical software, version 4.1.3, and SPSS, version 26.0. A logistic regression algorithm was conducted using the “plyr” package. Nomogram construction was conducted using the “rms” package. ROC curves were generated using the “pROC”, “tidyverse” and “rms” package. Calibration plots were conducted using the “gbm”, “rlang”, “magrittr” and “rms” packages. DCA was performed with the “rmda” package. Mean + standard deviation (SD) or median (min-max) are used for continuous variables, and categorical variables are expressed as percentages or numbers. Based on χ2 tests, Fisher exact tests, and logistic regression models, associations were assessed between re-RD and variables. Statistical analyses were two-tailed with 95% confidence intervals (CI). In all statistical tests, P < 0.05 was considered significant.
Results
Patient Characteristics
The final study cohort was composed of 403 patients. The characteristics of the patients with RDD are summarized in Table 1. The mean age was 52.5 ± 13.65 years, and 205 (50.90%) patients were men. 8.2% of patients previously underwent closed ocular trauma, and 5.2% had a previous retinal detachment in the contralateral eye. More than half of the patients (65.5%) had central vision loss for more than seven days. Besides, the lens status of patients was almost in Phakic (92.6%). The axial length of 32.2% of patients was more than 26.00mm. The rate of inferior breaks in patients was 27.0%, while retinal break diameter ≥ 3PD was 21.3%. Moreover, 55.1% of patients were treated with PPV+SO, while only 8.7% underwent SB. Of 403 patients, re-RD occurred in 34 (8.4%) patients.
 |
Table 1 Baseline Characteristics of the Investigated Patients (n = 403) |
Risk Factors for Recurrent Retinal Detachment
Univariate and multivariate logistic regression analyses performed to determine the relationship between variables and re-RD are shown in Table 2. Only 4 out of 15 candidate variables were associated with re-RD in the univariate logistic regression analyses with a P < 0.05. These were axial length (AL), inferior breaks, the diameter of retinal breaks, and surgical methods. The risk of re-RD among patients with AL ≥ 26.00mm was higher than that of patients with AL< 26.00mm (OR= 4.43, 95% CI: 2.12–9.28, P < 0.0001). Compared with the SB group, more patients in the PPV+ gas group developed re-RD, with a significantly increased risk (OR= 3.38, 95% CI: 0.93–12.29, P = 0.065). Patients with inferior breaks were more likely to suffer from re-RD than those with superior breaks (OR= 3.93, 95% CI: 1.92–8.05, P <0.0001). Besides, patients with a diameter of retinal breaks ≥ 3PD increased the risk of re-RD (OR= 2.51, 95% CI: 1.20–5.25, P =0.014). In addition, the incidence of re-RD did not differ significantly based on sex, age, diabetes, hypertension, laterality, history of eye trauma, surgical history, duration of central vision loss, lens status, or macular status. Multivariate logistic regression was conducted using all variables with a bivariate association with re-RD with P < 0.05, resulting in the adjusted odds ratios shown in Table 2. The significant predictors of re-RD in the multivariate model were: AL ≥ 26.00mm (OR= 4.25, 95% CI:1.79–10.1, P = 0.001), the diameter of retinal breaks ≥3PD (OR= 3.47, 95% CI:1.33–9.06, P = 0.011), inferior breaks (OR= 7.19, 95% CI:2.85–18.18, P <0.0001), and surgical methods (OR= 9.04, 95% CI:2.02–40.43, P = 0.004). All possible two-way interactions between variables were examined in the multivariable model, but no statistically significant interaction was found (P > 0.05).
 |
Table 2 Univariate and Multivariate Logistic Regression Analysis of Clinical Candidate Predictors |
Nomogram for Recurrent Retinal Detachment
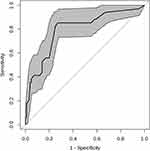
Fifteen clinical variables were analyzed to determine their association with re-RD. Four of the initial 15 variables were screened out: axial length, inferior breaks, the diameter of retinal breaks, and surgical methods. In this study, the backward stepwise selected model was computed as follows: −4.809+1.447×(axial length ≥ 26mm) +1.244×(diameter of retinal breaks ≥ 3PD) +1.973×(inferior breaks = yes) +2.202×(PPV+ gas) −1.900 (PPV+SO) + 0.957 (PPV+SO +SOR). The probability of re-RD can be estimated using the nomogram, as described in Figure 1. Based on the ROC curve analysis of this nomogram, the AUC was 0.892, indicating an excellent diagnostic performance (Figure 2) with a sensitivity of 79.4% and a specificity of 87.3% at the optimal cut-off value.
