Back to Journals » Cancer Management and Research » Volume 12
A Modified ypTNM Staging System–Development and External Validation of a Nomogram Predicting the Overall Survival of Gastric Cancer Patients Received Neoadjuvant Chemotherapy
Authors Li Z , Xiao Q , Wang Y , Wang W, Li S, Shan F , Zhou Z, Ji J
Received 30 October 2019
Accepted for publication 21 February 2020
Published 19 March 2020 Volume 2020:12 Pages 2047—2055
DOI https://doi.org/10.2147/CMAR.S236696
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Seema Singh
Ziyu Li, 1,* Qiyan Xiao, 1,* Yinkui Wang, 1,* Wei Wang, 2,* Shuangxi Li, 1 Fei Shan, 1 Zhiwei Zhou, 2 Jiafu Ji 1
1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Gastrointestinal Cancer Center, Peking University Cancer Hospital & Institute, Beijing 100142, People’s Republic of China; 2State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Department of Gastric Surgery, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiafu Ji
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Gastrointestinal Cancer Center, Peking University Cancer Hospital & Institute, No. 52 Fucheng Road, Beijing 100142, People’s Republic of China
Tel +86-10-88196598
Fax +86-10-8819-6698
Email [email protected]
Zhiwei Zhou
State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Department of Gastric Surgery, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China
Tel +86-20-87343123
Fax +86-20-87343910
Email [email protected]
Purpose: Neoadjuvant chemotherapy is now widely used in gastric cancer patients. However, the current 8th ypTNM staging system is developed based on patients with less extensive lymph node dissection and the predictive value is relatively limited. In this study, we aim to develop and validate a nomogram that predicts overall survival in gastric cancer patients received neoadjuvant chemotherapy.
Patients and Methods: From January, 2007 to December, 2014, 471 patients receiving neoadjuvant chemotherapy at our center were enrolled in the study. Based on the Cox proportional hazard model, a nomogram was developed from them and then an external validation was conducted on a cohort of 239 patients from another cancer center.
Results: The overall survival (OS) rates of 1 year and 3 years were 90.0% and 64.1%, respectively. Body mass index category, tumor location, T stage and N stage were independent prognostic factors for the survival outcome. The C-index of the model was 0.74 in the development cohort and 0.69 in the validation cohort. Our nomogram also showed good calibration in both cohorts.
Conclusion: We developed and validated a nomogram to predict the 1- and 3-year OS of patients who received neoadjuvant chemotherapy and radical gastrectomy with D2 lymph node dissection. This nomogram predicts survival more accurately than the AJCC TNM staging system, which is the current golden standard.
Keywords: stomach neoplasms, perioperative chemotherapy, survival, nomograms
Introduction
Gastric cancer is the fifth common cancer and the third leading cause of cancer-related deaths worldwide.1 Nowadays, surgery is the most widely used treatment for patients with localized gastric cancer.2–4 However, after curative resection, the survival rate for locally advanced gastric cancer (AGC) remains to be unsatisfactory.5–7 To improve patients’ survival, a variety of studies have examined the treatment effect of additional chemotherapy and radiotherapy.8–10 Among these, neoadjuvant chemotherapy (or perioperative chemotherapy) was first advocated by Wilke et al.11 It is now widely accepted that neoadjuvant chemotherapy (NAC) can help improve patients’ tolerance, increase curative resection rate, decrease tumor metastasis, and thus increase the survival rate.12–14 As a result of its increasing popularity, there is now an increasing need for practical tools to predict individual survival after NAC.
To our knowledge, the only predictive system for patients received neoadjuvant chemotherapy was the American Joint Committee on Cancer (AJCC) ypTNM staging system, which was established according to the local invasion depth, the number of positive lymph nodes, and distant metastasis.2 However, this system was developed from patients with less extensive lymph node dissection (less than D2) and thus may not be well applied to patients underwent D2 lymphadenectomy. In our previous study, we conducted a validation of this system (patients at T0 stage excluded)15 and demonstrated that although ypTNM staging system was effective for staging, its predictive value was limited with a relatively low C-index (0.657). In addition, patients with a T0 stage were not included in ypTNM staging system and thus cannot be evaluated. Furthermore, other prognostic factors related to individual survival have not been taken into consideration, such as age, body mass index (BMI), tumor size, histology, and chemotherapy regimen. Thus, new tools are needed to predict individual survival.