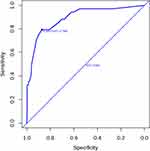 |
Figure 2 Receiver operating characteristic curve. Abbreviation: AUC, area under the receiver operating characteristic curve. |
Model Validation
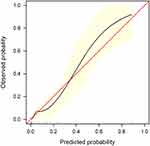
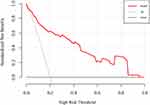
We further validated the nomogram using internal bootstrapping. Based on bootstrapping for 500 repetitions, the AUC of the bootstrap model was 0.797 (95% CI: 0.712–0.881), with similar statistical power to the original model (Figure 3). A calibration curve derived from internal bootstrap validation demonstrated that our model had excellent fitting and calibration with the ideal curve (Figure 4). Moreover, the internal bootstrap decision curve analysis revealed net positive benefits in the predictive model under a threshold probability of 0.85, indicating the predictive model’s favorable potential clinical effect (Figure 5).
Discussion
Artificial intelligence has received increasing attention in ophthalmology, and diagnostics for diabetic retinopathy, age-related macular degeneration, glaucoma, and proliferative vitreoretinopathy have demonstrated expert-level accuracy.19–21 However, as models increase in complexity, such models are not only inherently challenging to audit for quality but also limit the user’s ability to “debug” or make informed adjustments to the underlying algorithm.22 Nomogram-based clinical modelling is a reliable statistical and straightforward visual tool. A comprehensive analysis of all related risk factors can accurately calculate and predict disease occurrence, development, prognosis, and survival by nomogram.23
This study aimed to investigate the risk factors for re-RD and develop a model for predicting the re-RD. This study analyzed 15 potential variables of re-RD in 403 RRD patients who underwent initial surgical treatment and revealed that axial length, inferior breaks, the diameter of retinal breaks, and surgical methods were independent predictors of re-RD. Using multivariate analyses, we have developed a simple and easy-to-use prediction nomogram for re-RD. Four variables were screened out for the nomogram using backward stepwise regression. This nomogram had excellent diagnostic performance (AUC = 0.892, sensitivity = 79.4%, and specificity = 87.3%) and was validated internally using the bootstrap sampling method. Moreover, according to the results of the decision curve analysis, this prediction model demonstrated superior performance in the clinical setting.
RRD is often repaired with an SB, PPV, PR, or a combination of techniques. Although each method has advantages and disadvantages, its primary purpose is alleviating vitreous traction. The comparative efficacy of PPV, SB, and PR has often been studied.2 However, there are inconsistencies between the findings of various studies. For example, in 2335 cases, the Primary Retinal Detachment Outcomes Study found that the primary reattachment rates of SB (91.7%) and PPV supplemented with SB (PPV/SB, 91.2%) were higher than PPV (83.1%) in phakic eyes, but the rate of PPV/SB (92.0%) was higher than that of PPV (84.0%) in aphakic eyes.24,25 At the same time, a recent 2022 meta-analysis found no difference between SB, PPV, and PPV/SB at the final follow-up.1 Besides, when choosing PPV for treating RRD, it is typically accompanied by a postoperative intraocular tamponade agent.26 Tamponades have unique benefits and risks, and the choice of tamponades should be individualized according to the characteristics of the patient’s condition.27–29 Our study indicated that PPV+ gas is the most at risk for re-RD compared with SB (OR= 9.04, 95% CI:2.02–40.43, P = 0.004). The re-RD rates of PPV+SO+SOR were higher than SB, although the comparison was insignificant (P = 0.233).
Retinal breaks in RRD are usually distributed in more than one quadrant. Previous studies have demonstrated that retinal breaks are most likely to occur in the superotemporal quadrant, and the inferonasal quadrant is the least likely location for a break and the most potential for attached breaks.30 However, in a series of studies, the primary reattachment rate of RRD with inferior breaks is lower than those with superior breaks when treated by similar techniques.31–33 Moreover, significant breaks of more prominent than three disk diameters were confirmed as an independent risk for re-RD.8,34 Our study also found inferior breaks were an independent risk for re-RD (OR= 7.19, 95% CI:2.85–18.18, P <0.0001), consistent with previous studies. Moreover, this study demonstrated that giant retinal breaks (diameter ≥3 PD) are a statistically significant risk factor for re-RD (OR= 3.47, 95% CI:1.33–9.06, P =0.011).
Our study’s four independent re-RD predictors were axial length (AL). It has been demonstrated that myopic eyes are at a greater risk of developing RRD than emmetropic or hyperopic eyes, and the risk of RD increases with increasing axial length.35,36 An annual RRD incidence of 15 to 34 per 100,000 for mild myopia, 15 to 73 for moderate myopia, 102 to 128 for high myopia, and 287 in very highly myopic eyes were reported in a 2021 systematic review.37 In addition, previous studies have reported that AL increases significantly after SB or PPV.38,39 The change in AL was found to be between 0.1 and 0.6 mm postoperatively.40 Previous studies focused on the correlation between AL and RRD and AL change after surgical intervention, not only on the relationship between preoperative AL and re-RD in RRD patients who underwent initial surgical intervention. Herein, based on the results of our research, AL ≥ 26.00 mm was a remarkable prognosticator for re-RD compared to AL< 26.00 mm (OR= 4.25, 95% CI:1.79–10.1, P = 0.001). Therefore, surgeons should be cautious when choosing surgical techniques for highly myopic patients (AL ≥ 26.00 mm) with RRD.
Although several previous reports on recurrent retinal detachment have been published,41–43 none of the studies have constructed intuitive nomograms to calculate probability and validated their prediction models (internal or external). This study used a nomogram to calculate the overall likelihood of re-RD for an individual patient. This prediction model is essential for risk estimation, improving communication between patients and physicians, and clinical decision-making. In the present study, four independent variables were screened using stepwise regression, and the nomogram was established to predict the risk of re-RD in RRD patients. Nomograms showed excellent diagnostic performance (AUC= 0.892) and yielded a sensitivity of 79.4% and specificity of 87.3% at the optimal cut-off value. It is the first study to evaluate a nomogram that can be used to predict re-RD in patients with RRD. The nomogram might be a statistical tool to calculate the overall probability of re-RD in patients who underwent initial surgical treatment. This nomogram might serve as an essential early warning sign of re-RD in RRD patients.
Conclusion
Axial length, inferior breaks, retinal break diameter, and surgical methods could be risk factors for re-RD. Based on these four significant risk factors, we developed a practical and reliable nomogram to predict re-RD.
Data Sharing Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Ethics Approval and Informed Consent
The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of the First Affiliated Hospital of Soochow University (Approval No. 2022346). The patients/participants provided written informed consent to participate in this study.
Funding
This study was supported by grants from the Suzhou Municipal Health Commission (No: KJXW2020077) and the Science and Technology Program of Suzhou (No: SKJY2021015, SYSD2020048).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dhoot AS, Popovic MM, Nichani PAH, et al. Pars plana vitrectomy versus scleral buckle: a comprehensive meta-analysis of 15,947 eyes. Surv Ophthalmol. 2022;67(4):932–949. doi:10.1016/j.survophthal.2021.12.005
2. Popovic MM, Muni RH, Nichani P, Kertes PJ. Pars plana vitrectomy, scleral buckle, and pneumatic retinopexy for the management of rhegmatogenous retinal detachment: a meta-analysis. Surv Ophthalmol. 2022;67(1):184–196.
3. Rosenberg DM, Ghayur HS, Deonarain DM, et al. Supplemental scleral buckle for the management of rhegmatogenous retinal detachment by pars plana vitrectomy: a meta-analysis of randomized controlled trials. Ophthalmologica. 2022;245(2):101–110.