Previously, no survival nomograms of gastric cancer patients focused on patients received neoadjuvant chemotherapy.16,17 In this study, through evaluating data from 471 consecutive patients undergoing neoadjuvant chemotherapy, we aimed to develop a nomogram to predicts overall survival. External validation was then conducted to test the generalizability of our model on a cohort of 239 patients from a different center.
Materials and Methods
Patients
From January 1st, 2007 to December 31st, 2014, a total of 484 gastric cancer patients at Peking University Cancer Hospital in Beijing, China were retrospectively enrolled in this study. The patients were pathologically diagnosed with gastric adenocarcinoma and received no treatment before neoadjuvant chemotherapy. All patients included in this study were proved to be locally advanced gastric cancer of clinical stage II–III by CT and diagnostic staging laparoscopy. Many of our patients were enrolled in clinical trials for neoadjuvant chemotherapy. For other patients, we would suggest both neoadjuvant chemotherapy and surgery plus adjuvant chemotherapy, a shared decision would then be made after a discussion with patients. The extent of resection for gastric cancer was total or distal gastrectomy with D2 lymphadenectomy. After surgery, all of the patients were recommended to receive adjuvant chemotherapy until perioperative chemotherapy cycles added up to eight cycles. Patients with distant metastasis were excluded from the study. Other exclusion criteria included 1) patients with gastrointestinal stromal tumors, lymphoma, neuroendocrine carcinoma, carcinoid tumor; 2) patients with remnant gastric cancer; 3) patients died within the perioperative period; 4) patients received chemotherapy for other diseases within 6 months; 5) patients whose dissected lymph node are less than 15; 6) patients received neoadjuvant radiotherapy, molecular targeted therapy, or intraperitoneal chemotherapy. Eventually, 471 out of 484 patients were selected and enrolled in our study.
Previous information on demographic, treatment, and pathology were collected, including age, sex, BMI, ASA score, ECOG score, family history, chemotherapy regimen, surgery method, surgery approach, anastomosis way, blood loss, tumor location, tumor diameter, T stage, number of dissected lymph node, number of positive lymph node, histological type, differentiated type, and cancerous embolus situation.
For validation, we enrolled 239 patients who met the same inclusion and exclusion criteria at Sun Yat-sen University Cancer Center (Guangzhou, China) in the validation cohort. In this cohort, data of risk factors in the final nomogram were collected.
Follow Up
After the surgery, patients were followed up regularly via physical examination, radiological examination, endoscopic examination, and laboratory examination. These examinations were performed every 3 to 6 months during the first 2 years, then every 6 months until the fifth year, and then once every year.
Statistic Analysis
To build the nomogram for survival prediction, the univariate Cox regression model was applied to each variate and those with a two-sided p-value <0.05 were then included in the multivariable model. A backward stepwise selection method was used for variable selection in binary Cox regression. A nomogram was then developed based on the selected variables.
The performance of the nomogram was measured by its discrimination and calibration. The discrimination of the nomogram was measured by the concordance index (CI). Calibration, which compares predicted survival with actual survival, was also used to evaluate the model. We plotted the calibration curves for the actual survival against the nomogram predicted survival probabilities to assess the agreement, using 1000 bootstrap re-samples to decrease the overfitting bias.
We used restricted cubic splines to fit the continuous variables to allow for nonlinearity in the relationship between these variables and survival time.
We conducted all analyses using R 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided P value <0.05 was considered statistically significant.
Ethical Standards
The Ethics Committee of Peking University Cancer Hospital approved this study. All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Written informed consent was obtained from all patients prior to inclusion in the study. This study does not involve animal study.
Results
Descriptive Statistics of the Training Cohort
A total of 471 patients were included in this cohort. The baseline characteristics of the participants were provided in Table 1. Overall, 360 (76.4%) patients were males. The average age was 59 (±10.1) years old, with 153 (32.5%) over 65 years old. The average preoperative chemotherapy duration was 2.79 (±1.00) cycles, and surgery was then performed. Most patients (450, 95.5%) were in good pre-operatory conditions with an ECOG score of 0 or 1. After surgery, most patients (79.9%) were proved to be at pathologic stage II/III. The median follow-up duration was 38.5 (±21.7) months, with 193 patients died during the follow-up period (41%). Overall, the 1-year and 3-year OS rates were 90.0% and 64.1%, respectively. The pathology complete remission (pCR) rate was 6.4%.
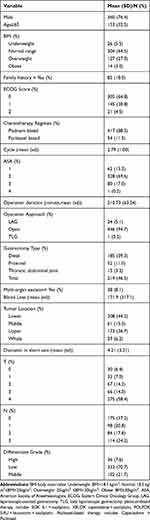 |
Table 1 Baseline Characteristics of the Study Population (n=471) |
Development of the Nomogram
Clinicopathological factors were further evaluated by univariate analysis with the Cox regression model. BMI, chemotherapy cycles, tumor location, multi-organ resection, T stage, N stage, and diameter in the long axis were identified as risk factors for OS (Table 2).
 |
Table 2 Univariant & Multivariant Analysis for Overall Survival |
All the variables above were included in the multivariant analysis, and after the stepwise regression process, T stage, N stage, BMI group, and tumor location were included in the final multivariable model for OS. A nomogram was then developed based on our Cox proportional hazard model (Figure 1).
Validation of the Nomogram
In the training cohort, the C-index of the OS model was 0.74 in the training cohort and 0.75 in bootstrap validations. The calibration curves for 1-year and 3-year OS were shown in Figures 2A and B. The x-axis was the nomogram predicted survival, and the y-axis was the actual survival calculated by the Kaplan–Meier method.
In the validation cohort, the C index was 0.693 (95% CI, 0.671–0.715). Good calibration was also shown for the 1-year, 3-year OS (Figure 2C and D).
Our model also showed superiority in discrimination compared with the AJCC TNM system (8th edition). In our previous study, the discrimination of the TNM staging system was evaluated and the C-index was 0.657.15
Discussion
To make an appropriate clinical decision, it is critical for physicians to determine the prognosis of patients who have received neoadjuvant chemotherapy. Prognostic nomograms based on clinicopathologic factors have been developed for patients who received neoadjuvant chemotherapy for breast,18 esophageal,19 and colorectal cancer.20 However, no nomogram for gastric cancer was available due to limited data.
To our knowledge, although prognostic factors for gastric cancer patients received neoadjuvant chemotherapy had been widely studied before,21,22 the ypTNM staging system was the only predictive model available. However, this system was developed from patients with less extensive lymphadenectomy (less than D2), and thus, the predictive value might be limited. In our previous study, it was shown that the discriminative ability of this system was not high enough to meet clinical demand.15 Moreover, the new ypTNM staging system did not address pCR and ypT0N1 patients. In this study, we developed a nomogram to predict the OS for targeting patients and conducted validation to prove its efficacy.
In our final model, T stage, N stage, BMI group and tumor location were independent prognostic factors for survival. It was not surprising to find that T and N stages both independently affect the prognosis. The prognostic role of T and N had been widely discussed and consensus had been reached that a higher stage correlated with a worse prognosis.
BMI was the only demographic factor correlated with overall survival in our final model and individuals with a higher BMI had a better prognosis. Several studies are in line with our finding on this point. Kong et al and Tokunaga et al reported a higher 5-year survival after gastrectomy for overweight patients.23,24 However, in some other cases, BMI was associated with less lymph node dissection, more surgical complications and higher perioperative morbidity.25,26 Possible explanations for the positive influence of BMI on survival might be that a patient with a higher BMI tends to have a better nutrition status, which increases the tolerance of both neoadjuvant chemotherapy and gastrectomy and thus improve overall survival. In addition, the negative influence of the BMI partly attributed to the increased surgery difficulty and insufficient lymph node dissection. However, all patients included in our research received enough lymph node dissection (D2); thus, the negative influence of a higher BMI might partly be offset.
Tumor location was also selected in the final model. Patients with tumors at the lower third lived longer than those with an upper part disease, and those with a tumor diffused at the whole stomach suffered the worst prognosis. This phenomenon was in accordance with many previous studies. The negative influence on survival of an upper part disease was shown in both single and multivariant analyses.27,28 In a meta-analysis conducted by Petrelli et al, it was shown that compared with distal tumors, proximal tumors suffered a 25% increased risk of mortality.29 Tumors spreading throughout the whole stomach also showed a negative influence, which was also reported in other pieces of research.30,31 Although somehow controversial, this phenomenon may be attributed to two aspects, the biological nature of the tumor and different gastrectomy. For the biology nature, some pieces of research correlated an upper part disease with a higher incidence of HER2 positivity, which is an independent risk factor for overall survival.32,33 And the increased risk of a diffused disease may be attributed to the aggressive biological features. For gastrectomy, patients with a proximal or diffused cancer always receive a total gastrectomy instead of a distal gastrectomy, which may lead to more complications and worse survival outcomes.34
Based on the prognostic factors above, a nomogram was then developed. With pT0 patients included and more risk factors considered, this nomogram may be applied to a broader population of patients. Besides, with a c-index of 0.74 in the training cohort and 0.69 in the validating cohort and good calibration, this nomogram predicted more accurately than the 8th AJCC stage system, whose c-index was 0.66. In addition, compared with the TNM system, our nomogram provided a visible tool easy to use. Thus, our nomogram may contribute to prognosis prediction and decision-making.