4. Nagpal M, Chaudhary P, Wachasundar S, Eltayib A, Raihan A. Management of recurrent rhegmatogenous retinal detachment. Indian J Ophthalmol. 2018;66(12):1763–1771.
5. Wykoff CC, Schwartz SG, Adelman RA, Brucker AJ, Flynn HW
6. Lai CT, Kung WH, Lin CJ, et al. Outcome of primary rhegmatogenous retinal detachment using microincision vitrectomy and sutureless wide-angle viewing systems. BMC Ophthalmol. 2019;19(1):230.
7. Speicher MA, Fu AD, Martin JP, von Fricken MA. Primary vitrectomy alone for repair of retinal detachments following cataract surgery. Retina. 2000;20(5):459–464.
8. Rootman DB, Luu S, Conti SM, et al. Predictors of treatment failure for pneumatic retinopexy. Can J Ophthalmol. 2013;48(6):549–552.
9. Han DP, Covert DJ, Wirostko WJ, Hammersley JA, Lindgren KE. Scleral buckle removal in the vitrectomy era: a 20-year clinical experience. Retina. 2013;33(2):387–391.
10. Wickham L, Ho-Yen GO, Bunce C, Wong D, Charteris DG. Surgical failure following primary retinal detachment surgery by vitrectomy: risk factors and functional outcomes. Br J Ophthalmol. 2011;95(9):1234–1238. doi:10.1136/bjo.2010.190306
11. Teke MY, Balikoglu-Yilmaz M, Yuksekkaya P, et al. Surgical outcomes and incidence of retinal redetachment in cases with complicated retinal detachment after silicone oil removal: univariate and multiple risk factors analysis. Retina. 2014;34(10):1926–1938. doi:10.1097/IAE.0000000000000204
12. Ambiya V, Rani PK, Narayanan R, et al. Outcomes of recurrent retinal detachment surgery following pars plana vitrectomy for rhegmatogenous retinal detachment. Semin Ophthalmol. 2018;33(5):657–663. doi:10.1080/08820538.2017.1395893
13. Nagpal MP, Videkar RP, Nagpal KM. Factors having implications on re-retinal detachments after silicone oil removal. Indian J Ophthalmol. 2013;61(9):534. doi:10.4103/0301-4738.119463
14. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Br J Cancer. 2015;112(2):251–259. doi:10.1038/bjc.2014.639
15. Moons KGM, Kengne AP, Grobbee DE, et al. Risk prediction models: II. External validation, model updating, and impact assessment. Heart. 2012;98(9):691–698. doi:10.1136/heartjnl-2011-301247
16. Steyerberg. Clinical prediction models: a practical approach to development, validation, and updating. Development. 2010;66(2):661–662.
17. Liu H, Li J, Guo J, Shi Y, Wang L. A prediction nomogram for neonatal acute respiratory distress syndrome in late-preterm infants and full-term infants: a retrospective study. EClinicalMedicine. 2022;50:101523.
18. Wang S, Tian S, Li Y, et al. Development and validation of a novel scoring system developed from a nomogram to identify malignant pleural effusion. EBioMedicine. 2020;58:102924.
19. Antaki F, Kahwati G, Sebag J, et al. Predictive modeling of proliferative vitreoretinopathy using automated machine learning by ophthalmologists without coding experience. Sci Rep. 2020;10(1):19528.
20. Rahimy E. Deep learning applications in ophthalmology. Curr Opin Ophthalmol. 2018;29(3):254–260.
21. Ting DSW, Pasquale LR, Peng L, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103(2):167–175.
22. Hanif AM, Beqiri S, Keane PA, Campbell JP. Applications of interpretability in deep learning models for ophthalmology. Curr Opin Ophthalmol. 2021;32(5):452–458.
23. Jimenez AE, Feghali J, Schilling AT, Azad TD. Deployment of clinical prediction models: a practical guide to nomograms and online calculators. Acta Neurochir Suppl. 2022;134:101–108.
24. Joseph DP, Ryan EH, Ryan CM, et al. Primary retinal detachment outcomes study: pseudophakic retinal detachment outcomes: primary retinal detachment outcomes study report number 3. Ophthalmology. 2020;127(11):1507–1514.