There were also several limitations to our study. First, our study did not contain patients at stage IV, so the implications in those patients were limited Second, due to the limited samples and the retrospective nature of our research, bias might exist. Lastly, the samples of ypT0 patients were limited. Thus, the predictive value of our model remained to be seen within them.
Conclusion
We developed and validated a nomogram to predict the 1 year and 3-year OS of patients undergoing neoadjuvant chemotherapy, radical gastrectomy, and D2 lymph node dissection. This nomogram uses readily available clinicopathologic factors and predicts survival more accurately than the AJCC TNM staging system.
Acknowledgments
This study was supported by Beijing Municipal Hospital Authority nos. ZYLX201701.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. NCCN Guidelines Version 1. 2017 staging gastric cancer.
3. Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol. 2014;40(5):584–591. doi:10.1016/j.ejso.2013.09.020
4. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19.
5. Nashimoto A, Akazawa K, Isobe Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16(1):1–27. doi:10.1007/s10120-012-0163-4
6. Ahn HS, Lee HJ, Hahn S, et al. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2010;116(24):5592–5598. doi:10.1002/cncr.25550
7. Zhong Q, Chen QY, Li P, et al. Prediction of conditional probability of survival after surgery for gastric cancer: a study based on eastern and western large data sets. Surgery. 2018;163(6):1307–1316. doi:10.1016/j.surg.2018.02.011
8. Ahn HS, Jeong SH, Son YG, et al. Effect of neoadjuvant chemotherapy on postoperative morbidity and mortality in patients with locally advanced gastric cancer. Br J Surg. 2014;101(12):1560–1565. doi:10.1002/bjs.9632
9. Eom BW, Kim S, Kim JY, et al. Survival benefit of perioperative chemotherapy in patients with locally advanced gastric cancer: a propensity score matched analysis. J Gastric Cancer. 2018;18(1):69–81. doi:10.5230/jgc.2018.18.e9
10. Coccolini F, Nardi M, Montori G, et al. Neoadjuvant chemotherapy in advanced gastric and esophago-gastric cancer. Meta-analysis of randomized trials. Int J Surg. 2018;51:120–127. doi:10.1016/j.ijsu.2018.01.008
11. Wilke H, Preusser P, Fink U, et al. Preoperative chemotherapy in locally advanced and nonresectable gastric cancer: a Phase II study with etoposide, doxorubicin, and cisplatin. J Clin Oncol. 1989;7(9):1318–1326. doi:10.1200/JCO.1989.7.9.1318
12. Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305(22):2335–2342. doi:10.1001/jama.2011.749
13. Kojima N, Yonemura Y, Bando E, et al. Optimal extent of lymph node dissection for T1 gastric cancer, with special reference to the distribution of micrometastasis, and accuracy of preoperative diagnosis for wall invasion. Hepato-Gastroenterology. 2008;55(84):1112–1117.
14. Kim JJ, Song KY, Hur H, Hur JI, Park SM, Park CH. Lymph node micrometastasis in node negative early gastric cancer. Eur J Surg Oncol. 2009;35(4):409–414. doi:10.1016/j.ejso.2008.05.004
15. Li Z, Wang Y, Shan F, et al. ypTNM staging after neoadjuvant chemotherapy in the Chinese gastric cancer population: an evaluation on the prognostic value of the AJCC eighth edition cancer staging system. Gastric Cancer. 2018;21(6):977–987. doi:10.1007/s10120-018-0830-1
16. Ogura K, Fujiwara T, Yasunaga H, et al. Development and external validation of nomograms predicting distant metastases and overall survival after neoadjuvant chemotherapy and surgery for patients with nonmetastatic osteosarcoma: A multi-institutional study. Cancer. 2015;121(21):3844–3852. doi:10.1002/cncr.29575
17. Eom BW, Ryu KW, Nam BH, et al. Survival nomogram for curatively resected Korean gastric cancer patients: multicenter retrospective analysis with external validation. PLoS One. 2015;10(2):e0119671. doi:10.1371/journal.pone.0119671
18. Hwang HW, Jung H, Hyeon J, et al. A nomogram to predict pathologic complete response (pCR) and the value of tumor-infiltrating lymphocytes (TILs) for prediction of response to neoadjuvant chemotherapy (NAC) in breast cancer patients. Breast Cancer Res Treat. 2018.
19. Deng W, Wang Q, Xiao Z, et al. A prognostic nomogram for overall survival after neoadjuvant radiotherapy or chemoradiotherapy in thoracic esophageal squamous cell carcinoma: a retrospective analysis. Oncotarget. 2017;8(25):41102–41112. doi:10.18632/oncotarget.17062
20. Sun Y, Zhang Y, Wu X, et al. Prognostic significance of neoadjuvant rectal score in locally advanced rectal cancer after neoadjuvant chemoradiotherapy and construction of a prediction model. J Surg Oncol. 2018;117(4):737–744. doi:10.1002/jso.24907
21. Biondi A, Agnes A, Del Coco F, et al. Preoperative therapy and long-term survival in gastric cancer: one size does not fit all. Surg Oncol. 2018;27(3):575–583. doi:10.1016/j.suronc.2018.07.006
22. Tang C, Cheng X, Yu S, et al. Platelet-to-lymphocyte ratio and lymphocyte-to-white blood cell ratio predict the efficacy of neoadjuvant chemotherapy and the prognosis of locally advanced gastric cancer patients treated with the oxaliplatin and capecitabine regimen. Onco Targets Ther. 2018;11:7061–7075. doi:10.2147/OTT.S176768
23. Kong F, Li H, Fan Y, et al. Overweight patients achieve ideal body weight following curative gastrectomy resulting in better long-term prognosis. Obes Surg. 2013;23(5):650–656. doi:10.1007/s11695-012-0847-1
24. Tokunaga M, Hiki N, Fukunaga T, Ohyama S, Yamaguchi T, Nakajima T. Better 5-year survival rate following curative gastrectomy in overweight patients. Ann Surg Oncol. 2009;16(12):3245–3251. doi:10.1245/s10434-009-0645-8
25. Yasunaga H, Horiguchi H, Matsuda S, Fushimi K, Hashimoto H, Ayanian JZ. Body mass index and outcomes following gastrointestinal cancer surgery in Japan. Br J Surg. 2013;100(10):1335–1343. doi:10.1002/bjs.9221
26. Bickenbach KA, Denton B, Gonen M, Brennan MF, Coit DG, Strong VE. Impact of obesity on perioperative complications and long-term survival of patients with gastric cancer. Ann Surg Oncol. 2013;20(3):780–787. doi:10.1245/s10434-012-2653-3
27. Talamonti MS, Kim SP, Yao KA, et al. Surgical outcomes of patients with gastric carcinoma: the importance of primary tumor location and microvessel invasion. Surgery. 2003;134(4):720–727. doi:10.1016/S0039-6060(03)00337-4
28. Han D-S, Suh Y-S, Kong S-H, et al. Nomogram predicting long-term survival after D2 gastrectomy for gastric cancer. J Clin Oncol. 2012;30(31):3834–3840. doi:10.1200/JCO.2012.41.8343
29. Petrelli F, Ghidini M, Barni S, et al. Prognostic role of primary tumor location in non-metastatic gastric cancer: a systematic review and meta-analysis of 50 studies. Ann Surg Oncol. 2017;24(9):2655–2668. doi:10.1245/s10434-017-5832-4
30. Chou HH, Kuo CJ, Hsu JT, et al. Clinicopathologic study of node-negative advanced gastric cancer and analysis of factors predicting its recurrence and prognosis. Am J Surg. 2013;205(6):623–630. doi:10.1016/j.amjsurg.2012.04.014
31. Hsu JT, Lin CJ, Sung CM, et al. Prognostic significance of the number of examined lymph nodes in node-negative gastric adenocarcinoma. Eur J Surg Oncol. 2013;39(11):1287–1293. doi:10.1016/j.ejso.2013.07.183
32. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a Phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi:10.1016/S0140-6736(10)61121-X
33. Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes–a systematic review. Int J Cancer. 2012;130(12):2845–2856. doi:10.1002/ijc.26292
34. Liu Z, Feng F, Guo M, et al. Distal gastrectomy versus total gastrectomy for distal gastric cancer. Medicine. 2017;96(5).
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