25. Ryan EH, Ryan CM, Forbes NJ, et al. Primary retinal detachment outcomes study report number 2: phakic retinal detachment outcomes. Ophthalmology. 2020;127(8):1077–1085.
26. Vaziri K, Schwartz SG, Kishor KS, Flynn HW
27. Huang Q, Cheng Y. The effectiveness of the supine position in managing inferior breaks in rhegmatogenous retinal detachment after vitrectomy with gas tamponade. Int J Gen Med. 2021;14:1179–1184.
28. Moussa G, Mathews N, Makhzoum O, Park DY. Retinal detachment repair with vitrectomy: air tamponade integration to a vitreoretinal service, comparison with gas tamponade, and literature review. Ophthalmic Surg Lasers Imaging Retina. 2022;53(2):87–95.
29. Tang Y, Lin B, Chen J, Chen D, Wu R. Outcomes of 25-gauge pars plana vitrectomy alone with air tamponade for the management of rhegmatogenous retinal detachment with inferior breaks. BMC Ophthalmol. 2022;22:1.
30. Shunmugam M, Shah AN, Hysi PG, Williamson TH. The pattern and distribution of retinal breaks in eyes with rhegmatogenous retinal detachment. Am J Ophthalmol. 2014;157(1):221–226.
31. Martínez-Castillo VJ, García-Arumí J, Boixadera A. Pars plana vitrectomy alone for the management of pseudophakic rhegmatogenous retinal detachment with only inferior breaks. Ophthalmology. 2016;123(7):1563–1569.
32. Tetsumoto A, Imai H, Hayashida M, et al. The comparison of the surgical outcome of 27-gauge pars plana vitrectomy for primary rhegmatogenous retinal detachment between air and SF6 gas tamponade. Eye. 2020;34(2):299–306.
33. Goto T, Nakagomi T, Iijima H. A comparison of the anatomic successes of primary vitrectomy for rhegmatogenous retinal detachment with superior and inferior breaks. Acta Ophthalmol. 2013;91(6):552–556.
34. Li KX, Carducci N, Moinuddin O, et al. Contemporary management of complex and non-complex rhegmatogenous retinal detachment due to giant retinal tears. Clin Ophthalmol. 2021;15:1013–1022.
35. Bechrakis NE, Dimmer A. Rhegmatogenous retinal detachment: epidemiology and risk factors. Ophthalmologe. 2018;115(2):163–178.
36. Tanihara H, Negi A, Kawano S, et al. Axial length of eyes with rhegmatogenous retinal detachment. Ophthalmologica. 1993;206(2):76–82.
37. Ullrich M, Zwickl H, Findl O. Incidence of rhegmatogenous retinal detachment in myopic phakic eyes. J Cataract Refract Surg. 2021;47(4):533–541.
38. Ozal SA, Garip R, Ozal E, Kupeli A. Evaluation of axial length changes after combined phacovitrectomy for macula-off rhegmatogenous retinal detachment. Beyoglu Eye J. 2019;4(3):136–140.
39. Shi J, Wu K, Wen H, et al. Change in axial length after vitrectomy with silicone oil tamponade for rhegmatogenous retinal detachment. BMC Ophthalmol. 2022;22(1):257.
40. Huang C, Zhang T, Liu J, Ji Q, Tan R. Changes in axial length, central cornea thickness, and anterior chamber depth after rhegmatogenous retinal detachment repair. BMC Ophthalmol. 2016;16:121.
41. Mitry D, Awan MA, Borooah S, et al. Surgical outcome and risk stratification for primary retinal detachment repair: results from the Scottish retinal detachment study. Br J Ophthalmol. 2012;96(5):730–734.
42. Smith JM, Ward LT, Townsend JH, et al. Rhegmatogenous retinal detachment in children: clinical factors predictive of successful surgical repair. Ophthalmology. 2019;126(9):1263–1270.
43. Hirata N, Iwase T, Kobayashi M, Yamamoto K, Ra E, Terasaki H. Correlation between preoperative factors and final visual acuity after successful rhegmatogenous retinal reattachment. Sci Rep. 2019;9(1):3217.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.